COVID-19 Vaccination within the Context of Reactogenicity and Immunogenicity of ChAdOx1 Vaccine Administered to Teachers in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population and Setting
2.2. Study Instrument
2.3. Laboratory Assays
2.4. Vaccination Immunogenicity Assessment
2.5. Statistical Analysis
3. Results
3.1. Adverse Effects
3.2. Dosing Interval, Time of Evaluation
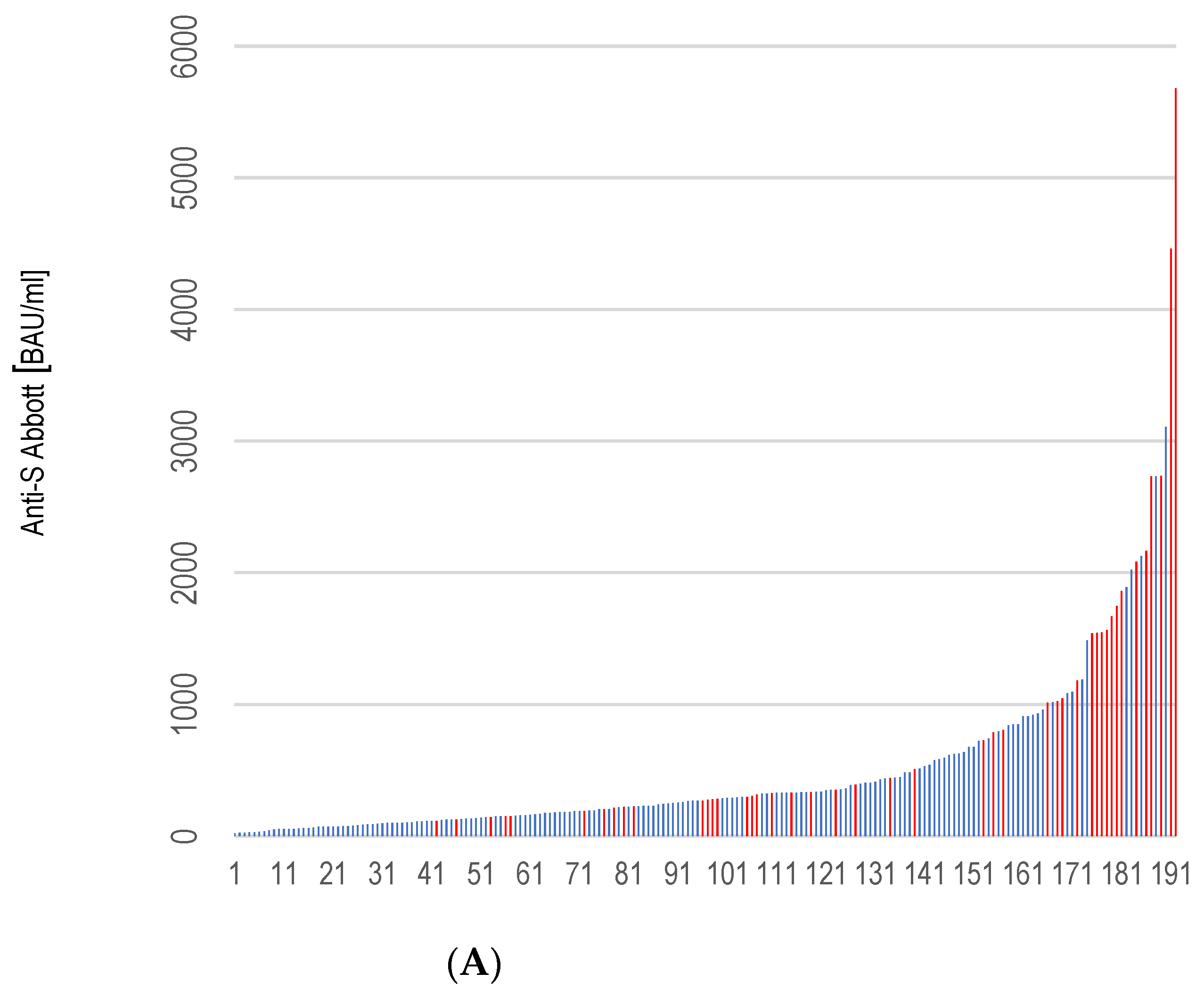
3.3. Antibody Response
3.4. Correlation of the Rate of Anti-S Antibody Titers and Selected Variables
3.5. Predictors of Immunogenicity
4. Discussion
4.1. Dosing Interval, Time of Evaluation
4.2. Frequencies and Intensity of Adverse Reactions
4.3. Anti-RBD Antibody Titers
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, H.Y.; Wang, S.H.; Tang, Y.; Sheng, W.; Zuo, C.J.; Wu, D.W.; Fang, H.; Du, Q.; Li, N. Landscape and progress of global COVID-19 vaccine development. Hum. Vaccin. Immunother. 2021, 17, 3276–3280. [Google Scholar] [CrossRef] [PubMed]
- Polish National Immunization Program–COVID-10 Immunization. Available online: https://www.gov.pl/szczepimysie/narodowy-program-szczepien-przeciw-covid-19 (accessed on 21 November 2021).
