Direct Medical Costs of Parkinson’s Disease in Southern China: A Cross-Sectional Study Based on Health Insurance Claims Data in Guangzhou City
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Design
2.3. Cost Estimation and Its Predictors
2.4. Measures and Variables
2.5. Statistical Analysis
2.6. Ethical Consideration
3. Results
3.1. Patient Characteristics
3.2. Direct Medical Costs and Costs Composition by Insurance Types
3.3. Patient Characteristics Related to Inpatient Costs by Types of Insurance
3.4. Predictors of Inpatient Costs
4. Discussion
4.1. Costs Comparisons with Previous Studies in Other Countries
4.2. Costs Comparisons with Previous Studies in China
4.3. Comparisons of Cost Composition
4.4. Difference in Costs between Two Insurance Schemes
4.5. Influential Factors of Inpatient Costs
4.6. Limitations
4.7. Practical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Parkinson’s Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [Green Version]
- Kalia, L.V.; Lang, A.E. Parkinsons disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Hollerhage, M. Secondary parkinsonism due to drugs, vascular lesions, tumors, trauma, and other insults. Int. Rev. Neurobiol. 2019, 149, 377–418. [Google Scholar]
- Lopez-Sendon, J.; Mena, M.A.; de Yebenes, J.G. Drug-induced parkinsonism. Expert. Opin. Drug Saf. 2013, 12, 487–496. [Google Scholar] [CrossRef]
- GBD Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [Green Version]
- Qi, S.; Yin, P.; Wang, L.; Qu, M.; Kan, G.L.; Zhang, H.; Zhang, Q.; Xiao, Y.; Deng, Y.; Dong, Z.; et al. Prevalence of Parkinson’s Disease: A Community-Based Study in China. Mov. Disord. 2021, 36, 2940–2944. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.P.; Ziegler, U.; Deuschl, G.; Dodel, R.; Doblhammer-Reiter, G. Projected numbers of people with movement disorders in the years 2030 and 2050. Mov. Disord. 2011, 26, 2286–2290. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Tsai, S.-T. The Epidemiology of Parkinson’s Disease. Tzu Chi Med. J. 2010, 22, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Hamilton, J.L.; Kopil, C.; Beck, J.C.; Tanner, C.M.; Albin, R.L.; Ray Dorsey, E.; Dahodwala, N.; Cintina, I.; Hogan, P.; et al. Current and projected future economic burden of Parkinson’s disease in the U.S. NPJ Parkinson’s Dis. 2020, 6, 15. [Google Scholar] [CrossRef]
- Li, G.; Ma, J.; Cui, S.; He, Y.; Xiao, Q.; Liu, J.; Chen, S. Parkinson’s disease in China: A forty-year growing track of bedside work. Transl. Neurodegener. 2019, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.; Fang, H.; Liu, X.; Yuan, B.; Xu, J. Consolidating the social health insurance schemes in China: Towards an equitable and efficient health system. Lancet 2015, 386, 1484–1492. [Google Scholar] [CrossRef]
- Chen, R.; Li, N.X.; Liu, X. Study on the equity of medical services utilization for elderly enrolled in different basic social medical insurance systems in an underdeveloped city of Southwest China. Int. J. Equity Health 2018, 17, 147. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.G.; Vortherms, S.A.; Hong, X. China’s Health Reform Update. Annu. Rev. Public Health 2017, 38, 431–448. [Google Scholar] [CrossRef] [Green Version]
- Dahodwala, N.; Li, P.X.; Jahnke, J.; Ladage, V.P.; Pettit, A.R.; Kandukuri, P.L.; Bao, Y.J.; Zamudio, J.; Jalundhwala, Y.J.; Doshi, J.A. Burden of Parkinson’s Disease by Severity: Health Care Costs in the US Medicare Population. Mov. Disord. 2021, 36, 133–142. [Google Scholar] [CrossRef]
- Bohingamu Mudiyanselage, S.; Watts, J.J.; Abimanyi-Ochom, J.; Lane, L.; Murphy, A.T.; Morris, M.E.; Iansek, R. Cost of Living with Parkinson’s Disease over 12 Months in Australia: A Prospective Cohort Study. Parkinsons Dis. 2017, 2017, 5932675. [Google Scholar] [CrossRef] [PubMed]
