UV-C Light-Based Surface Disinfection: Analysis of Its Virucidal Efficacy Using a Bacteriophage Model
Abstract
:1. Introduction
2. Materials and Methods
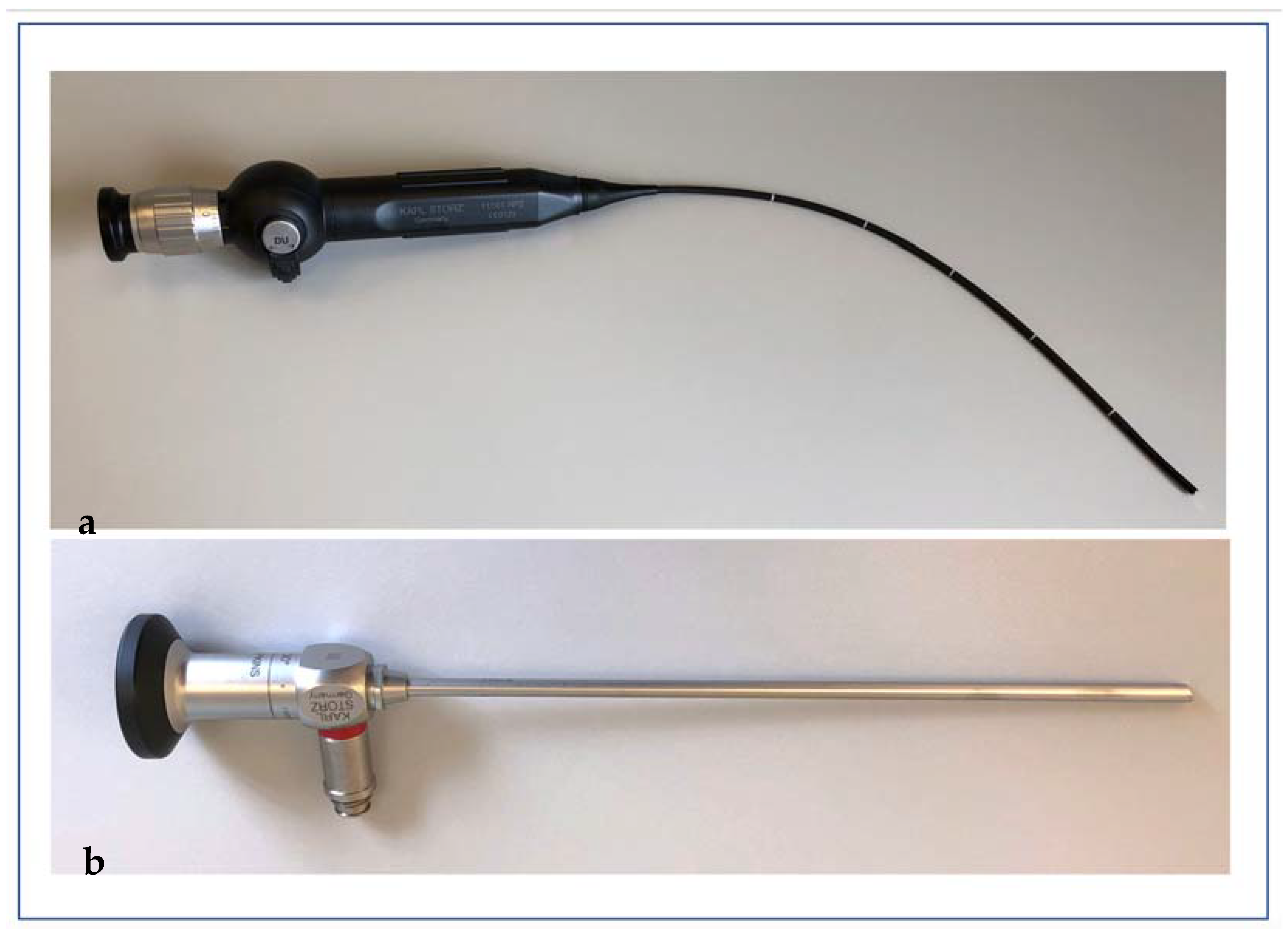
2.1. The D25 UV Light System
2.2. Statistics and Ethics Approval
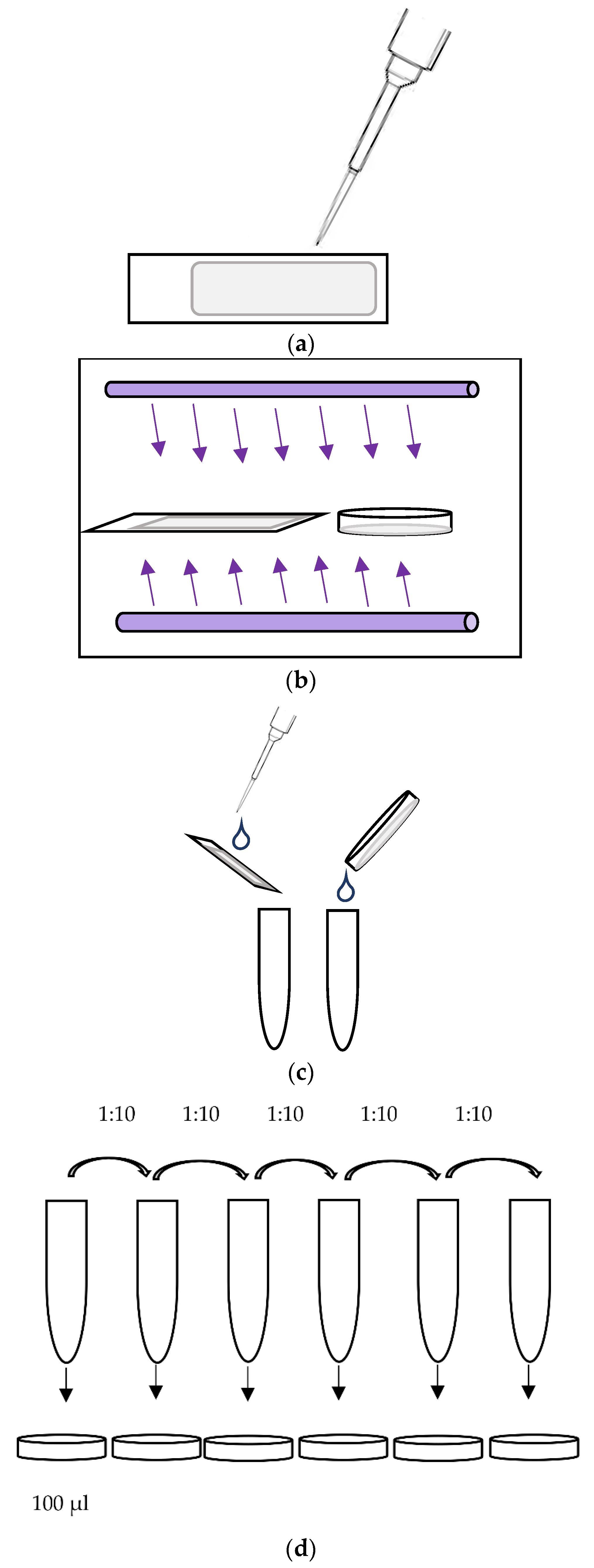
2.3. Virucidal Testing
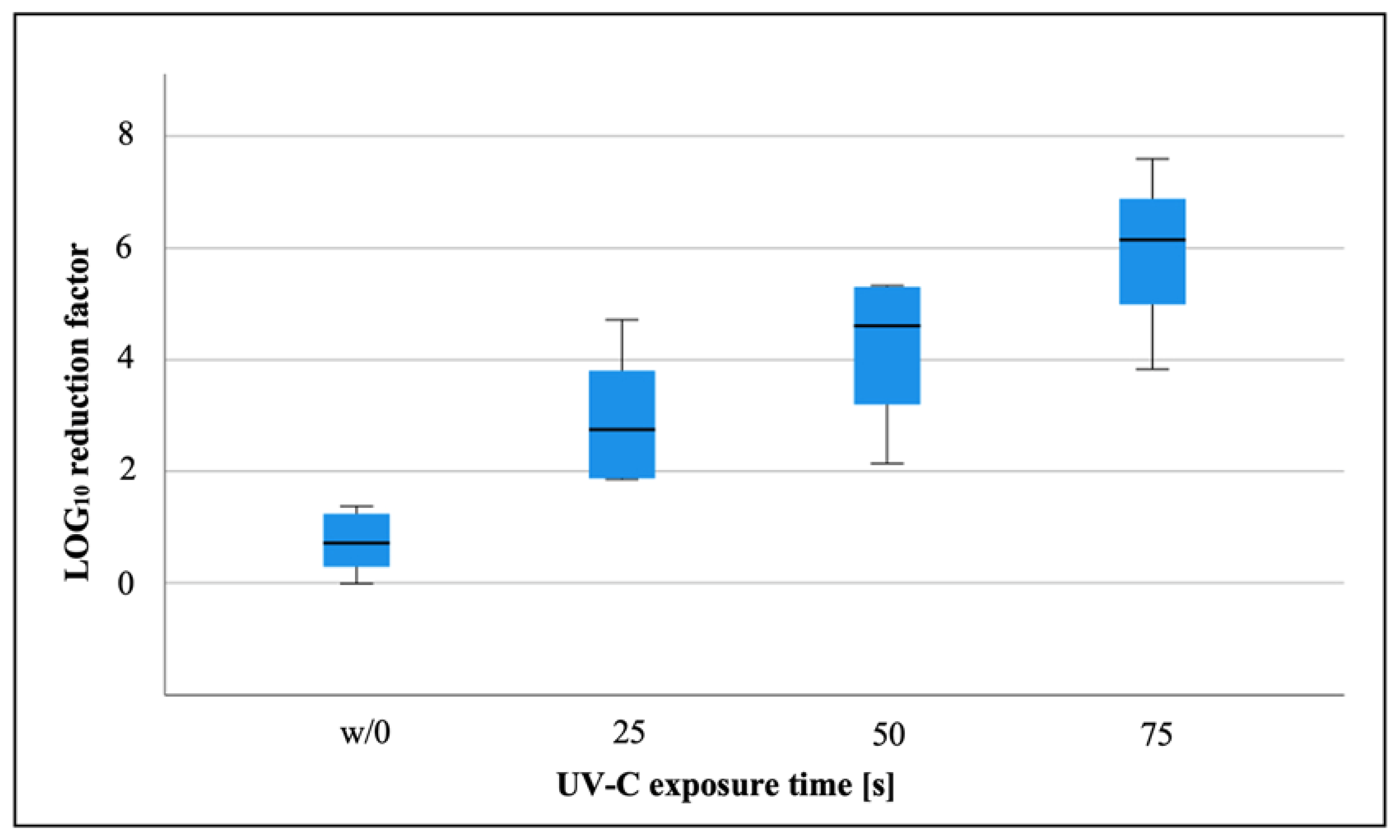
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spaulding, E.H. Chemical disinfection of medical and surgical materials. In Disinfection, Sterilization, and Preservation; Lawrence, C., Block, S.S., Eds.; Lea & Febiger: Philadelphia, PA, USA, 1968; pp. 517–531. [Google Scholar]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
- Parasher, A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef]
- Li, Y.; Ren, B.; Peng, X.; Hu, T.; Li, J.; Gong, T.; Tang, B.; Xu, X.; Zhou, X. Saliva is a non-negligible factor in the spread of COVID-19. Mol. Oral Microbiol. 