Assessment of the Impact of Selected Industrial Wastewater on the Nitrification Process in Short-Term Tests
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
- (1)
- Nitrification inhibition;
- (2)
- Changes in the structure of activated sludge flocs;
- (3)
- Toxic effect on activated sludge microorganisms.
4. Conclusions
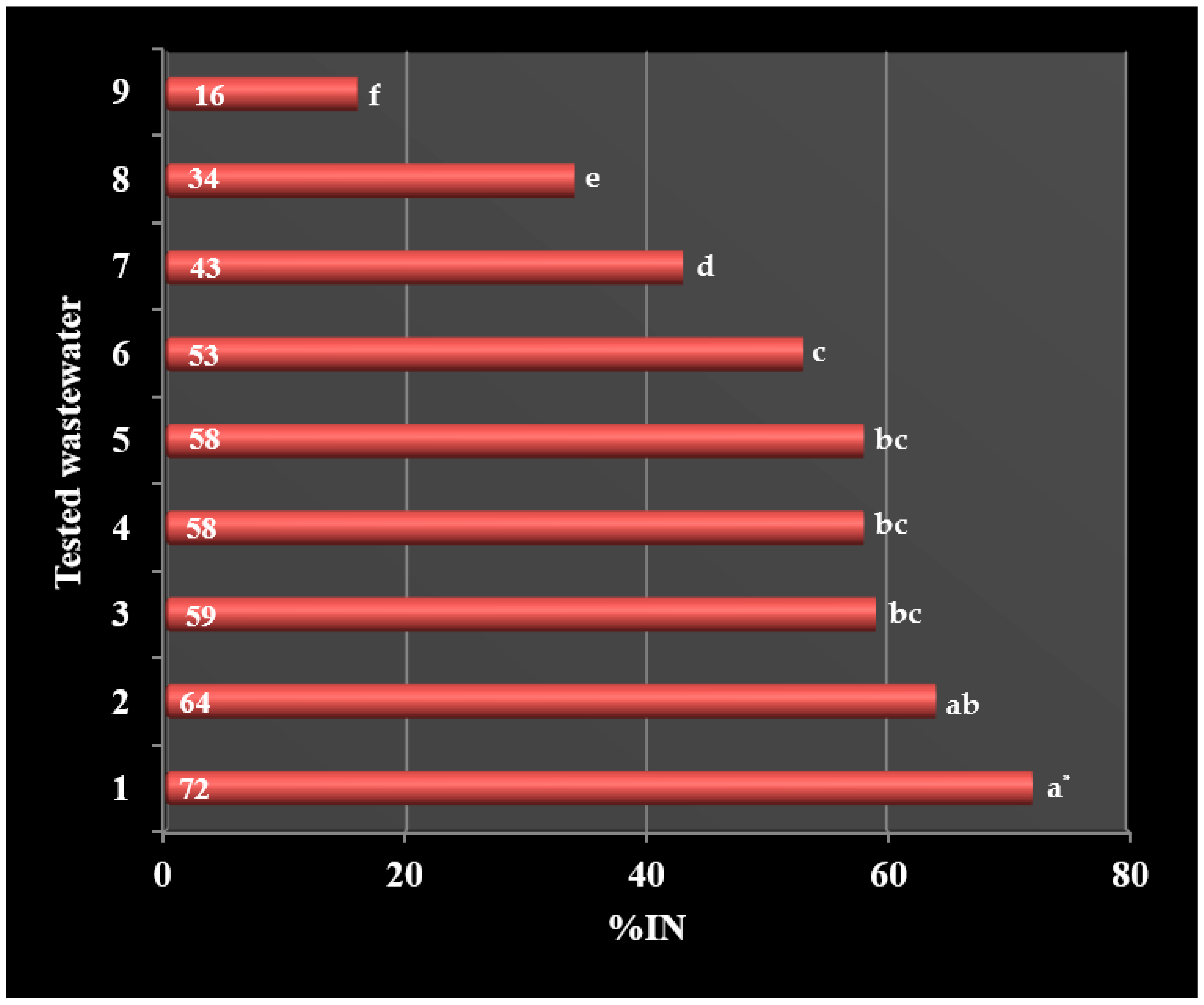
- Industrial wastewater from the chemical company Synthos S.A. contain numerous toxic substances that significantly inhibit the nitrification process.
- The most dangerous industrial streams include wastewater from the production of styrene butadiene rubbers, solvents (butyl acetate, ethyl acetate), styrene, acrylonitrile rubbers, terpenes and emulsifiers, which inhibit nitrification above 50%.
- To a slightly lower degree, the nitrification process is inhibited by wastewater from the production of polyvinyl acetate (43%) and polyvinyl chloride (33%).
- Wastewater from methacrylate production turned out to be the least toxic to nitrifying bacteria and showed the lowest degree of nitrification inhibition (16%).