- Hjalte, F.; Norlin, J.M.; Kellerborg, K.; Odin, P. Parkinson’s disease in Sweden-resource use and costs by severity. Acta Neurol. Scand. 2021, 144, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Von Campenhausen, S.; Winter, Y.; Rodrigues e Silva, A.; Sampaio, C.; Ruzicka, E.; Barone, P.; Poewe, W.; Guekht, A.; Mateus, C.; Pfeiffer, K.P.; et al. Costs of illness and care in Parkinson’s disease: An evaluation in six countries. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2011, 21, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Tamas, G.; Gulacsi, L.; Bereczki, D.; Baji, P.; Takats, A.; Brodszky, V.; Pentek, M. Quality of life and costs in Parkinson’s disease: A cross sectional study in Hungary. PLoS ONE 2014, 9, e107704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamabe, K.; Liebert, R.; Flores, N.; Pashos, C. Health-related quality-of-life, work productivity, and economic burden among patients with Parkinson’s disease in Japan. J. Med. Econ. 2018, 21, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Tan, L.C.; Li, S.C.; Au, W.L.; Seah, S.H.; Lau, P.N.; Luo, N.; Wee, H.L. Economic burden of Parkinson’s disease in Singapore. Eur. J. Neurol. 2011, 18, 519–526. [Google Scholar] [CrossRef]
- Prado, M.; Jamora, R.D. Cost of Parkinson’s disease among Filipino patients seen at a public tertiary hospital in Metro Manila. J. Clin. Neurosci. 2020, 74, 41–46. [Google Scholar] [CrossRef]
- Bovolenta, T.M.; de Azevedo Silva, S.M.C.; Saba, R.A.; Borges, V.; Ferraz, H.B.; Felicio, A.C. Average annual cost of Parkinson’s disease in Sao Paulo, Brazil, with a focus on disease-related motor symptoms. Clin. Interv. Aging 2017, 12, 2095–2108. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.X.; Chen, L. Economic Burden Analysis of Parkinson’s Disease Patients in China. Parkinsons Dis. 2017, 2017, 8762939. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Cheng, Q.; Zheng, R.; Tan, Y.Y.; Sun, X.K.; Zhou, H.Y.; Ye, X.L.; Wang, Y.; Wang, Z.; Sun, B.M.; et al. Economic burden of Parkinson’s disease in a developing country: A retrospective cost analysis in Shanghai, China. Mov. Disord. 2006, 21, 1439–1443. [Google Scholar] [CrossRef]
- Yang, Y.; Nicholas, S.; Li, S.; Huang, Z.; Chen, X.; Ma, Y.; Shi, X. Health care utilization for patients with stroke: A 3-year cross-sectional study of China’s two urban health insurance schemes across four cities. BMC Public Health 2021, 21, 531. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Wang, Y.; Ye, X.; Zhu, D.; Shi, X.; He, P. Antidepressant use and expenditure in the treatment of patients with depression: Evidence from China urban medical claims data. J. Affect. Disord. 2022, 296, 603–608. [Google Scholar] [CrossRef]
- Yin, X.J.; Xu, Y.; Man, X.W.; Liu, L.M.; Jiang, Y.; Zhao, L.Y.; Cheng, W. Direct costs of both inpatient and outpatient care for all type cancers: The evidence from Beijing, China. Cancer Med. 2019, 8, 3250–3260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Sun, Y.; Zhang, D.; Zhang, C.; Chen, G. Direct medical costs for patients with schizophrenia: A 4-year cohort study from health insurance claims data in Guangzhou city, Southern China. Int. J. Ment. Health Syst. 2018, 12, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhang, D.; Yin, Y.; Zhang, C.; Huang, Y. Costs of Hospitalization for Dementia in Urban China: Estimates from Two Urban Health Insurance Scheme Claims Data in Guangzhou City. Int. J. Environ. Res. Public Health 2019, 16, 2781. [Google Scholar] [CrossRef] [Green Version]
- GuangzhouStatisticsBureau. Guangzhou City Statistics Bulletin 2013. Available online: http://112.94.72.17/portal/queryInfo/statisticsYearbook/index (accessed on 13 December 2021). (In Chinese).
- Zhang, H.; Yin, Y.; Zhang, C.; Zhang, D. Costs of hospitalization for stroke from two urban health insurance claims data in Guangzhou City, southern China. BMC Health Serv. Res. 2019, 19, 671. [Google Scholar] [CrossRef]
- Andersen, R.M. Revisiting the behavioral model and access to medical care: Does it matter? J. Health Soc. Behav. 1995, 36, 1–10. [Google Scholar] [CrossRef]
- Heider, D.; Matschinger, H.; Muller, H.; Saum, K.U.; Quinzler, R.; Haefeli, W.E.; Wild, B.; Lehnert, T.; Brenner, H.; Konig, H.H. Health care costs in the elderly in Germany: An analysis applying Andersen’s behavioral model of health care utilization. BMC Health Serv. Res. 2014, 14, 71. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.I.; Kim, S. The determinants of caregiver use and its costs for elderly inpatients in Korea: A study applying Andersen’s behavioral model of health care utilization and replacement cost method. BMC Health Serv. Res. 2021, 21, 631. [Google Scholar] [CrossRef]
