On Health Effects of Resveratrol in Wine
Abstract
:1. Introduction
2. Dataset and Methods
2.1. Dataset
2.2. Methods
3. Results
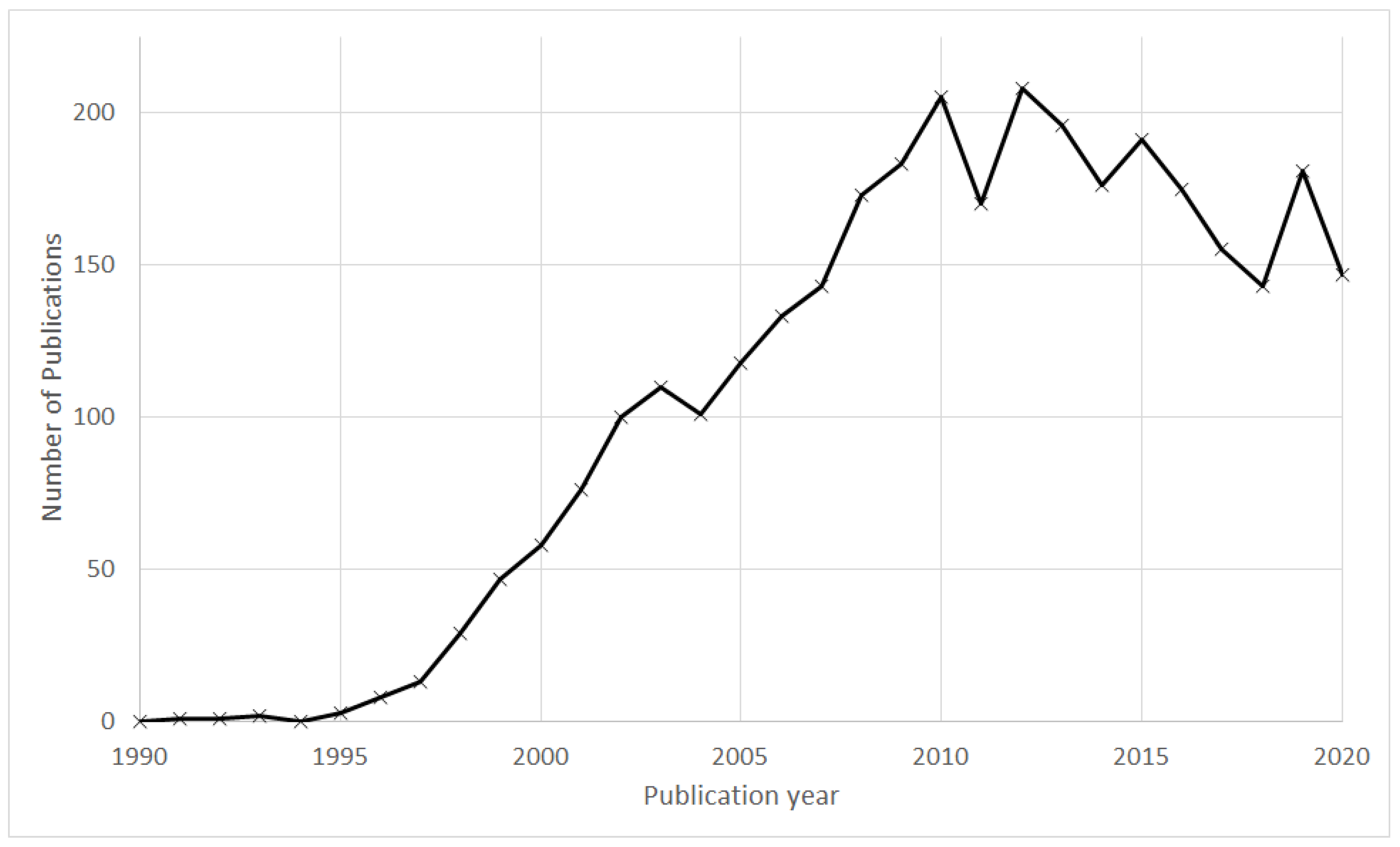
3.1. Annual Publication Profile
3.2. Keyword Analysis
- Beverages related: wine (133), red wine (108), grapes (72), vitis vinifera (37), grape juice (22), grape seed extract (18), grapevine (13), vitis amurensis (12), vitaceae (12).