2020, 35, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Kutter, J.S.; Spronken, M.I.; Fraaij, P.L.; Fouchier, R.A.; Herfst, S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018, 28, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Finsen, N.R. Om Anvendelse i Medicinen af Koncentrerede Kemiske Lysstraaler; Gyldendal: Copenhagen, Denmark, 1896; pp. 1–64. [Google Scholar]
- Lectures, N. Physiology or Medicine 1901–1921; Elsevier: Amsterdam, The Netherlands, 1967; pp. 121–128. [Google Scholar]
- Fenton, L.; Moseley, H. UV emissions from low energy artificial light sources. Photodermatol. Photoimmunol. Photomed. 2014, 30, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyon, B.A.; Milsk, R.Y.; DeAngelo, A.B.; Simmons, J.E.; Moyer, M.P.; Weinberg, H.S. Integrated chemical and toxicological investigation of UV-chlorine/chloramine drinking water treatment. Environ. Sci. Technol. 2014, 48, 6743–6753. [Google Scholar] [CrossRef]
- Marra, A.R.; Schweizer, M.L.; Edmond, M.B. No-Touch Disinfection Methods to Decrease Multidrug-Resistant Organism Infections: A Systematic Review and Meta-analysis. Infect. Control. Hosp. Epidemiol. 2018, 39, 20–31. [Google Scholar] [CrossRef]
- Chen, L.H.; Li, Y.; Qi, Y.; Wang, S.N.; Gao, C.Q.; Wu, Y. Evaluation of a pulsed xenon ultraviolet light device for reduction of pathogens with biofilm-forming ability and impact on environmental bioburden in clinical laboratories. Photodiagn. Photodyn. Ther. 2020, 29, 101544. [Google Scholar] [CrossRef]
- Lindblad, M.; Tano, E.; Lindahl, C.; Huss, F. Ultraviolet-C decontamination of a hospital room: Amount of UV light needed. Burns 2020, 46, 842–849. [Google Scholar] [CrossRef]
- Hamzavi, I.H.; Lyons, A.B.; Kohli, I.; Narla, S.; Parks-Miller, A.; Gelfand, J.M.; Lim, H.W.; Ozog, D.M. Ultraviolet germicidal irradiation: Possible method for respirator disinfection to facilitate reuse during the COVID-19 pandemic. J. Am. Acad. Dermatol. 2020, 82, 1511–1512. [Google Scholar] [CrossRef]
- Rudhart, S.A.; Günther, F.; Dapper, L.; Thangavelu, K.; Gehrt, F.; Stankovic, P.; Wilhelm, T.; Guenzel, T.; Stuck, B.A.; Hoch, S. UV light-based decontamination: An effective and fast way for disinfection of endoscopes in otorhinolaryngology? Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2363–2369. [Google Scholar] [CrossRef] [PubMed]
- Park, G.W.; Linden, K.G.; Sobsey, M.D. Inactivation of murine norovirus, feline calicivirus and echovirus 12 as surrogates for human norovirus (NoV) and coliphage (F+) MS2 by ultraviolet light (254 nm) and the effect of cell association on UV inactivation. Lett. Appl. Microbiol. 2011, 52, 162–167. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Ultraviolet Disinfection Guidance Manual for the Final Long Term 2Enhanced Surface Water Treatment Rule (4601); Office of Water: Washington, DC, USA, 2006.