- Kowal, S.L.; Dall, T.M.; Chakrabarti, R.; Storm, M.V.; Jain, A. The current and projected economic burden of Parkinson’s disease in the United States. Mov. Disord. 2013, 28, 311–318. [Google Scholar] [CrossRef]
- McCrone, P.; Allcock, L.M.; Burn, D.J. Predicting the cost of Parkinson’s disease. Mov. Disord. 2007, 22, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Winter, Y.; von Campenhausen, S.; Reese, J.P.; Balzer-Geldsetzer, M.; Longo, K.; Spiga, G.; Boetzel, K.; Eggert, K.; Oertel, W.H.; Dodel, R.; et al. Costs of Parkinson’s disease and antiparkinsonian pharmacotherapy: An Italian cohort study. Neurodegener. Dis. 2010, 7, 365–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, Y.; von Campenhausen, S.; Brozova, H.; Skoupa, J.; Reese, J.P.; Botzel, K.; Eggert, K.; Oertel, W.H.; Dodel, R.; Ruzicka, E. Costs of Parkinson’s disease in eastern Europe: A Czech cohort study. Parkinsonism Relat. Disord. 2010, 16, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Winter, Y.; von Campenhausen, S.; Popov, G.; Reese, J.P.; Klotsche, J.; Botzel, K.; Gusev, E.; Oertel, W.H.; Dodel, R.; Guekht, A. Costs of illness in a Russian cohort of patients with Parkinson’s disease. Pharmacoeconomics 2009, 27, 571–584. [Google Scholar] [CrossRef]
- Yoritaka, A.; Fukae, J.; Hatano, T.; Oda, E.; Hattori, N. The Direct Cost of Parkinson Disease at Juntendo Medical University Hospital, Japan. Intern. Med. 2016, 55, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Findley, L.; Aujla, M.; Bain, P.G.; Baker, M.; Beech, C.; Bowman, C.; Holmes, J.; Kingdom, W.K.; MacMahon, D.G.; Peto, V.; et al. Direct economic impact of Parkinson’s disease: A research survey in the United Kingdom. Mov. Disord. 2003, 18, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Man, X.; Nicholas, S.; Li, S.; Bai, Q.; Huang, L.; Ma, Y.; Shi, X. Utilisation of health services among urban patients who had an ischaemic stroke with different health insurance—A cross-sectional study in China. BMJ Open 2020, 10, e040437. [Google Scholar] [CrossRef]
- Weir, S.; Samnaliev, M.; Kuo, T.C.; Tierney, T.S.; Walleser Autiero, S.; Taylor, R.S.; Schrag, A. Short- and long-term cost and utilization of health care resources in Parkinson’s disease in the UK. Mov. Disord. 2018, 33, 974–981. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wang, S.; Jia, W.; Han, K.; Song, Y.; Liu, S.; Li, X.; Liu, M.; He, Y. Spatiotemporal Analysis of the Prevalence and Pattern of Multimorbidity in Older Chinese Adults. Front. Med. 2021, 8, 806616. [Google Scholar] [CrossRef]
- Nwabuobi, L.; Zhang, C.; Henchcliffe, C.; Shah, H.; Sarva, H.; Lee, A.; Kamel, H. Characteristics and Outcomes of Parkinson’s Disease Individuals Hospitalized with COVID-19 in a New York City Hospital System. Mov. Disord. Clin. Pract. 2021, 8, 1100–1106. [Google Scholar] [CrossRef]
- Orozco, J.L.; Valderrama-Chaparro, J.A.; Pinilla-Monsalve, G.D.; Molina-Echeverry, M.I.; Pérez Castaño, A.M.; Ariza-Araújo, Y.; Prada, S.I.; Takeuchi, Y. Parkinson’s disease prevalence, age distribution and staging in Colombia. Neurol. Int. 2020, 12, 8401. [Google Scholar] [CrossRef]
- Kuusimäki, T.; Al-Abdulrasul, H.; Kurki, S.; Hietala, J.; Hartikainen, S.; Koponen, M.; Tolppanen, A.M.; Kaasinen, V. Increased Risk of Parkinson’s Disease in Patients With Schizophrenia Spectrum Disorders. Mov. Disord. 2021, 36, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Smeland, O.B.; Shadrin, A.; Bahrami, S.; Broce, I.; Tesli, M.; Frei, O.; Wirgenes, K.V.; O’Connell, K.S.; Krull, F.; Bettella, F.; et al. Genome-wide Association Analysis of Parkinson’s Disease and Schizophrenia Reveals Shared Genetic Architecture and Identifies Novel Risk Loci. Biol. Psychiatry 2021, 89, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Chendo, I.; Fabbri, M.; Godinho, C.; Moiron Simoes, R.; Severiano Sousa, C.; Coelho, M.; Voon, V.; Ferreira, J.J. High frequency of psychosis in late-stage Parkinsons disease. Clin. Park. Relat. Disord. 2021, 5, 100119. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Rathouz, P.J. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics 2005, 6, 93–109. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.C.; Miller, G.E. Health expenditure estimation and functional form: Applications of the generalized gamma and extended estimating equations models. Health Econ. 2010, 19, 608–627. [Google Scholar] [CrossRef]