- Compounds related: resveratrol (1531), polyphenols (306), stilbenes (90), nitric oxide (86), phenolics (57), flavonoids (56), reactive oxygen species (56), quercetin (54), alcohol (53), phytochemical (48), piceatannol (40), hplc (34), pterostilbene (34), curcumin (29), ethanol (26), egcg (21), cytokines (20), sirtuins (20), ampk (19), glutathione (16), phytoestrogen (15), red wine polyphenols (15), calcium (13), cytochrome p450 (13), stilbenoids (13), anthocyanins (12), caspase-3 (12), cholesterol (12), piceid (12), bcl-2 (11), catechin (11), cisplatin (11), cyclooxygenase (11), genistein (11), grape polyphenols (11), phytoalexin (11), superoxide dismutase (11), beta amyloid (10), doxorubicin (10), epsilon viniferin (10), hydrogen peroxide (10), lipopolysaccharide (10), melatonin (10), polydatin (10), polygonum cuspidatum (10).
- Diseases related: atherosclerosis (63), cardiovascular disease (63), cancer (58), alzheimer’s disease (46), diabetes (34), obesity (32), breast cancer (28), colon cancer (26), hypertension (25), liver (25), prostate cancer (23), diet (18), blood pressure (17), colorectal cancer (15), ischemia (15), metastasis (15), brain (14), heart (14), parkinson’s disease (14), nf2 (13), hippocampus (12), ischemial/reperfusion (12), cardiovascular (11), cerebral ischemia (11), neuroinflammation (11), microglia (10), neurodegeneration (10), osteoblast (10).
- Effects related: apoptosis (240), oxidative stress (201), antioxidant (189), inflammation (93), chemoprevention (68), neuroprotection (65), antioxidant activity (51), rats (41), cardioprotection (24), mediterranean diet (24), nutraceutical (23), anti-inflammatory (20), invasion (16), antioxidant capacity (13), prevention (13), cancer chemoprevention (12), clinical trials (12), health (12), anticancer (11), french paradox (11), neuroinflammation (11), high-fat diet (10), insulin resistance (10).
- Mechanisms related: cell cycle (39), sirt1 (55), nf-kappa b (38), angiogenesis (33), cytotoxicity (33), mitochondria (32), bioavailability (31), autophagy (28), metabolism (27), pharmacokinetics (27), endothelium (26), free radicals (26), p53 (25), lipid peroxidation (24), endothelial cells (22), platelets (16), gene expression (15), molecular docking (15), endothelial function (14), platelet aggregation (14), tnf-alpha (14), dna damage (13), macrophages (12), vegf (12), blood platelets (11), caco-2 cells (10), endothelial dysfunction (10), estrogen receptor (10), inducible nitric oxide synthase (10).
- Broader terms: aging (41), proliferation (28), akt (21), cell proliferation (20), longevity (12), migration (11), differentiation (10), natural products (10), ros (10), toxicity (10).