- Madar, R.; Novakova, E.; Baska, T. The role of non-critical health-care tools in the transmission of nosocomial infections. Bratisl. Lek. Listy 2005, 106, 348. [Google Scholar] [PubMed]
- Harris, G.D.; Adams, V.D.; Sorensen, D.L.; Curtis, M.S. Ultraviolet inactivation of selected bacteria and viruses with photoreactivation of the bacteria. Water Res. 1987, 21, 687–692. [Google Scholar] [CrossRef]
- Wurtmann, E.J.; Wolin, S.L. RNA under attack: Cellular handling of RNA damage. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 34–49. [Google Scholar] [CrossRef] [Green Version]
- Rudhart, S.A.; Günther, F.; Dapper, L.; Thangavelu, K.; Geisthoff, U.W.; Stankovic, P.; Wilhelm, T.; Stuck, B.A.; Hoch, S. UV light-based reprocessing of flexible endoscopes without working channel in Oto-Rhino-Laryngology: An effective method? Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 4075–4080. [Google Scholar] [CrossRef]
- Heilingloh, C.S.; Aufderhorst, U.W.; Schipper, L.; Dittmer, U.; Witzke, O.; Yang, D.; Zheng, X.; Sutter, K.; Trilling, M.; Alt, M.; et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am. J. Infect. Control. 2020, 48, 1273–1275. [Google Scholar] [CrossRef]
- Heimbuch, B.; Harnish, D. Research to mitigate a shortage of respiratory protection devices during public health emergencies. Appl. Res. Assoc. 2019, 275, 14–25. [Google Scholar]
- MaCkey, E.D.; Hargy, T.M.; Wright, H.B.; Malley, J.P., Jr.; Cushing, R.S. Comparing Cryptosporidium and MS2 bioassays—implications for UV reactor validation. J.-Am. Water Works Assoc. 2002, 94, 62–69. [Google Scholar] [CrossRef]
- Beck, S.E.; Rodriguez, R.A.; Hawkins, M.A.; Hargy, T.M.; Larason, T.C.; Linden, K.G. Comparison of UV-Induced Inactivation and RNA Damage in MS2 Phage across the Germicidal UV Spectrum. Appl. Environ. Microbiol. 2015, 82, 1468–1474. [Google Scholar] [CrossRef] [Green Version]
- Commission for Hospital Hygiene and Infection Prevention (KRINKO). Hygiene requirements for the reprocessing of medical devices. Recommendation of the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch Institute (RKI) and the Federal Institute for Drugs and Medical Devices (BfArM). Bundesgesundheitsblatt Gesundh. Gesundh. 2012, 55, 1244–1310. [Google Scholar] [CrossRef] [Green Version]
- Simmons, B.P. CDC guidelines for the prevention and control of nosocomial infections. Guideline for hospital environmental control. Am. J. Infect. Control 1983, 11, 97–120. [Google Scholar] [CrossRef]
- Muscarella, L.F. High-level disinfection or “sterilization” of endoscopes? Infect. Control Hosp. Epidemiol. 1996, 17, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Katara, G.; Hemvani, N.; Chitnis, S.; Chitnis, V.; Chitnis, D.S. Surface disinfection by exposure to germicidal UV light. Indian J. Med. Microbiol. 2008, 26, 241–242. [Google Scholar] [CrossRef]
- Deshmukh, J.; Pofahl, R.; Haase, I. Epidermal Rac1 regulates the DNA damage response and protects from UV-light-induced keratinocyte apoptosis and skin carcinogenesis. Cell Death Dis. 2017, 8, e2664. [Google Scholar] [CrossRef] [Green Version]
- Boyce, J.M.; Havill, N.L.; Moore, B.A. Terminal decontamination of patient rooms using an automated mobile UV light unit. Infect. Control Hosp. Epidemiol. 2011, 32, 737–742. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudhart, S.A.; Günther, F.; Dapper, L.; Stuck, B.A.; Hoch, S. UV-C Light-Based Surface Disinfection: Analysis of Its Virucidal Efficacy Using a Bacteriophage Model. Int. J. Environ. Res. Public Health 2022, 19, 3246. https://doi.org/10.3390/ijerph19063246
Rudhart SA, Günther F, Dapper L, Stuck BA, Hoch S. UV-C Light-Based Surface Disinfection: Analysis of Its Virucidal Efficacy Using a Bacteriophage Model. International Journal of Environmental Research and Public Health. 2022; 19(6):3246. https://doi.org/10.3390/ijerph19063246
Chicago/Turabian StyleRudhart, Stefan A., Frank Günther, Laura Dapper, Boris A. Stuck, and Stephan Hoch. 2022. "UV-C Light-Based Surface Disinfection: Analysis of Its Virucidal Efficacy Using a Bacteriophage Model" International Journal of Environmental Research and Public Health 19, no. 6: 3246. https://doi.org/10.3390/ijerph19063246
APA StyleRudhart, S. A., Günther, F., Dapper, L., Stuck, B. A., & Hoch, S. (2022). UV-C Light-Based Surface Disinfection: Analysis of Its Virucidal Efficacy Using a Bacteriophage Model. International Journal of Environmental Research and Public Health, 19(6), 3246. https://doi.org/10.3390/ijerph19063246