- Spector, W.D.; Limcangco, R.; Owens, P.L.; Steiner, C.A. Marginal Hospital Cost of Surgery-related Hospital-acquired Pressure Ulcers. Med. Care 2016, 54, 845–851. [Google Scholar] [CrossRef]
- Spector, W.D.; Limcangco, R.; Furukawa, M.F.; Encinosa, W.E. The Marginal Costs of Adverse Drug Events Associated With Exposures to Anticoagulants and Hypoglycemic Agents During Hospitalization. Med. Care 2017, 55, 856–863. [Google Scholar] [CrossRef]
- CCEMG-EPPI-CentreCostConverter. Available online: http://eppi.ioe.ac.uk/costconversion/default.aspx (accessed on 13 December 2021).
- Bovolenta, T.M.; de Azevedo Silva, S.M.; Arb Saba, R.; Borges, V.; Ferraz, H.B.; Felicio, A.C. Systematic Review and Critical Analysis of Cost Studies Associated with Parkinson’s Disease. Parkinsons Dis. 2017, 2017, 3410946. [Google Scholar] [CrossRef]
- Gong, L.B. A Comparative Study between the National Basic Medical Insurance, Industrial Injury Insurance, and Maternity Insurance Medicines List in 2009 Edition and 2004 Edition. Drug Eval. 2010, 7, 24–30. (In Chinese) [Google Scholar]
- Liu, X.; Wong, H.; Liu, K. Outcome-based health equity across different social health insurance schemes for the elderly in China. BMC Health Serv. Res. 2016, 16, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Qiu, Y.; He, Z. Is universal and uniform health insurance better for China? Evidence from the perspective of supply-induced demand. Health Econ. Policy Law 2020, 15, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lei, X.; Strauss, J.; Zhao, Y. Health Insurance and Health Care among the Mid-Aged and Older Chinese: Evidence from the National Baseline Survey of CHARLS. Health Econ. 2017, 26, 431–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Xiong, X.; Xue, Q.; Yao, L.; Luo, F.; Xiang, L. The impact of medical insurance policies on the hospitalization services utilization of people with schizophrenia: A case study in Changsha, China. Pak. J. Med. Sci. 2013, 29, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Schuepbach, W.M.; Rau, J.; Knudsen, K.; Volkmann, J.; Krack, P.; Timmermann, L.; Hälbig, T.D.; Hesekamp, H.; Navarro, S.M.; Meier, N.; et al. Neurostimulation for Parkinson’s disease with early motor complications. N. Engl. J. Med. 2013, 368, 610–622. [Google Scholar] [CrossRef] [Green Version]
- Stroupe, K.T.; Smith, B.; Weaver, F.M.; Gonzalez, B.; Huo, Z.; Cao, L.; Ippolito, D.; Follett, K.A. Healthcare Utilization and Costs for Patients With Parkinson’s Disease After Deep Brain Stimulation. Mov. Disord. Clin. Pract. 2019, 6, 369–378. [Google Scholar] [CrossRef]
- Lopez-Sendon, J.L.; Mena, M.A.; de Yebenes, J.G. Drug-induced parkinsonism in the elderly: Incidence, management and prevention. Drugs Aging 2012, 29, 105–118. [Google Scholar] [CrossRef]
- Chang, S.S.; Wu, J.H.; Liu, Y.; Zhang, T.; Du, X.; Dong, J.Z.; Lip, G.Y.H.; Ma, C.S. In-hospital direct costs for thromboembolism and bleeding in Chinese patients with atrial fibrillation. Chronic. Dis. Transl. Med. 2018, 4, 127–134. [Google Scholar] [CrossRef]
- Wei, J.W.; Heeley, E.L.; Jan, S.; Huang, Y.; Huang, Q.; Wang, J.G.; Cheng, Y.; Xu, E.; Yang, Q.; Anderson, C.S.; et al. Variations and determinants of hospital costs for acute stroke in China. PLoS ONE 2010, 5, e13041. [Google Scholar] [CrossRef]
- Liu, H.P.; Zhu, C.; Cao, J.; Jiao, J.; Song, B.Y.; Jin, J.F.; Liu, Y.L.; Wen, X.X.; Cheng, S.Z.; Wu, X.J. Hospitalization costs among immobile patients with hemorrhagic or ischemic stroke in China: A multicenter cross-sectional study. BMC Health Serv. Res. 2020, 20, 905. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, D. Management of psychiatric disorders in Parkinson’s disease: Neurotherapeutics—Movement Disorders Therapeutics. Neurother. J. Am. Soc. Exp. Neurother. 2020, 17, 1511–1524. [Google Scholar] [CrossRef] [PubMed]