3.3. Reference Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Label | Replaced by |
|---|---|
| antioxidants | antioxidant |
| cardiovascular diseases | cardiovascular disease |
| epigallocatechin gallate | egcg |
| grape | grapes |
| nutraceuticals | nutraceutical |
| phenolic compounds | phenolics |
| phytochemicals | phytochemical |
| polyphenol | polyphenols |
| rat | rats |
| stilbene | stilbenes |
| trans-resveratrol | resveratrol |
References
- Siemann, E.H.; Creasy, L.L. Concentration of the phytoalexin resveratrol in wine. Am. J. Enol. Vitic. 1992, 43, 49–52. [Google Scholar]
- Lucarini, M.; Durazzo, A.; Lombardi-Boccia, G.; Souto, E.B.; Cecchini, F.; Santini, A. Wine polyphenols and health: Quantitative research literature analysis. Appl. Sci. 2021, 11, 4762. [Google Scholar] [CrossRef]
- Takaoka, M. The phenolic substances of white hellebore (veratrum grandiflorum loes. Fill). V synthesis of resveratrol (3, 5, 4-trioxystilbene) and its derivatives. Nippon. Kagaku Kaishi 1940, 61, 1067–1069. [Google Scholar] [CrossRef]
- Takaoka, M. Resveratrol, a new phenolic compound, from veratrum grandiflorum. J. Chem. Soc. Jpn. 1939, 60, 1090–1100. [Google Scholar]
- Kulashekar, M.; Stom, S.M.; Peuler, J.D. Resveratrol’s potential in the adjunctive management of cardiovascular disease, obesity, diabetes, alzheimer disease, and cancer. J. Am. Osteopath. Assoc. 2018, 118, 596–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.M.; Wang, Z.R.; Hsieh, T.C.; Bruder, J.L.; Zou, J.G.; Huang, Y.Z. Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (review). Int. J. Mol. Med. 2001, 8, 3–17. [Google Scholar] [CrossRef]
- Simini, B. Serge renaud: From french paradox to cretan miracle. Lancet 2000, 355, 48. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (rv): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Galton, F. Vox populi. Nature 1907, 75, 450–451. [Google Scholar] [CrossRef]
- Marx, W.; Bornmann, L.; Barth, A.; Leydesdorff, L. Detecting the historical roots of research fields by reference publication year spectroscopy (rpys). J. Assoc. Inf. Sci. Technol. 2014, 65, 751–764. [Google Scholar] [CrossRef] [Green Version]
- Marx, W.; Bornmann, L.; Barth, A. Detecting the historical roots of research fields by reference publication year spectroscopy (rpys). In Proceedings of the 14th International-Society-of-Scientometrics-and-Informetrics Conference (ISSI), Vienna, Austria, 5–20 July 2013; 2013; pp. 493–506. [Google Scholar]
- Marx, W.; Bornmann, L. Change of perspective: Bibliometrics from the point of view of cited references, a literature overview on approaches to the evaluation of cited references in bibliometrics. Scientometrics 2016, 109, 1397–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bornmann, L.; Marx, W. The wisdom of citing scientists. J. Am. Soc. Inf. Sci. Technol. 2014, 65, 1288–1292. [Google Scholar] [CrossRef] [Green Version]
- Birkle, C.; Pendlebury, D.A.; Schnell, J.; Adams, J. Web of science as a data source for research on scientific and scholarly activity. Quant. Sci. Stud. 2020, 1, 363–376. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: Vosviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thor, A.; Bornmann, L.; Marx, W.; Mutz, R. Identifying single influential publications in a research field: New analysis opportunities of the crexplorer. Scientometrics 2018, 116, 591–608. [Google Scholar] [CrossRef] [Green Version]
- Thor, A.; Marx, W.; Leydesdorff, L.; Bornmann, L. New features of citedreferencesexplorer (crexplorer). Scientometrics 2016, 109, 2049–2051. [Google Scholar] [CrossRef] [Green Version]
- Thor, A.; Marx, W.; Leydesdorff, L.; Bornmann, L. Introducing citedreferencesexplorer (crexplorer): A program for reference publication year spectroscopy with cited references standardization. J. Informetr. 2016, 10, 503–515. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing, 3.5.0; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Haunschild, R.; Bibplots: Plot Functions for Use in Bibliometrics. R Package Version 0.0.8. Available online: https://cran.r-project.org/web/packages/BibPlots/index.html (accessed on 26 October 2021).