- ECDC. Overview of the Implementation of COVID-19 Vaccination Strategies and Vaccine Deployment Plans in the EU/EEA. Available online: http://uploads/sites/124/2021/02/Overview-of-COVID-19-vaccination-strategies-deployment-plans-in-the-EU-EEA.pdf (accessed on 25 November 2021).
- UNESCO Urges All Countries to Prioritize Teachers in National COVID-19 Vaccine Rollout Plans to Ensure Education Can Continue Safely and Schools Remain Open. Prioritization of Teachers in COVID-19 Vaccine Rollout. Available online: https://en.unesco.org/covid-19/educationresponse/teacher-vaccination (accessed on 15 November 2021).
- Confusion over Vaccination at School. Zamieszanie Wokół Szczepień w Szkole. Available online: http://www.rp.pl/diagnostyka-i-terapie/art293781-zamieszanie-wokół-szczepien-w-szkole (accessed on 5 June 2021).
- Summary of Product Characteristics for Vaxzevria-GOV.UK. Available online: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca/information-for-healthcare-professionals-on-covid-19-vaccine-astrazeneca (accessed on 21 September 2021).
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Eyre, D.W.; Lumley, S.F.; Wei, J.; Cox, S.; James, T.; Justice, A.; Jesuthasan, G.; O’Donnell, D.; Howarth, A.; Hatch, S.B.; et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status. Clin. Microbiol. Infect. 2021, 27, 1516.e7–1516.e14. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, L.; Pulendran, B. The immunology of SARS-CoV-2 infections and vaccines. Semin. Immunol. 2020, 50, 101422. [Google Scholar] [CrossRef] [PubMed]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Brüggen, M.C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef] [PubMed]
- Parry, H.; Bruton, R.; Stephens, C.; Brown, K.; Amirthalingam, G.; Otter, A.; Hallis, B.; Zuo, J.; Moss, P. Differential immunogenicity of BNT162b2 or ChAdOx1 vaccines after extended-interval homologous dual vaccination in older people. Immun. Ageing 2021, 18, 34. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Pettini, E.; Pastore, G.; Fiorino, F.; Medaglini, D.; Ciabattini, A. Short or Long Interval between Priming and Boosting: Does It Impact on the Vaccine Immunogenicity? Vaccines 2021, 9, 289. [Google Scholar] [CrossRef]
- Vaccination against COVID-19. From Today We Will Get the Second Dose Faster. Available online: https://polskieradio24.pl/5/1222/artykul/2735472 (accessed on 28 June 2021).
- Is AstraZeneca a Dangerous Vaccine? Czy AstraZeneca to Groźna w Skutkach Szczepionka? Available online: https://oko.press/czy-astrazeneca-togroźnawskutkachszczepionka (accessed on 5 July 2021).
- Bhuyan, P.; Medin, J.; da Silva, H.G.; Yadavalli, M.; Shankar, N.K.; Mullerova, H.; Arnold, M.; Nord, M. Very rare thrombosis with thrombocytopenia after second AZD1222 dose: A global safety database analysis. Lancet 2021, 398, 577–578. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Oxford COVID Vaccine Trial Group. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. NGS-SA Group; Wits-VIDA COVID Group. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. AstraZeneca AZD1222 Clinical Study Group. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Wei, J.; Stoesser, N.; Matthews, P.C.; Ayoubkhani, D.; Studley, R.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat. Microbiol. 2021, 6, 1140–1149. [Google Scholar] [CrossRef]
- English, E.; Cook, L.E.; Piec, I.; Dervisevic, S.; Fraser, W.D.; John, W.G. Performance of the Abbott SARS-CoV-2 IgG II Quantitative Antibody Assay Including the New Variants of Concern, VOC 202012/V1 (United Kingdom) and VOC 202012/V2 (South Africa), and First Steps towards Global Harmonization of COVID-19 Antibody Methods. J. Clin. Microbiol. 2021, 59, e0028821. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Bernal, J.L.; Andrews, N.; Gower, C.; Stowe, J.; Robertson, C.; Tessier, E.; Simmons, R.; Cottrell, S.; Robertson, R.; O’Doherty, M.; et al. Early effectiveness of COVID-19 vaccination with BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on symptomatic disease, hospitalisations and mortality in older adults in England. BMJ 2021, 373, n1088. [Google Scholar]