- Hermanowicz, N.; Edwards, K. Parkinson’s disease psychosis: Symptoms, management, and economic burden. Am. J. Manag. Care 2015, 21 (Suppl. S10), s199–s206. [Google Scholar] [PubMed]
- Szeto, J.Y.; Lewis, S.J. Current Treatment Options for Alzheimer’s Disease and Parkinson’s Disease Dementia. Curr. Neuropharmacol. 2016, 14, 326–338. [Google Scholar] [CrossRef]
- Winter, Y.; Balzer-Geldsetzer, M.; Spottke, A.; Reese, J.P.; Baum, E.; Klotsche, J.; Rieke, J.; Simonow, A.; Eggert, K.; Oertel, W.H.; et al. Longitudinal study of the socioeconomic burden of Parkinson’s disease in Germany. Eur. J. Neurol. 2010, 17, 1156–1163. [Google Scholar] [CrossRef]
- Herrmann, N.; Marras, C.; Fischer, H.D.; Wang, X.; Anderson, G.M.; Rochon, P.A. Management of neuropsychiatric symptoms in long-term care residents with Parkinson’s disease: A retrospective cohort study. Drugs Aging 2013, 30, 19–22. [Google Scholar] [CrossRef]
- Teggi, D. Care homes as hospices for the prevalent form of dying: An analysis of long-term care provision towards the end of life in England. Soc. Sci. Med. 2020, 260, 113150. [Google Scholar] [CrossRef]
- Yang, W.; Wu, B.; Tan, S.Y.; Li, B.; Lou, V.W.Q.; Chen, Z.A.; Chen, X.; Fletcher, J.R.; Carrino, L.; Hu, B.; et al. Understanding Health and Social Challenges for Aging and Long-Term Care in China. Res. Aging 2021, 43, 127–135. [Google Scholar] [CrossRef]
- Zhu, Y.; Österle, A. China’s policy experimentation on long-term care insurance: Implications for access. Int. J. Health Plann. Manag. 2019, 34, e1661–e1674. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Glinskaya, E.; Chen, H.; Gong, S.; Qiu, Y.; Xu, J.; Yip, W. Long-term care system for older adults in China: Policy landscape, challenges, and future prospects. Lancet 2020, 396, 1362–1372. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Z.; Yu, Y. Does long-term care insurance reduce hospital utilization and medical expenditures? Evidence from China. Soc. Sci. Med. 2020, 258, 113081. [Google Scholar] [CrossRef] [PubMed]
- Barbour, P.J.; Arroyo, J.; High, S.; Fichera, L.B.; Staska-Pier, M.M.; McMahon, M.K. Telehealth for patients with Parkinson’s disease: Delivering efficient and sustainable long-term care. Hosp. Pract. 2016, 44, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Quercioli, C.; Nistico, F.; Troiano, G.; Maccari, M.; Messina, G.; Barducci, M.; Carriero, G.; Golinelli, D.; Nante, N. Developing a new predictor of health expenditure: Preliminary results from a primary healthcare setting. Public Health 2018, 163, 121–127. [Google Scholar] [CrossRef] [PubMed]
| UEBMI | URBMI | |||
|---|---|---|---|---|
| Inception year | 2002 | 2008 | ||
| Eligible population | Urban employed workers (including employees and the retired) | Urban unemployed residents (including children, students, unemployed adults and elderly residents not covered by UEBMI) | ||
| Financing sources | Contributor (8% of annual wage, 6% from employers and 2% from employees) | Government subsidy (70%) Individual premium (30%) | ||
| Accounts | Medical Savings Account (employee contributions and 30% of employer contributions) for outpatient care; Social Risk-Pooling Account (70% of employer contribution) for inpatient care and critical (i.e., chronic or fatal diseases including PD) outpatient care | Social Risk-Pooling Account(all funds) for inpatient care and critical (i.e., chronic or fatal diseases including PD) outpatient care | ||
| Employees | Children and students | |||
| Deductible (inpatient care) | Primary hospital | CNY 400 | Primary hospital | CNY 120 |
| Secondary hospital | CNY 800 | Secondary hospital | CNY 240 | |
| Tertiary hospital | CNY 1600 | Tertiary hospital | CNY 480 | |
| The retired | Unemployed adults and elderly residents | |||
| Primary hospital | CNY 280 | Primary hospital | CNY 280 | |
| Secondary hospital | CNY 560 | Secondary hospital | CNY 560 | |
| Tertiary hospital | CNY 1120 | Tertiary hospital | CNY 1120 | |
| Employees | Children and students | |||
| Primary hospital | 90% | Primary hospital | 85% | |
| Reimbursed rate * (inpatient care) | Secondary hospital | 85% | Secondary hospital | 75% |