- Vanderkam, D.; Allaire, J.J.; Owen, J.; Gromer, D.; Thieurmel, B. Dygraphs: Interface to ‘Dygraphs’ Interactive Time Series Charting Library. R Package Version 1.1.1.6. Available online: https://CRAN.R-project.org/package=dygraphs (accessed on 19 August 2021).
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley Publishing Company: Boston, MA, USA, 1977. [Google Scholar]
- Thor, A.; Bornmann, L.; Haunschild, R. Citedreferencesexplorer (Crexplorer) Manual. Available online: https://andreas-thor.github.io/cre/manual.pdf (accessed on 19 December 2019).
- Haunschild, R.; Bornmann, L. Reference Publication Year Spectroscopy (RPYS) in practice: A software tutorial. arXiv 2021, arXiv:2109.00969. [Google Scholar]
- Langcake, P.; Pryce, R.J. Production of resveratrol by vitis-vinifera and other members of vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Frankel, E.N.; Waterhouse, A.L.; Kinsella, J.E. Inhibition of human ldl oxidation by resveratrol. Lancet 1993, 341, 1103–1104. [Google Scholar] [CrossRef]
- Frankel, E.N.; Kanner, J.; German, J.B.; Parks, E.; Kinsella, J.E. Inhibition of oxidation of human low-density-lipoprotein by phenolic substances in red wine. Lancet 1993, 341, 454–457. [Google Scholar] [CrossRef]
- Fitzpatrick, D.F.; Hirschfield, S.L.; Coffey, R.G. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am. J. Physiol.-Heart Circ. Physiol. 1993, 265, H774–H778. [Google Scholar] [CrossRef] [PubMed]
- Pace-Asciak, C.R.; Hahn, S.; Diamandis, E.P.; Soleas, G.; Goldberg, D.M. The red wine phenolics trans-resveratrol and quercetin block human platelet-aggregation and eicosanoid synthesis-Implications for protection against coronary heart-disease. Clin. Chim. Acta 1995, 235, 207–219. [Google Scholar] [CrossRef]
- Bertelli, A.A.E.; Giovannini, L.; Giannessi, D.; Migliori, M.; Bernini, W.; Fregoni, M.; Bertelli, A. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int. J. Tissue React.-Exp. Clin. Asp. 1995, 17, 1–3. [Google Scholar]
- Jang, M.S.; Cai, E.N.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehm, B.D.; McAndrews, J.M.; Chien, P.Y.; Jameson, J.L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 14138–14143. [Google Scholar] [CrossRef] [Green Version]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Resveratrol: A molecule whose time has come? And gone? Clin. Biochem. 1997, 30, 91–113. [Google Scholar] [CrossRef]