- Wastewater from the production of styrene-butadiene and acrylonitrile rubbers, filtered in ash basins, show a much lower degree of nitrification inhibition than the same wastewater directed to the sewage treatment plant without the filtration process.
- The wastewater filtering process in ash basins significantly reduces nitrification inhibition, even about 60% for some wastewater.
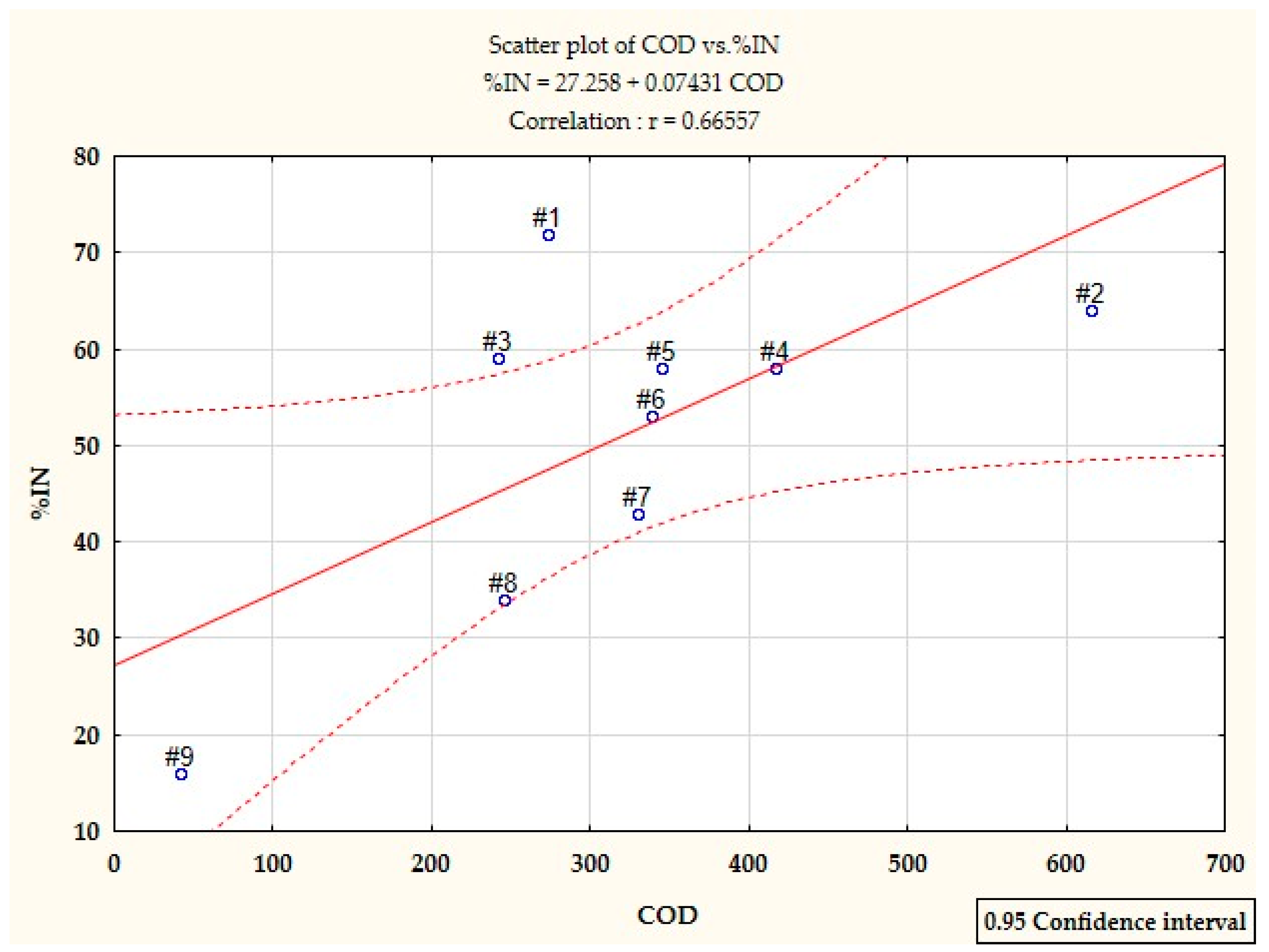
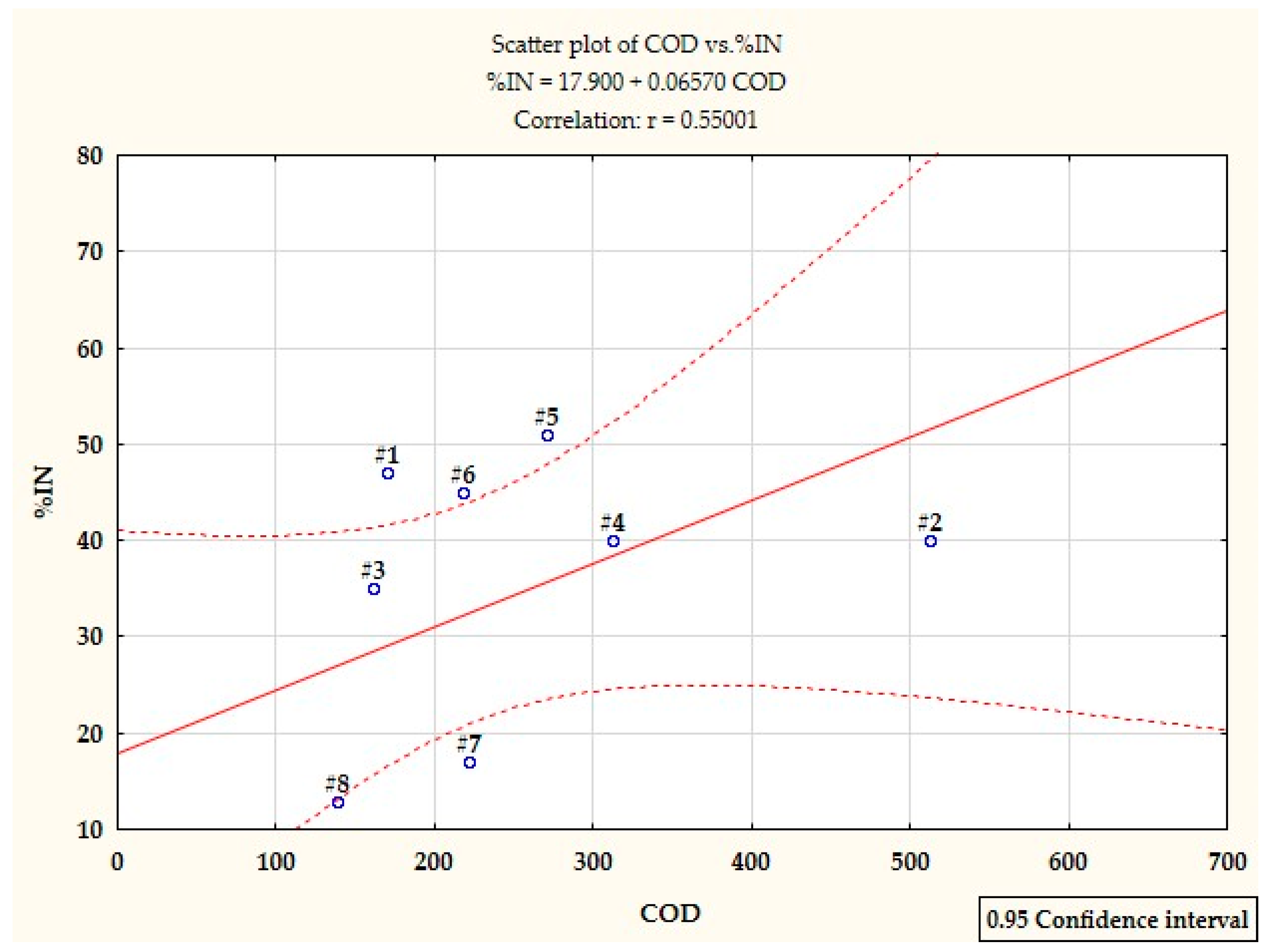
- Under static testing, wastewater from the production of styrene butadiene rubbers has a much higher degree of nitrification inhibition than the wastewater from the production of acrylonitrile rubbers. This phenomenon is not confirmed in the biological purification system, because as the production time of acrylonitrile rubbers increases, the efficiency of the nitrification process is significantly reduced or even completely ceases, which proves that a long time of exposure of the toxic factor enhances the inhibition of nitrification. This is also confirmed by the analysis of the correlation between %IN and COD of these wastewater, both before and after filtration.
- The conducted research allowed to select the most toxic industrial wastewater. The performed analyses suggest that the main cause of the inhibition of nitrification are the toxins contained in the sewage.