| Tertiary hospital | 80% | Tertiary hospital | 65% | |
| The retired | Unemployed adults and elderly residents | |||
| Primary hospital | 93% | Primary hospital | 75% | |
| Secondary hospital | 89.50% | Secondary hospital | 65% | |
| Tertiary hospital | 86% | Tertiary hospital | 55% | |
| Reimbursed ceiling (inpatient care) | Six times local employees’ annual average wage(CNY 295,680)(in 2012) | Six times local household disposable income(CNY 206,628)(in 2012) | ||
| Deductible (outpatient care) | CNY 0 | CNY 0 | ||
| Reimbursed rate * (outpatient care) | Community health centers 85% Non-community institutes 65% | Community health centers 85% Non-community institutes 65% | ||
| Reimbursed ceiling (outpatient care) | CNY 150 per person per month | CNY 100 per person per month | ||
| Costs | Definition | Calculation Equations |
|---|---|---|
| Direct medical costs (Type of health services use perspective) | Direct medical costs refer to the annual average medical costs per patient with PD, including outpatient costs and inpatient costs. | =Outpatient costs + Inpatient costs |
| Outpatient costs | Outpatient costs are the annual average costs for outpatient services. | |
| Inpatient costs | Inpatient costs are the annual average costs for inpatient services. | |
| Direct medical costs (Payer perspective) | =Individual out-of-pocket spending + Reimbursement | |
| Individual out-of-pocket spending | Individual out-of-pocket spending is costs that the insurance scheme does not cover and that the individual must pay on their own. | |
| Reimbursement | Reimbursement is costs reimbursed by the health insurance scheme. | |
| Direct medical costs (Cost composition perspective) | =Bed fees + Medication costs + Laboratory and diagnostic costs + Non-medication costs + other fees | |
| Bed fees | Bed fees are spending on accommodation when hospitalized. | |
| Medication costs | Medication costs consist of traditional Chinese medicine and Western medicine expenses. | |
| Laboratory and diagnostic costs | Laboratory and diagnostic costs are spending on biochemical tests and physical examinations. | |
| Non-medication treatment costs | Non-medication treatment costs are the costs of blood transfusion, surgery and the other forms of treatment, excluding drug therapy. | |
| Other fees | Other fees are expenses on other services, such as air conditioning. |
| Variables | Measures |
|---|---|
| Healthcare services use (Dependent variable) | |
| Inpatient costs | Inpatient costs are the annual average costs for inpatient services, including bed fees, medication costs, laboratory and diagnostic costs, non-medication costs and other fees. |
| Individual characteristics (Independent variables) | |
| Predisposing characteristics | |
| Gender | Female = 0, Male = 1 |
| Age | 18 ≤ age < 60, 60 ≤ age < 75, 75 ≤ age < 90, age ≥ 90 |
| Enabling characteristics | |
| Insurance types | URBMI = 0, UEBMI = 1 |
| Need characteristics | |
| Disease subtypes | IPD = 0, SP = 1 |
| Hospital level | Primary hospital = 1, Secondary hospital = 2, Tertiary hospital = 3 |
| LOS | Days < 15, 15 ≤ Days < 30, Days ≥ 30 |
| Comorbidities | None, Hypertension, Diabetes, Coronary heart disease, Alzheimer’s disease, Schizophrenia, Mood disorders |
| Overall | UEBMI | URBMI | |
|---|---|---|---|
| No. Patients | 2660 | 2448 | 212 |
| Gender, n(%) | |||
| Female | 1318.0 (49.5) | 1178.0 (48.1) | 140.0 (66.0) |
| Male | 1342.0 (50.5) | 1270.0 (51.9) | 72.0 (34.0) |
| Age (years) | |||
| Mean ± SD | 71.4 ± 9.9 | 71.5 ± 9.8 | 70.5 ± 10.3 |
| Median (25th–75th) | 73.0 (65.0–78.0) | 73.0 (66.0–78.0) | 71.0 (63.0–78.0) |
| Age groups, n(%) | |||
| 18 ≤ age < 60 | 335.0 (12.6) | 302.0 (12.3) | 33.0 (15.6) |
| 60 ≤ age < 75 | 1145.0 (43.0) | 1047.0 (42.8) | 98.0 (46.2) |
| 75 ≤ age < 90 | 1160.0 (43.6) | 1080.0 (44.1) | 80.0 (37.7) |
| ≥90 | 20.0 (0.8) | 19.0 (0.8) | 1.0 (0.5) |
| Insurance types, n(%) | |||
| URBMI | 212.0 (8.0) | / | 212.0 (100.0) |
| UEBMI | 2448.0 (92.0) | 2448.0 (100.0) | / |
| Disease subtypes, n(%) | |||
| SP | 1319.0 (49.6) | 1211.0 (49.5) | 108.0 (50.9) |
| IPD | 1341.0 (50.4) | 1237.0 (50.5) | 104.0 (49.1) |
| Hospital levels, n(%) | |||