- Fremont, L. Minireview-Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol suppresses tnf-induced activation of nuclear transcription factors nf-kappa b, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef] [Green Version]
- Hung, L.M.; Chen, J.K.; Huang, S.S.; Lee, R.S.; Su, M.J. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc. Res. 2000, 47, 549–555. [Google Scholar] [CrossRef]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; Forstermann, U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 2002, 106, 1652–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, J.F.; Rottinghaus, G.E.; Simonyi, A.; Lubahn, D.; Sun, G.Y.; Sun, A.Y. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002, 958, 439–447. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating sirt1 and pgc-1 alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010, 70, 9003. [Google Scholar] [CrossRef] [Green Version]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar]
- Pacholec, M.; Bleasdale, J.E.; Chrunyk, B.; Cunningham, D.; Flynn, D.; Garofalo, R.S.; Griffith, D.; Griffor, M.; Loulakis, P.; Pabst, B.; et al. Srt1720, srt2183, srt1460, and resveratrol are not direct activators of sirt1. J. Biol. Chem. 2010, 285, 8340–8351. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.R.; Brown, V.A.; Jones, D.J.L.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, H.H.S.; Garland, L.L.; Hsu, C.H.; Vining, D.R.; Chew, W.M.; Miller, J.A.; Perloff, M.; Crowell, J.A.; Alberts, D.S. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev. Res. 2010, 3, 1168–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbaramaiah, K.; Chung, W.J.; Michaluart, P.; Telang, N.; Tanabe, T.; Inoue, H.; Jang, M.; Pezzuto, J.M.; Dannenberg, A.J. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J. Biol. Chem. 1998, 273, 21875–21882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, P.S.; Maulik, G.; Cordis, G.A.; Bertelli, A.A.E.; Bertelli, A.; Das, D.K. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic. Biol. Med. 1999, 27, 160–169. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Clement, M.V.; Hirpara, J.L.; Chawdhury, S.H.; Pervaiz, S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers cd95 signaling-dependent apoptosis in human tumor cells. Blood 1998, 92, 996–1002. [Google Scholar] [CrossRef]