- The obtained results indicate the need for further research to determine the inhibitory concentration (IC) of the nitrification process for the most toxic wastewater from the production of styrene butadiene rubbers, solvents: butyl acetate, ethyl acetate, styrene, acrylonitrile rubbers and terpenes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grunditz, C.; Gumaelius, L.; Dalhammar, G. Comparison of inhibition assays using nitrogen removing bacteria: Application to industrial wastewater. Water Res. 1998, 32, 2995–3000. [Google Scholar] [CrossRef]
- Kapoor, V.; Elk, M.; Li, X.; Santo Domingo, J.W. Inhibitory effect of cyanide on wastewater nitrification determined using SOUR and RNA-based gene-specific assays. Lett. Appl. Microbiol. 2016, 63, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, L.; Svojitka, J.; Wanner, J.; Wintgens, T. Nitrification performance in a membrane bioreactor treating industrial wastewater. Water Res. 2013, 47, 4412–4421. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wang, S.; Yang, Q.; Yang, P.; Peng, Y. Start up partial nitrification at low temperature with a real-time control strategy based on blower frequency and pH. Bioresour. Technol. 2012, 112, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, K.; Grunditz, C.; Dalhammar, G.; Jansen, J.L.C. Occurrence of nitrification inhibition in Swedish municipal wastewaters. Water Res. 2000, 34, 2455–2462. [Google Scholar] [CrossRef]
- Kelly, R.T.; Henriques, I.D.S.; Love, N.G. Chemical inhibition of nitrification in activated sludge. Biotechnol. Bioeng. 2004, 85, 683–694. [Google Scholar]
- Okayasu, Y.; Tanaka, H.; Inui, T.; Tanaka, Y. Biosensor-based control of nitrification inhibitor in municipal wastewater treatment plants. Water Sci. Technol. 2006, 53, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.L.; Chen, H. Nitrification at full-scale municipal wastewater treatment plants: Evaluation of inhibition and bioaugmentation of nitrifiers. Bioresour. Technol. 2015, 190, 76–81. [Google Scholar] [CrossRef]
- ISO 9509:1989; Water Quality—Method for Assessing the Inhibition of Nitrification of Activated Sludge Microorganisms by Chemicals and Waste Waters; Ref. No. EN ISO 9509:1995 E. European Committee for Standardization (CEN)/International Organization for Standardization (ISO): Geneva, Switzerland, 1989.