| Primary | 103.0 (3.9) | 94.0 (3.8) | 9.0 (4.2) |
| Secondary | 506.0 (19.0) | 472.0 (19.3) | 34.0 (16.0) |
| Tertiary | 2051.0 (77.1) | 1882.0 (76.9) | 169.0 (79.7) |
| Length of stay (days) | |||
| Mean ± SD | 20.0 ± 26.3 | 20.2 ± 26.9 | 17.8 ± 17.7 |
| Median(25th–75th) | 14.0 (10.0–20.0) | 14.0 (10.0–20.0) | 12.0 (9.0–19.0) |
| Days < 15, n(%) | 1457.0 (54.8) | 1325.0 (54.1) | 132.0 (62.3) |
| 15 ≤ Days < 30 | 855.0 (32.1) | 800.0 (32.7) | 55.0 (25.9) |
| Days ≥ 30 | 348.0 (13.1) | 323.0 (13.2) | 25.0 (11.8) |
| Comorbidities, n(%) | |||
| None | 1506.0 (56.6) | 1349.0 (55.1) | 157.0 (74.1) |
| Hypertension | 946.0 (35.6) | 900.0 (36.8) | 46.0 (21.7) |
| Diabetes | 306.0 (11.5) | 296.0 (12.1) | 10.0 (4.7) |
| Coronary heart disease | 177.0 (6.7) | 168.0 (6.9) | 9.0 (4.2) |
| Alzheimer’s disease | 70.0 (2.6) | 68.0 (2.8) | 2.0 (0.9) |
| Schizophrenia | 9.0 (0.3) | 9.0 (0.4) | 0.0 (0.0) |
| Mood disorders | 69.0 (2.6) | 67.0 (2.7) | 2.0 (0.9) |
| Admission year, n(%) | |||
| Year 2008 | 364.0 (13.7) | 351.0 (14.3) | 13.0 (6.1) |
| Year 2009 | 419.0 (15.8) | 382.0 (15.6) | 37.0 (17.5) |
| Year 2010 | 440.0 (16.5) | 409.0 (16.7) | 31.0 (14.6) |
| Year 2011 | 652.0 (24.5) | 598.0 (24.4) | 54.0 (25.5) |
| Year 2012 | 785.0 (29.5) | 708.0 (28.9) | 77.0 (36.3) |
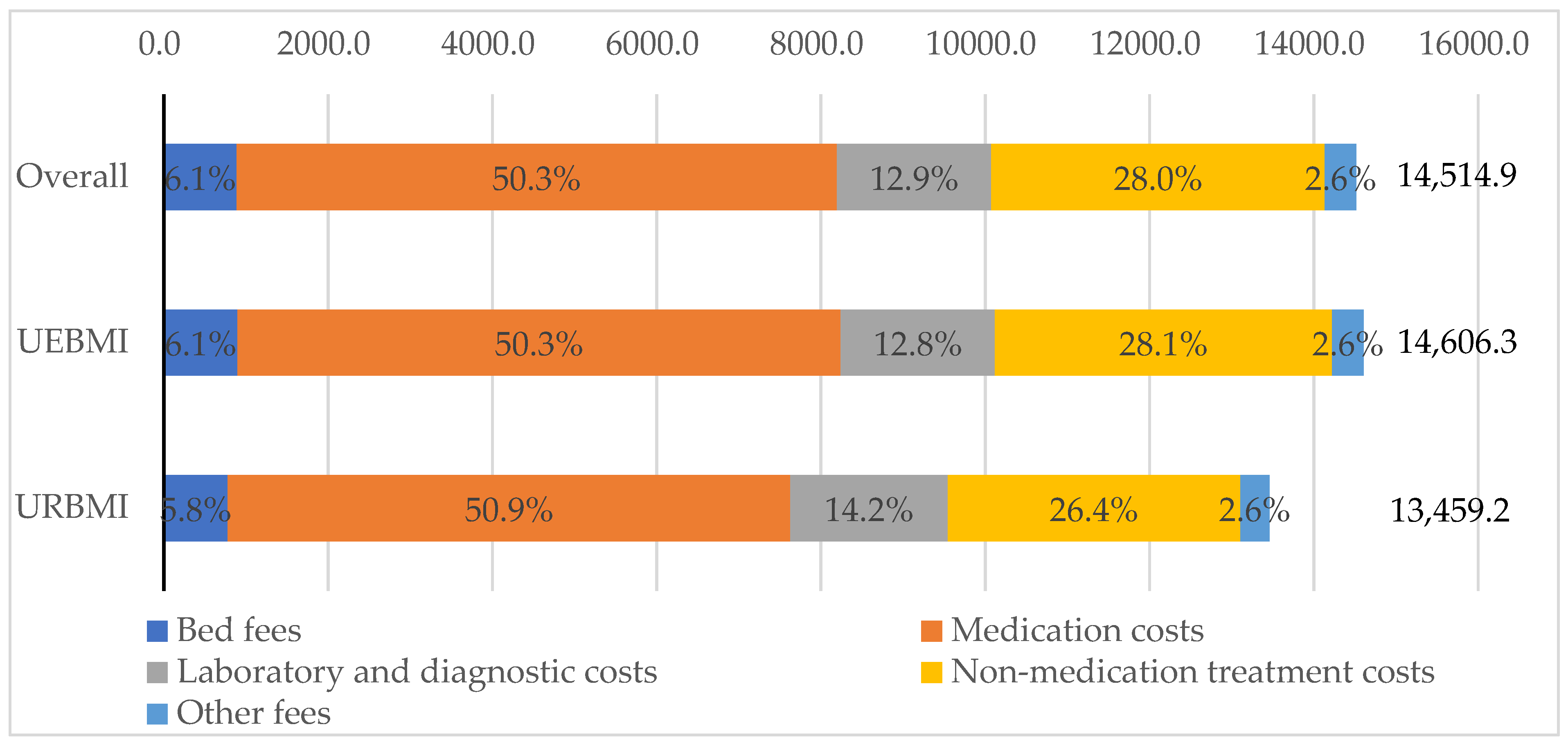
| Compositions | Overall | UEBMI | URBMI | p Value |
|---|---|---|---|---|
| No. Patients | 2660 | 2448 | 212 | |
| Direct medical costs | ||||
| Mean (CNY) | 14,514.9 | 14,606.3 | 13,459.2 | 0.060 |
| SD | 12,511.2 | 12,615.6 | 11,208.9 | |
| Bed fees | ||||
| Percentage of direct medical costs (%) | 6.1 | 6.1 | 5.8 | |
| Mean (CNY) | 887.3 | 896.6 | 779.6 | 0.012 |
| SD | 1011.7 | 1029.3 | 774.3 | |
| Medication costs | ||||
| Percentage of direct medical costs (%) | 50.3 | 50.3 | 50.9 | |
| Mean (CNY) | 7307.3 | 7347.0 | 6848.7 | 0.344 |
| SD | 7063.4 | 7151.1 | 5953.0 | |
| Laboratory and diagnostic costs | ||||
| Percentage of direct medical costs (%) | 12.9 | 12.8 | 14.2 | |
| Mean (CNY) | 1877.2 | 1873.8 | 1917.0 | 0.833 |
| SD | 1645.1 | 1627.3 | 1842.1 | |
| Non-medication treatment costs | ||||
| Percentage of direct medical costs (%) | 28.0 | 28.1 | 26.4 | |
| Mean (CNY) | 4062.0 | 4105.6 | 3558.7 | 0.003 |
| SD | 5700.9 | 5748.0 | 5111.2 | |
| Other fees | ||||
| Percentage of direct medical costs (%) | 2.6 | 2.6 | 2.6 | |
| Mean (CNY) | 381.0 | 383.2 | 355.2 | 0.048 |
| SD | 720.6 | 730.3 | 598.0 | |
| Out-of-pocket spending | ||||
| Percentage of direct medical costs (%) | 28.1 | 26.4 | 50.1 | |
| Mean (CNY) | 4085.3 | 3854.7 | 6747.9 | <0.001 |
| SD | 3234.3 | 2904.9 | 5120.7 | |
| Inpatient costs | ||||
| No. patients having hospitalization | 2660.0 (100.0) | 2448.0 (100.0) | 212.0 (100.0) | |
| Mean (CNY) | 13,551.4 | 13,651.0 | 12,402.2 | 0.041 |
| SD | 12,424.4 | 12,540.0 | 10,962.5 | |
| Out-of-pocket spending | ||||
| Percentage of inpatient costs (%) | 26.0 | 24.3 | 47.9 | |
| Mean (CNY) | 3527.8 | 3318.7 | 5942.2 | <0.001 |
| SD | 2942.7 | 2645.4 | 4648.0 | |
| Outpatient costs | ||||
| No. patients visiting outpatient | 1432.0 (53.8) | 1339.0 (54.7) | 93.0 (43.9) | |
| Mean (CNY) | 963.5 | 955.3 | 1057.0 | 0.024 |
| SD | 1461.6 | 1391.4 | 2112.5 | |
| Out-of-pocket spending | ||||
| Percentage of outpatient costs (%) | 57.9 | 56.1 | 76.2 | |
| Mean (CNY) | 557.5 | 536.0 | 805.7 | 0.118 |
| SD | 1126.7 | 1038.8 | 1848.2 | |
| Patient Characteristics | Overall | UEBMI | URBMI | p Value | |||
|---|---|---|---|---|---|---|---|
| No. Patients | n = 2660 | n = 2448 | n = 212 | ||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Gender | 0.040 | ||||||
| Female | 13,291.5 | 11,917.0 | 13,291.0 | 11,839.8 | 13,295.2 | 12,592.1 | |
| Male | 13,806.8 | 12,902.7 | 13,984.8 | 13,152.1 | 10,665.7 | 6488.1 | |
| Age groups | 0.030 | ||||||
| 18 ≤ age < 60 | 11,505.0 | 8643.7 | 11,452.6 | 8532.7 | 11,984.6 | 9732.7 | |
| 60 ≤ age < 75 | 13,205.4 | 12,166.6 | 13,363.5 | 12,264.3 | 11,515.8 | 10,984.1 | |
| 75 ≤ age < 90 | 14,443.9 | 13,493.1 | 14,500.8 | 13,633.3 | 13,676.5 | 11,478.5 | |
| ≥90 | 15,878.5 | 11,650.0 | 16,130.0 | 11,913.4 | 11,101.4 | / | |
| Disease subtypes | 0.040 | ||||||