- Turner, P.J.; Ansotegui, I.J.; Campbell, D.E.; Cardona, V.; Ebisawa, M.; El-Gamal, Y.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; et al. WAO Anaphylaxis Committee. COVID-19 vaccine-associated anaphylaxis: A statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ. J. 2021, 14, 100517. [Google Scholar] [CrossRef]
- Pottegård, A.; Lund, L.C.; Karlstad, Ø.; Dahl, J.; Andersen, M.; Hallas, J.; Lidegaard, Ø.; Tapia, G.; Gulseth, H.L.; Ruiz, P.L.; et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: Population based cohort study. BMJ 2021, 373, n1114. [Google Scholar] [CrossRef]
- Gordon, S.F.; Clothier, H.J.; Morgan, H.; Buttery, J.P.; Phuong, L.K.; Monagle, P.; Chunilal, S.; Wood, E.M.; Tran, H.; Szer, J.; et al. SAEFVIC and VicSIS investigators. Immune thrombocytopenia following immunisation with Vaxzevria ChadOx1-S (AstraZeneca) vaccine, Victoria, Australia. Vaccine 2021, 39, 7052–7057. [Google Scholar] [CrossRef]
- ChAdOx1 S (recombinant) vaccine: Thrombosis and thrombocytopenia. Drug Ther Bull. 2021, 59, 101. [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef] [PubMed]
- Saadat, S.; Rikhtegaran Tehrani, Z.; Logue, J.; Newman, M.; Frieman, M.B.; Sajadi, M.M. Binding and Neutralization Antibody Titers After a Single Vaccine Dose in Health Care Workers Previously Infected With SARS-CoV-2. JAMA. 2021, 325, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Wise, J. COVID-19: People who have had infection might only need one dose of mRNA vaccine. BMJ 2021, 372, n308. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Abu Jabal, K.; Ben-Amram, H.; Beiruti, K.; Batheesh, Y.; Sussan, C.; Zarka, S.; Edelstein, M. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: Real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Eurosurveillance 2021, 26, 2100096. [Google Scholar] [CrossRef]
- Gobbi, F.; Buonfrate, D.; Moro, L.; Rodari, P.; Piubelli, C.; Caldrer, S.; Riccetti, S.; Sinigaglia, A.; Barzon, L. Antibody Response to the BNT162b2 mRNA COVID-19 Vaccine in Subjects with Prior SARS-CoV-2 Infection. Viruses. 2021, 13, 422. [Google Scholar] [CrossRef]
- Havervall, S.; Marking, U.; Greilert-Norin, N.; Ng, H.; Gordon, M.; Salomonsson, A.C.; Hellström, C.; Pin, E.; Blom, K.; Mangsbo, S.; et al. Antibody responses after a single dose of ChAdOx1 nCoV-19 vaccine in healthcare workers previously infected with SARS-CoV-2. EBioMedicine 2021, 70, 103523. [Google Scholar] [CrossRef]
- Payne, R.P.; Longet, S.; Austin, J.A.; Skelly, D.T.; Dejnirattisai, W.; Adele, S.; Meardon, N.; Faustini, S.; Al-Taei, S.; Moore, S.C.; et al. PITCH Consortium. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021, 184, 5699–5714.e11. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Aging of the Immune System. Mechanisms and Therapeutic Targets. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 5), S422–S428. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Duan, K.; Zhang, Y. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: Interim analysis of 2 randomized clinical trials. JAMA 2020, 324, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Jalkanen, P.; Kolehmainen, P.; Häkkinen, H.K.; Huttunen, M.; Tähtinen, P.A.; Vapalahti, J.L.; Kakkola, L.; Kantele, A.; Julkunen, I. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat. Commun. 2021, 12, 3991. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H. A review of vaccine effects on women in light of the COVID-19 pandemic. Taiwan J. Obstet. Gynecol. 2020, 59, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Silva, J.; Reis, T.; Lopes, C.; Batista-Silva, R.; Ribeiro, R.; Marques, G.; Pacheco, V.; Rodrigues, T.; Afonso, A.; Pinheiro, V.; et al. Humoral response to the SARS-CoV-2 BNT162b2 mRNA vaccine: Real-world data from a large cohort of healthcare workers. Vaccine 2022, 40, 650–655. [Google Scholar] [CrossRef]
- National Bureau of Statistics. Education in 2019/2020. Full-Time and Part-Time Teachers by Type of Schools and Voivodships. Available online: https://GłównyUrządStatystyczny/Obszarytematyczne/Edukacja/Edukacja/Oświataiwychowaniewrok-uszkolnym2019/2020 (accessed on 26 November 2021).