- Pagga, U.; Bachner, J.; Strotmann, U. Inhibition of nitrification in laboratory tests and model wastewater treatment plants. Chemosphere 2006, 65, 1–8. [Google Scholar] [CrossRef]
- Hu, Z.; Chandran, K.; Grasso, D.; Smets, B.F. Effect of nickel and cadmium speciation on nitrification inhibition. Environ. Sci. Technol. 2002, 36, 3074–3078. [Google Scholar] [CrossRef]
- Kim, Y.M.; Park, D.; Lee, D.S.; Park, J.M. Inhibitory effects of toxic compounds on nitrification process for cokes wastewater treatment. J. Hazard. Mater. 2008, 152, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Lee, D.S.; Park, C.; Park, D.; Park, J.M. Effects of free cyanide on microbial communities and biological carbon and nitrogen removal performance in the industrial activated sludge process. Water Res. 2011, 45, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Park, H.; Chandran, K. Nitrification inhibition by hexavalent chromium Cr(VI)—Microbial ecology, gene expression and off-gas emissions. Water Res. 2016, 92, 254–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Kapoor, V.; Impellitteri, C.; Chandran, K.; Domingo, J.W.S. Measuring nitrification inhibition by metals in wastewater treatment systems: Current state of science and fundamental research Leeds. Crit. Rev. Environ. Sci. Technol. 2016, 46, 249–289. [Google Scholar] [CrossRef]
- Radniecki, T.S.; Dolan, M.E.; Semprini, L. Physiological and transcriptional responses of Nitrosomonas europaea to toluene and benzene inhibition. Environ. Sci. Technol. 2008, 42, 4093–4098. [Google Scholar] [CrossRef]
- Suárez-Ojeda, M.E.; Guisasola, A.; Carrera, J. Inhibitory impact of quinone-like compounds over partial nitrification. Chemosphere 2010, 80, 474–480. [Google Scholar] [CrossRef]
- Kroiss, H.; Schweighofer, P.; Frey, W.; Matsche, N. Nitrification inhibition-a source identification method for combined municipal and/or industrial wastewater treatment plants. Water Sci. Technol. 1992, 26, 1135–1146. [Google Scholar] [CrossRef] [Green Version]
- Nowak, O.; Svardal, K. Observations on the kinetics of nitrification under inhibiting conditions caused by industrial water compounds. Water Sci. Technol. 1993, 28, 115–123. [Google Scholar] [CrossRef]