| SP | 12,975.4 | 11,037.9 | 12,940.9 | 10,990.5 | 13,362.4 | 11,602.7 | |
| IPD | 14,118.1 | 13,631.9 | 14,346.2 | 13,859.9 | 11,405.1 | 10,216.0 | |
| Hospital levels | <0.001 | ||||||
| Primary | 9103.1 | 10,913.9 | 9097.4 | 11,344.3 | 9162.6 | 4754.9 | |
| Secondary | 11,944.7 | 13,582.5 | 11,872.0 | 13,435.2 | 12,953.4 | 15,676.9 | |
| Tertiary | 14,171.2 | 12,117.5 | 14,324.6 | 12,277.0 | 12,463.8 | 10,047.8 | |
| Length of stay (days) | 0.160 | ||||||
| Days < 15 | 8794.3 | 4209.8 | 8836.6 | 4260.9 | 8369.4 | 3644.6 | |
| 15 ≤ Days < 30 | 13,890.4 | 7331.3 | 13,966.3 | 7455.4 | 12,786.2 | 5124.5 | |
| Days ≥ 30 | 32,636.0 | 22,708.1 | 32,619.4 | 22,932.6 | 32,850.8 | 19,990.2 | |
| Comorbidities | <0.001 | ||||||
| None | 13,675.2 | 13,500.4 | 13,775.8 | 13,659.2 | 12,810.3 | 12,055.2 | |
| Hypertension | 13,180.0 | 10,073.1 | 13,262.7 | 10,192.9 | 11,561.3 | 7237.2 | |
| Diabetes | 14,108.4 | 12,110.7 | 14,190.7 | 12,198.0 | 11,672.8 | 9308.0 | |
| Coronary heart disease | 12,926.6 | 9070.6 | 13,020.5 | 9211.5 | 11,172.5 | 5927.0 | |
| Alzheimer’s disease | 14,306.8 | 8758.7 | 14,390.4 | 8840.5 | 11,464.9 | 6351.9 | |
| Schizophrenia | 14,997.9 | 9377.3 | 14,997.9 | 9377.3 | / | / | |
| Mood disorders | 14,644.6 | 11,905.0 | 14,739.5 | 12,045.6 | 11,464.9 | 6351.9 | |
| Predictors | Overall (n = 2660) | p Value | ||
|---|---|---|---|---|
| Coef. | Adjusted Std.err. | Marginal Effect | ||
| Gender | ||||
| Female (Reference) | ||||
| Male | 0.007 | 0.017 | 86.9 | 0.707 |
| Age group | ||||
| 18 ≤ age < 60 (Reference) | ||||
| 60 ≤ age < 75 | 0.077 *** | 0.028 | 1027.0 | 0.006 |
| 75 ≤ age < 90 | 0.131 *** | 0.029 | 1738.0 | <0.001 |
| ≥90 | 0.371 *** | 0.130 | 5660.5 | 0.004 |
| Insurance types | ||||
| URBMI (Reference) | ||||
| UEBMI | 0.069 ** | 0.031 | 888.1 | 0.027 |
| Disease subtypes | ||||
| SP (Reference) | ||||
| IPD | 0.010 | 0.017 | 127.4 | 0.565 |
| Hospital levels | ||||
| Secondary (Reference) | ||||
| Primary | −0.283 *** | 0.045 | −3378.7 | <0.001 |
| Tertiary | 0.459 *** | 0.027 | 5523.9 | <0.001 |
| Length of stay (days) | ||||
| Days < 15 (Reference) | ||||
| 15 ≤ Days < 30 | 0.454 *** | 0.023 | 6451.2 | <0.001 |
| Days ≥ 30 | 1.497 *** | 0.056 | 29,804.3 | <0.001 |
| Comorbidities | ||||
| None (Reference) | ||||
| Hypertension | −0.01 | 0.017 | −128.9 | 0.574 |
| Diabetes | 0.043 | 0.026 | 569.8 | 0.099 |
| Coronary | −0.004 | 0.031 | −52.3 | 0.898 |
| Alzheimer’s disease | −0.122 ** | 0.047 | −1543.6 | 0.010 |
| Schizophrenia | 0.087 | 0.128 | 1188.7 | 0.496 |
| Mood disorders | 0.164 *** | 0.047 | 2305.5 | <0.001 |
| Year | ||||
| Year 2008(Reference) | ||||
| Year 2009 | 0.091 ** | 0.036 | 1230.0 | 0.011 |
| Year 2010 | 0.110 *** | 0.031 | 1493.2 | <0.001 |
| Year 2011 | 0.108 *** | 0.029 | 1454.4 | <0.001 |
| Year 2012 | 0.158 *** | 0.028 | 2143.1 | <0.001 |
| λ | 0.213 ** | 0.092 | ||
| θ1 | 0.238 *** | 0.026 | ||
| θ2 | 2.547 *** | 0.134 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhou, W.; Zhang, D. Direct Medical Costs of Parkinson’s Disease in Southern China: A Cross-Sectional Study Based on Health Insurance Claims Data in Guangzhou City. Int. J. Environ. Res. Public Health 2022, 19, 3238. https://doi.org/10.3390/ijerph19063238
Zhang H, Zhou W, Zhang D. Direct Medical Costs of Parkinson’s Disease in Southern China: A Cross-Sectional Study Based on Health Insurance Claims Data in Guangzhou City. International Journal of Environmental Research and Public Health. 2022; 19(6):3238. https://doi.org/10.3390/ijerph19063238
Chicago/Turabian StyleZhang, Hui, Wenjing Zhou, and Donglan Zhang. 2022. "Direct Medical Costs of Parkinson’s Disease in Southern China: A Cross-Sectional Study Based on Health Insurance Claims Data in Guangzhou City" International Journal of Environmental Research and Public Health 19, no. 6: 3238. https://doi.org/10.3390/ijerph19063238
APA StyleZhang, H., Zhou, W., & Zhang, D. (2022). Direct Medical Costs of Parkinson’s Disease in Southern China: A Cross-Sectional Study Based on Health Insurance Claims Data in Guangzhou City. International Journal of Environmental Research and Public Health, 19(6), 3238. https://doi.org/10.3390/ijerph19063238