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab. Res. Rev. 2021, 38, e3465. [Google Scholar] [CrossRef]

| Variable | n | % |
|---|---|---|
| Sex | ||
| Female | 161 | 83.9 |
| Male | 31 | 16.1 |
| Age (years): * 50.5 (27–67) | ||
| 27–40 | 17 | 8.9 |
| 41–50 | 73 | 38.0 |
| >50 | 102 | 53.1 |
| BMI kg/m2 | ||
| <25 | 90 | 49.2 |
| 25.0–29.9 | 69 | 37.7 |
| ≥30 | 23 | 13.1 |
| Smoking | ||
| current | 23 | 12.0 |
| quit | 26 | 13.5 |
| never | 143 | 74.5 |
| Comorbidity | ||
| Cardiovascular disease | 13 | 6.8 |
| Respiratory disease | 8 | 4.2 |
| Diabetes | 19 | 9.9 |
| Other ** | 47 | 24.5 |
| No comorbidity | 105 | 54.6 |
| Previous SARS-CoV-2 Infection | ||
| Assessed by the test *** | 27 | 14.1 |
| Assessed by the participant | 22 | 11.4 |
| No infection | 143 | 74.5 |
| Variable | Previously Uninfected | Previously Infected with SARS-CoV-2 | ||||
|---|---|---|---|---|---|---|
| GMT | N | p | GMT | N | p | |
| Sex | ||||||
| Female | 347.7 | 121 | 0.51 | 1048.1 | 41 | 0.89 |
| Male | 413.2 | 24 | 984.7 | 7 | ||
| Age (years) | ||||||
| <40 | 355.7 | 13 | 0.95 | 741.8 | 4 | 0.0001 |
| 40–60 | 347.3 | 120 | 862.5 | 39 | ||
| ≥60 | 473.2 | 12 | 3053.6 | 4 | ||
| BMI | ||||||
| <25 kg/m2 | 336.5 | 67 | 0.86 | 803.5 | 24 | 0.04 |
| ≥25–29.9 kg/m2 | 350.0 | 57 | 866.1 | 15 | ||
| ≥30 kg/m2 | 462.7 | 15 | 1666.1 | 6 | ||
| Comorbidities * | ||||||
| Yes | 335.1 | 98 | 0.57 | 833.3 | 35 | 0.52 |
| No | 383.7 | 39 | 1150.1 | 7 | ||
| Current/Previous smoker | ||||||
| Yes | 440.5 | 19 | 0.37 | 942.3 | 36 | 0.30 |
| No | 536.6 | 128 | 1354.2 | 11 | ||
| Variable | Estimate | 95% CI | p |
|---|---|---|---|
| Intercept | −1173.84 | −2161.24–186.45 | 0.02 |
| Age | 10.83 | −1.79–23.64 | 0.09 |
| SARS-CoV-2 infection | 545.17 | 300.69–798.64 | <0.0001 |
| Dose interval | 14.31 | 3.43–25.20 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganczak, M.; Korzeń, M.; Sobieraj, E.; Goławski, J.; Pasek, O.; Biesiada, D. COVID-19 Vaccination within the Context of Reactogenicity and Immunogenicity of ChAdOx1 Vaccine Administered to Teachers in Poland. Int. J. Environ. Res. Public Health 2022, 19, 3111. https://doi.org/10.3390/ijerph19053111
Ganczak M, Korzeń M, Sobieraj E, Goławski J, Pasek O, Biesiada D. COVID-19 Vaccination within the Context of Reactogenicity and Immunogenicity of ChAdOx1 Vaccine Administered to Teachers in Poland. International Journal of Environmental Research and Public Health. 2022; 19(5):3111. https://doi.org/10.3390/ijerph19053111
Chicago/Turabian StyleGanczak, Maria, Marcin Korzeń, Ewa Sobieraj, Jakub Goławski, Oskar Pasek, and Daniel Biesiada. 2022. "COVID-19 Vaccination within the Context of Reactogenicity and Immunogenicity of ChAdOx1 Vaccine Administered to Teachers in Poland" International Journal of Environmental Research and Public Health 19, no. 5: 3111. https://doi.org/10.3390/ijerph19053111
APA StyleGanczak, M., Korzeń, M., Sobieraj, E., Goławski, J., Pasek, O., & Biesiada, D. (2022). COVID-19 Vaccination within the Context of Reactogenicity and Immunogenicity of ChAdOx1 Vaccine Administered to Teachers in Poland. International Journal of Environmental Research and Public Health, 19(5), 3111. https://doi.org/10.3390/ijerph19053111