- Svenson, A.; Sanden, B.; Dalhammar, G.; Remberger, M.; Kaj, L. Toxicity identification and evaluation of nitrification inhibitors in wastewaters. Environ. Toxicol. 2000, 15, 527–532. [Google Scholar] [CrossRef]
- Nowak, O.; Svardal, K.; Schweighofer, P. The dynamic behaviour of nitrifying activated sludge systems influenced by inhibiting wastewater compounds. Water Sci. Technol. 1995, 31, 115–124. [Google Scholar] [CrossRef]
- Hartmann, E. Nitrification inhibition in the treatment of sewage. Compiled by MERVYN RICHARDSON.—91 pp. London: The Royal Society of Chemistry 1985. ISBN 0-85186-596-8. $21.00. Hydrobiology 1987, 72, 375. [Google Scholar] [CrossRef]
- Surerus, V.; Giordano, G.; Teixeira, L.A.C. Activated sludge inhibition capacity index. Braz. J. Chem. Eng. 2014, 31, 385–392. [Google Scholar] [CrossRef]
- Okey, R.W.; Stensel, H.D.; Martis, M.C. Modeling nitrification inhibition. Water Sci. Technol. 1996, 33, 101–107. [Google Scholar] [CrossRef]
- König, A.; Riedel, K.; Metzger, J.W. A microbial sensor for detecting inhibitors of nitrification in wastewater. Biosens. Bioelectron. 1998, 13, 869–874. [Google Scholar] [CrossRef]
- Gwak, J.H.; Jung, M.Y.; Hong, H.; Kim, J.G.; Quan, Z.X.; Reinfelder, J.R.; Spasov, E.; Neufeld, J.D.; Wagner, M.; Rhee, S.K. Archaeal nitrification is constrained by copper complexation with organic matter in municipal wastewater treatment plants. ISMEJ 2020, 14, 335–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katipoglu-Yazan, T.; Cokgor, E.U.; Insel, G.; Orhon, D. Is ammonification the rate limiting step for nitrification kinetics? Bioresour. Technol. 2012, 114, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, H.; Du, M.; Wang, W. Inhibition and recovery of nitrification in treating real coal gasification wastewater with moving bed biofilm reaktor. J. Environ. Sci. 2011, 23, 568–574. [Google Scholar] [CrossRef]
- Salem, S.; Berends, D.H.J.G.; Heijnen, J.J.; Van Loosdrech, M.C.M. Bio-augmentation by nitrification with return sludge. Water Res. 2003, 37, 1794–1804. [Google Scholar] [CrossRef]
- Figuerola, E.L.M.; Erijman, L. Diversity of nitrifying bacteria in a full-scale petroleum refinery wastewater treatment plant experiencing unstable nitrification. J. Hazard. Mater. 2010, 181, 281–288. [Google Scholar] [CrossRef]
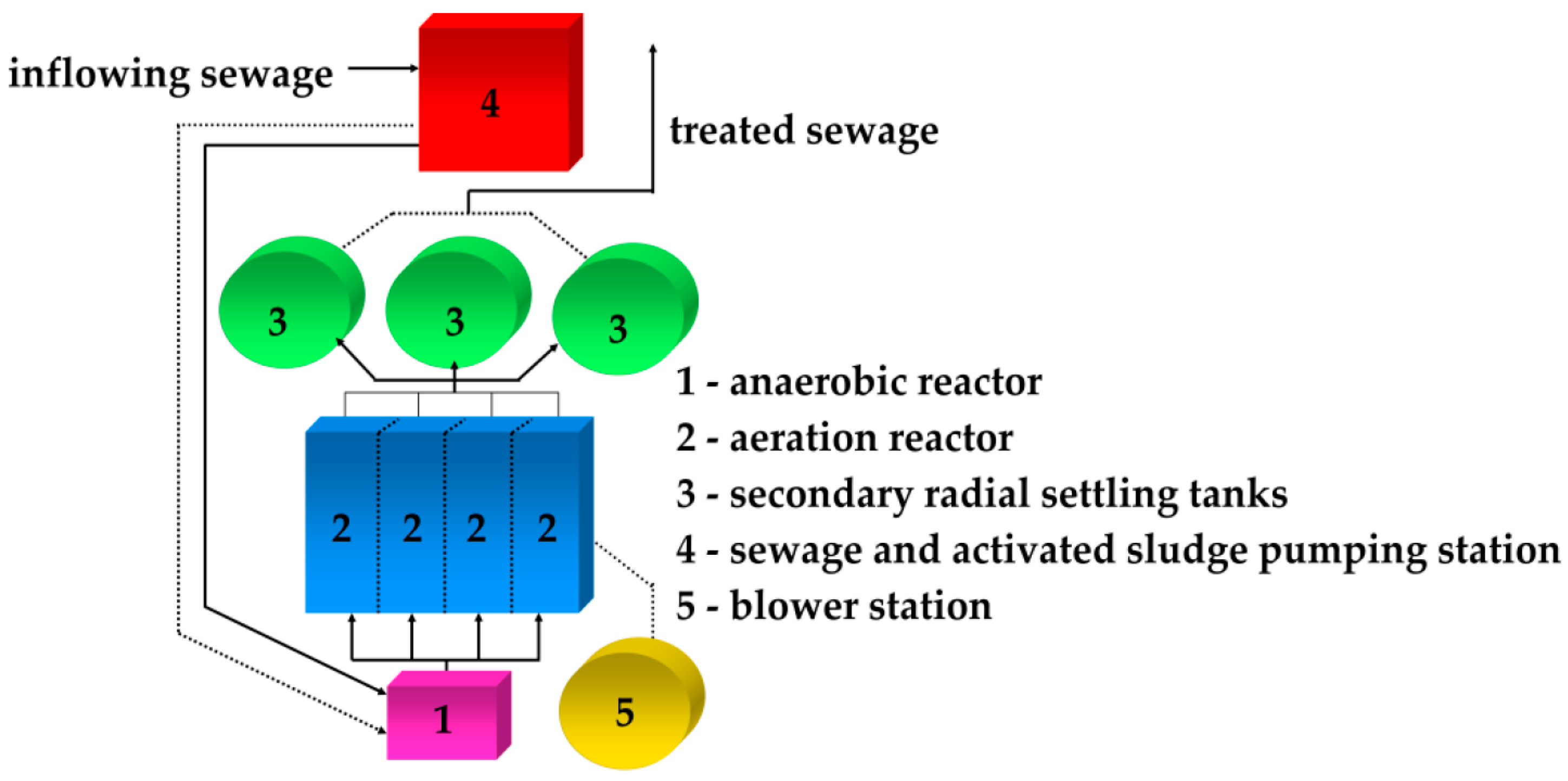
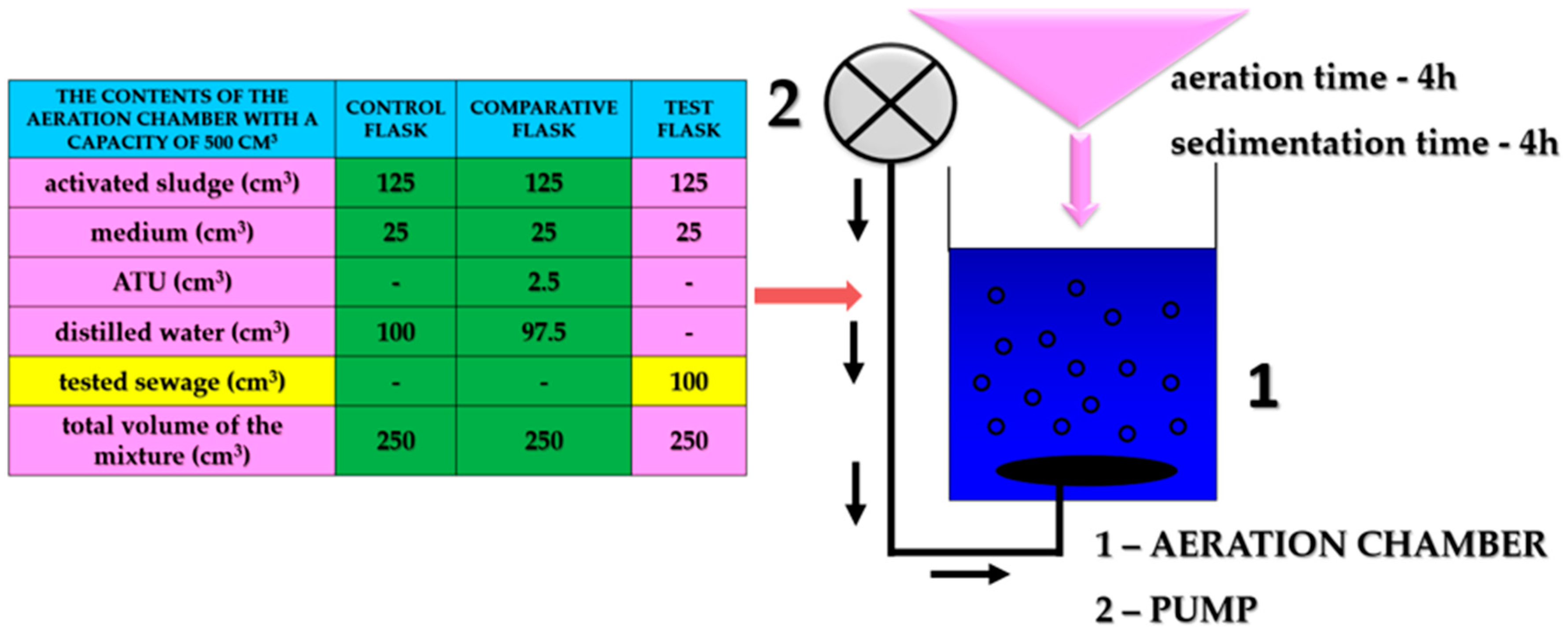