- Goldberg, D.A.; Yan, J.; Soleas, G.J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer. Res. 2004, 24, 2783–2840. [Google Scholar]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef]

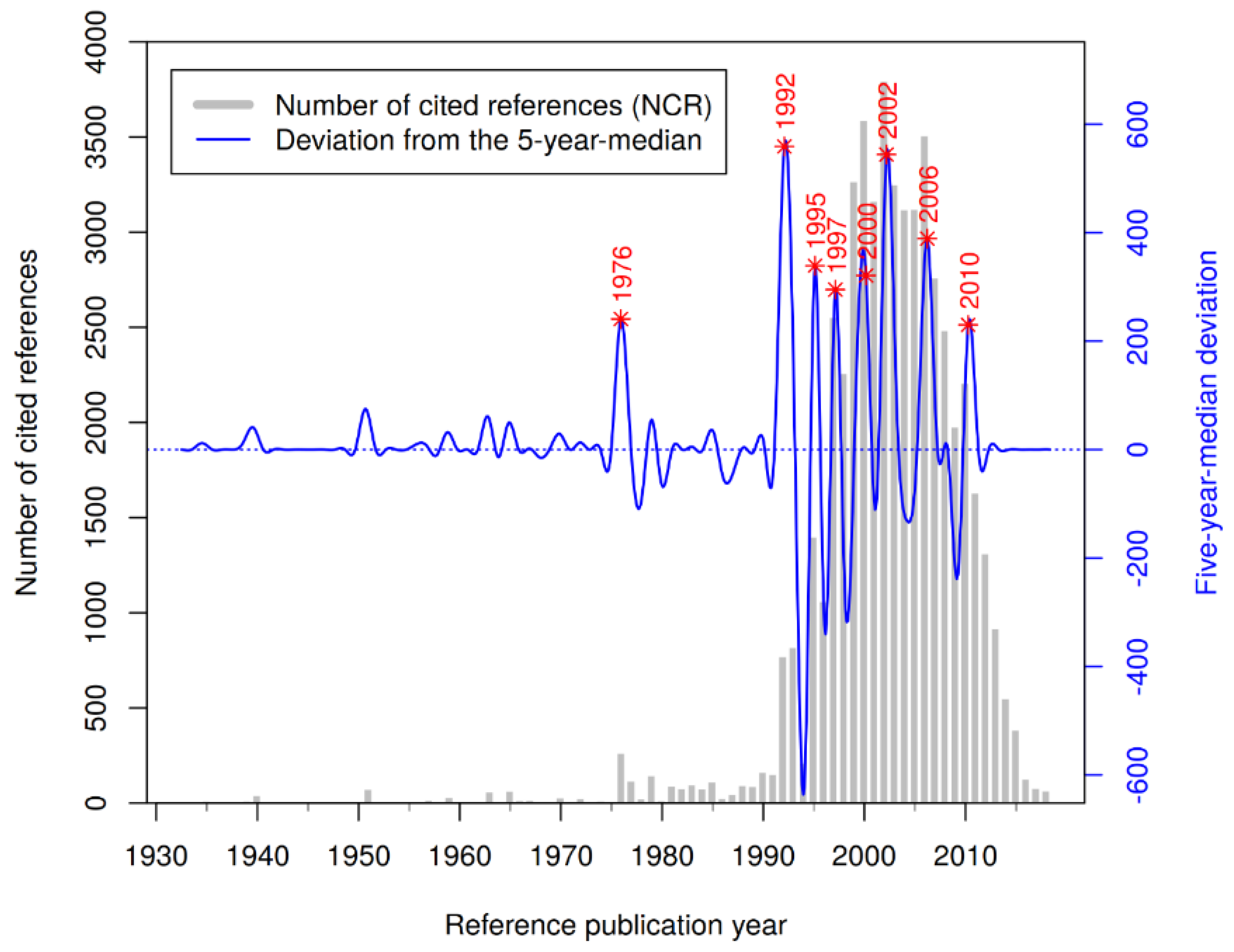
| #CR | Cited Reference | RPY | NCR |
|---|---|---|---|
| 1 | Takaoka M., 1939, Nippon Kagaku Kaishi, V60, P1090, DOI 10.1246/nikkashi1921.60.1090 [4] | 1939 | 12 |
| 2 | Takaoka M.J., 1940, J. Fac. Sci. Hokkaido Imperi, V3, P1 [3] | 1940 | 41 |
| 3 | Langcake P., 1976, Physiol. Plant Pathol., V9, P77, DOI 10.1016/0048-4059(76)90077-1 [26] | 1976 | 149 |
| 4 | Renaud S., 1992, Lancet, V339, P1523, DOI 10.1016/0140-6736(92)91277-F [8] | 1992 | 459 |
| 5 | Siemann E.H., 1992, Am. J. Enol. Viticult., V43, P49 [1] | 1992 | 200 |
| 6 | Frankel E.N., 1993, Lancet, V341, P1103, DOI 10.1016/0140-6736(93)92472-6 [27] | 1993 | 269 |
| 7 | Frankel E.N., 1993, Lancet, V341, P454, DOI 10.1016/0140-6736(93)90206-V [28] | 1993 | 120 |
| 8 | Fitzpatrick D.F., 1993, Am. J. Physiol., V265, pH774 [29] | 1993 | 87 |
| 9 | Pace-Asciak C.R., 1995, Clin. Chim. Acta, V235, P207, DOI 10.1016/0009-8981(95)06045-1 [30] | 1995 | 283 |
| 10 | Bertelli A.A.E., 1995, Int. J. Tissue React., V17, P1 [31] | 1995 | 166 |
| 11 | Jang M.S., 1997, Science, V275, P218, DOI 10.1126/science.275.5297.218 [32] | 1997 | 895 |
| 12 | Gehm B.D., 1997, PNAS USA, V94, P14138, DOI 10.1073/pnas.94.25.14138 [33] | 1997 | 289 |
| 13 | Soleas G.J., 1997, Clin. Biochem., V30, P91, DOI 10.1016/S0009-9120(96)00155-5 [34] | 1997 | 233 |
| 14 | Fremont L., 2000, Life Sci., V66, P663, DOI 10.1016/S0024-3205(99)00410-5 [35] | 2000 | 336 |
| 15 | Manna S.K., 2000, J. Immunol., V164, P6509, DOI 10.4049/jimmunol.164.12.6509 [36] | 2000 | 161 |
| 16 | Hung L.M., 2000, Cardiovasc. Res., V47, P549, DOI 10.1016/S0008-6363(00)00102-4 [37] | 2000 | 141 |
| 17 | Wallerath T., 2002, Circulation, V106, P1652, DOI 10.1161/01.CIR.0000029925.18593.5C [38] | 2002 | 189 |
| 18 | Burns J., 2002, J. Agr. Food Chem., V50, P3337, DOI 10.1021/jf0112973 [39] | 2002 | 111 |
| 19 | Wang Q., 2002, Brain Res., V958, P439, DOI 10.1016/S0006-8993(02)03543-6 [40] | 2002 | 108 |
| 20 | Baur J.A., 2006, Nat. Rev. Drug. Discov., V5, P493, DOI 10.1038/nrd2060 [41] | 2006 | 427 |
| 21 | Baur J.A., 2006, Nature, V444, P337, DOI 10.1038/nature05354 [42] | 2006 | 302 |
| 22 | Lagouge M., 2006, Cell, V127, P1109, DOI 10.1016/j.cell.2006.11.013 [43] | 2006 | 197 |
| 23 | Brown V.A., 2010, Cancer Res., V70, P9003, DOI 10.1158/0008-5472.CAN-10-2364 [44] | 2010 | 75 |
| 24 | Cottart C.H., 2010, Mol. Nutr. Food Res., V54, P7, DOI 10.1002/mnfr.200900437 [45] | 2010 | 66 |
| 25 | Pacholec M., 2010, J. Biol. Chem., V285, P8340, DOI 10.1074/jbc.M109.088682 [46] | 2010 | 56 |
| 26 | Patel K.R., 2010, Cancer. Res., V70, P7392, DOI 10.1158/0008-5472.CAN-10-2027 [47] | 2010 | 55 |
| 27 | Chow H.H.S., 2010, Cancer Prev. Res., V3, P1168, DOI 10.1158/1940-6207.CAPR-09-0155 [48] | 2010 | 48 |
| #CR | Cited Reference | RPY | NCR | N_TOP10 |
|---|---|---|---|---|
| 28 | Subbaramaiah K., 1998, J. Biol. Chem., V273, P21875, DOI 10.1074/jbc.273.34.21875 [49] | 1998 | 216 | 17 |
| 29 | Howitz K.T., 2003, Nature, V425, P191, DOI 10.1038/nature01960 [51] | 2003 | 275 | 17 |
| 30 | Clement M. V., 1998, Blood, V92, P996 [52] | 1998 | 223 | 16 |
| 31 | Goldberg D. A., 2003, Clin. Biochem., V36, P79, DOI 10.1016/S0009-9120(02)00397-1 [53] | 2003 | 156 | 16 |
| 32 | Walle T., 2004, Drug Metab. Dispos., V32, P1377, DOI 10.1124/dmd.104.000885 [54] | 2004 | 259 | 16 |
| 33 | Aggarwal B.B., 2004, Anticancer Res., V24, P2783 [55] | 2004 | 202 | 16 |
| 34 | Ray P.S., 1999, Free Radical Bio. Med., V27, P160, DOI 10.1016/S0891-5849(99)00063-5 [50] | 1999 | 158 | 15 |
| 35 | Bowers J.L., 2000, Endocrinology, V141, P3657, DOI 10.1210/en.141.10.3657 [56] | 2000 | 114 | 15 |
| 36 | Wu J.M., 2001, Int. J. Mol. Med., V8, P3 [6] | 2001 | 105 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haunschild, R.; Marx, W. On Health Effects of Resveratrol in Wine. Int. J. Environ. Res. Public Health 2022, 19, 3110. https://doi.org/10.3390/ijerph19053110
Haunschild R, Marx W. On Health Effects of Resveratrol in Wine. International Journal of Environmental Research and Public Health. 2022; 19(5):3110. https://doi.org/10.3390/ijerph19053110
Chicago/Turabian StyleHaunschild, Robin, and Werner Marx. 2022. "On Health Effects of Resveratrol in Wine" International Journal of Environmental Research and Public Health 19, no. 5: 3110. https://doi.org/10.3390/ijerph19053110
APA StyleHaunschild, R., & Marx, W. (2022). On Health Effects of Resveratrol in Wine. International Journal of Environmental Research and Public Health, 19(5), 3110. https://doi.org/10.3390/ijerph19053110