| Sewage from Production | Chemical Compounds of Sewage |
|---|---|
| styrene butadiene rubbers | latex, silicone emulsion, technical olein, butadiene, styrene, versenic acid, alkyl benzene sulfonic acid, acetic acid, butylcatechol, tricresol |
| solvents: butyl acetate, ethyl acetate | butanol, acetic acid, acetaldehyde, ethanol, carbide |
| styrene | ethylbenzene, p-tert-butylcatechol |
| acrylonitrile rubbers | acrylic acid, ethyl glycol, sodium acetate, butadiene, acrylonitrile, acetonitrile, stearin, versenic acid, tertiary dodecyl mercaptan, thiuram sodium |
| terpenes | balsamic turpentine |
| emulsifiers | balsamic rosin, balsamic turpentine |
| polyvinyl acetate | distilled vinyl acetate, polyvinyl alcohol, crude carbide, dibutyl phthalate, rectified methanol, lauroyl peroxide |
| polyvinyl chloride | distilled vinyl chloride |
| methacrylate | methyl methacrylate, alpha-azodi-isobutyronitrile, dioctyl phthalate, technical stearin, butanol, acrylic acid, methacrylamide, butyl acrylate, citric acid, distilled vinyl acetate, butyl methacrylate, toluene, benzoyl peroxide, ethyl acetate |
| Parameter | Unit | Value |
|---|---|---|
| temperature | °C | 14.9 |
| oxygenation | mg O2·dm−3 | 1–3 |
| aeration time | h | 4–5 |
| total suspension of the activated sludge in the aeration chamber | g·dm−3 | 3.5–5.0 |
| total suspension in excess sludge | g·dm−3 | 6.0–8.0 |
| excess sludge increase | m3·d−1 | 300–400 |
| recirculation | % | 120 |
| dry mass of activated sludge | % | 1.64 |
| the age of the activated sludge | days | 13–14 |
| BOD5 | kgBOD5·kgd.m.·d−1 | 0.11–0.19 |
| Sample | pH | COD (mg O2·dm−3) | Average Concentration NH4-N (mg·dm−3) | Average Concentration NO3-N (mg·dm−3) | ||
|---|---|---|---|---|---|---|
| Before Incubation | After 4 h of Incubation | Before Incubation | After 4 h of Incubation | |||
| C | 7.4 | - | 56.0 | 24.3 | 0.0 | 19.2 |
| ATU | 7.4 | - | 56.0 | 36.2 | 0.0 | 0.1 |
| 1 | 7.8 | 272.7 | 56.0 | 39.7 | 0.0 | 5.4 |
| 2 | 7.5 | 615.5 | 56.0 | 27.9 | 0.0 | 7.0 |
| 3 | 7.4 | 242.1 | 56.0 | 32.0 | 0.0 | 8.0 |
| 4 | 7.0 | 416.5 | 56.0 | 35.2 | 0.0 | 8.2 |
| 5 | 7.5 | 345.6 | 56.0 | 26.9 | 0.0 | 8.2 |
| 6 | 7.6 | 339.0 | 56.0 | 45.4 | 0.0 | 9.1 |
| 7 | 7.7 | 329.8 | 56.0 | 14.0 | 0.0 | 10.9 |
| 8 | 7.3 | 245.6 | 56.0 | 21.4 | 0.0 | 12.8 |
| 9 | 7.3 | 41.9 | 56.0 | 18.0 | 0.0 | 16.1 |
| Sample | pH | COD (mg O2·dm−3) | Average Concentration NH4-N (mg·dm−3) | Average Concentration NO3-N (mg·dm−3) | ||
|---|---|---|---|---|---|---|
| Before Incubation | After 4 h of Incubation | Before Incubation | After 4 h of Incubation | |||
| C | 7.6 | - | 56.0 | 21.4 | 0.0 | 22.7 |
| ATU | 7.6 | - | 56.0 | 39.5 | 0.0 | 0.1 |
| 1 | 7.3 | 170.0 | 56.0 | 28.5 | 0.0 | 12.0 |
| 2 | 7.6 | 512.7 | 56.0 | 18.6 | 0.0 | 13.6 |
| 3 | 7.2 | 161.8 | 56.0 | 25.7 | 0.0 | 14.7 |
| 4 | 7.1 | 312.6 | 56.0 | 29.1 | 0.0 | 13.6 |
| 5 | 7.4 | 271.3 | 56.0 | 19.6 | 0.0 | 11.2 |
| 6 | 7.1 | 218.0 | 56.00 | 32.6 | 0.0 | 12.6 |
| 7 | 7.0 | 221.4 | 56.0 | 10.3 | 0.0 | 18.8 |
| 8 | 7.2 | 138.5 | 56.0 | 13.3 | 0.0 | 19.7 |
| 9 | 7.4 | 16.4 | 56.0 | 11.4 | 0.0 | 21.4 |
| Sample 1 | %IN | Increase in the Efficiency of the Nitrification Process (%) | |
|---|---|---|---|
| Before Filtration | After Filtration | ||
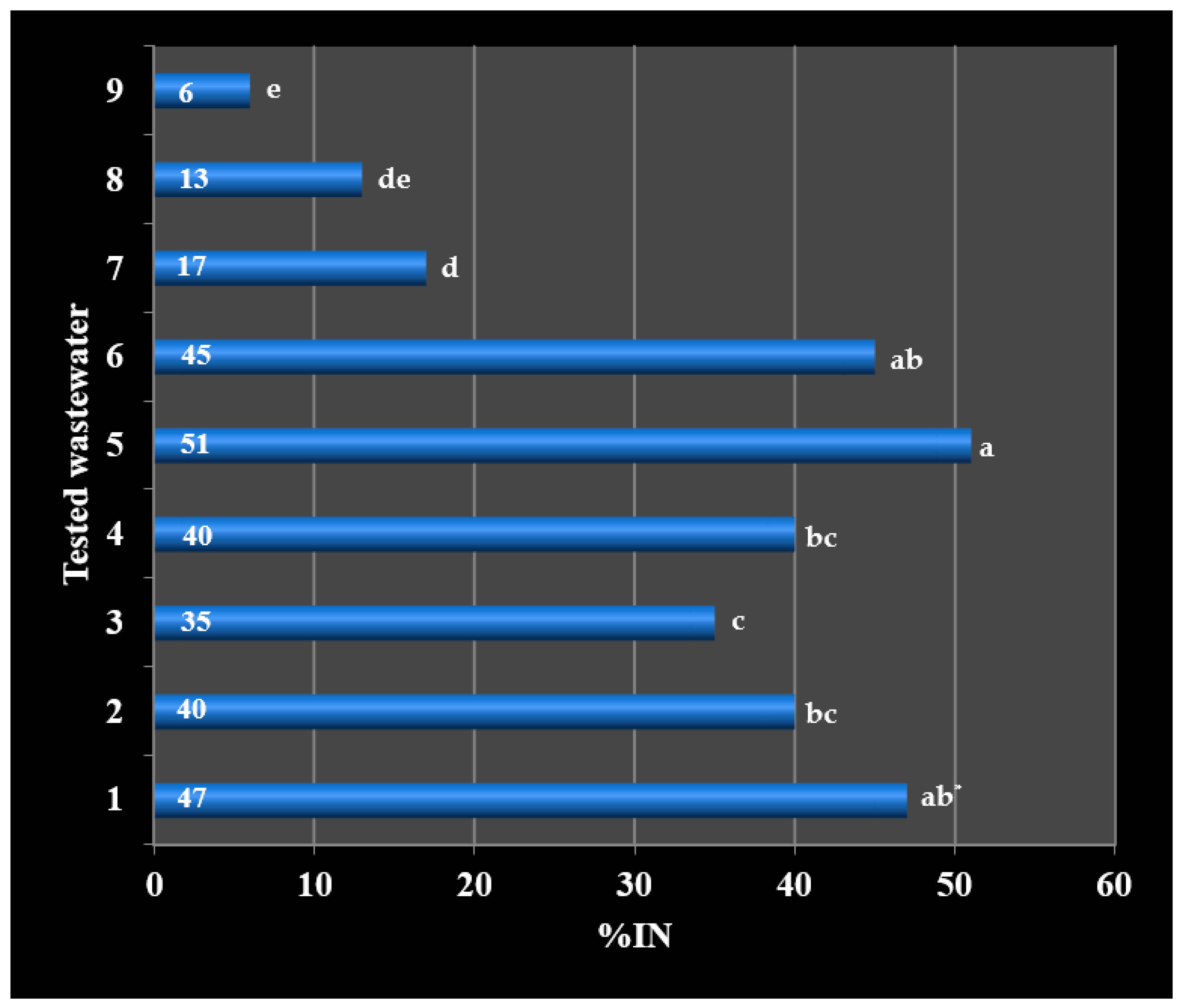
| 1 | 72 a * | 47 b | 35 |
| 2 | 64 a | 40 b | 37 |
| 3 | 59 a | 35 b | 41 |
| 4 | 58 a | 40 b | 31 |
| 5 | 58 a | 51 a | 12 |
| 6 | 53 a | 45 a | 15 |
| 7 | 43 a | 17 b | 60 |
| 8 | 34 a | 13 b | 61 |
| 9 | 16 a | 6 b | 62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paśmionka, I.B.; Gospodarek, J. Assessment of the Impact of Selected Industrial Wastewater on the Nitrification Process in Short-Term Tests. Int. J. Environ. Res. Public Health 2022, 19, 3014. https://doi.org/10.3390/ijerph19053014
Paśmionka IB, Gospodarek J. Assessment of the Impact of Selected Industrial Wastewater on the Nitrification Process in Short-Term Tests. International Journal of Environmental Research and Public Health. 2022; 19(5):3014. https://doi.org/10.3390/ijerph19053014
Chicago/Turabian StylePaśmionka, Iwona B., and Janina Gospodarek. 2022. "Assessment of the Impact of Selected Industrial Wastewater on the Nitrification Process in Short-Term Tests" International Journal of Environmental Research and Public Health 19, no. 5: 3014. https://doi.org/10.3390/ijerph19053014
APA StylePaśmionka, I. B., & Gospodarek, J. (2022). Assessment of the Impact of Selected Industrial Wastewater on the Nitrification Process in Short-Term Tests. International Journal of Environmental Research and Public Health, 19(5), 3014. https://doi.org/10.3390/ijerph19053014







