Efficient Production of Naringin Acetate with Different Acyl Donors via Enzymatic Transesterification by Lipases
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
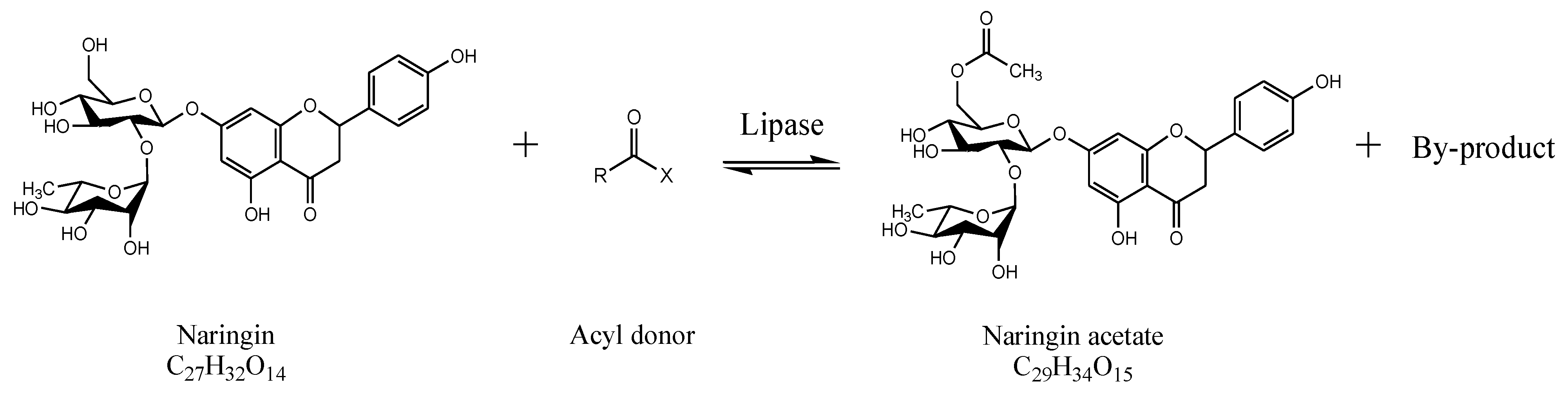
2.2. Enzymatic Synthesis of Naringin Acetate
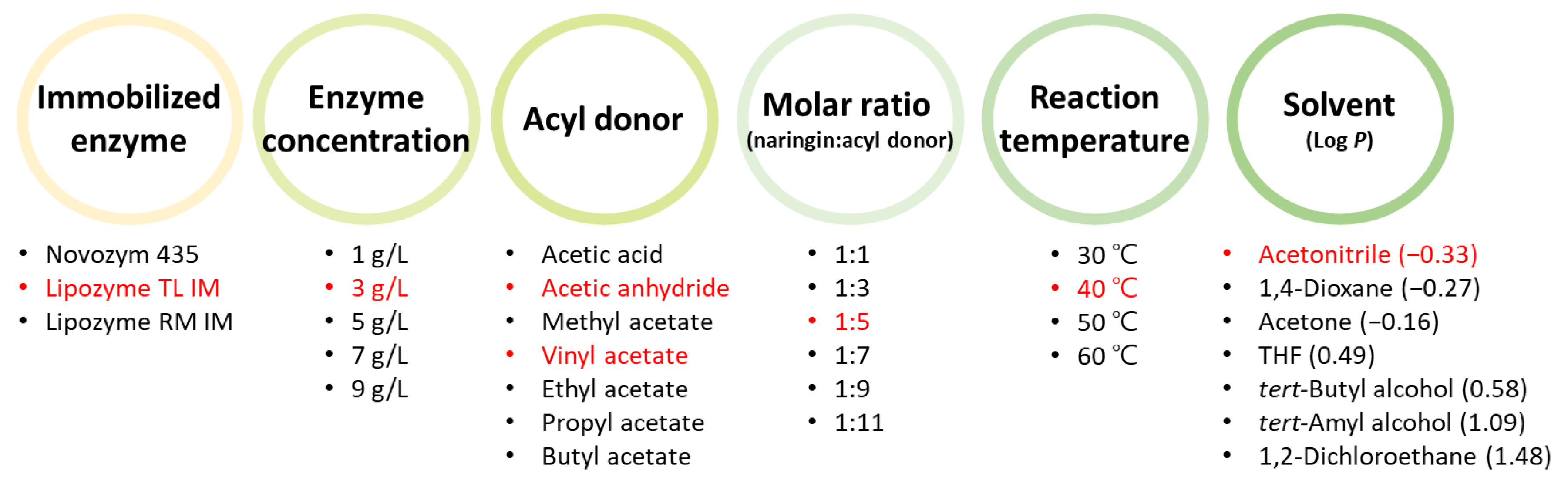
2.3. Optimization of Reaction Conditions
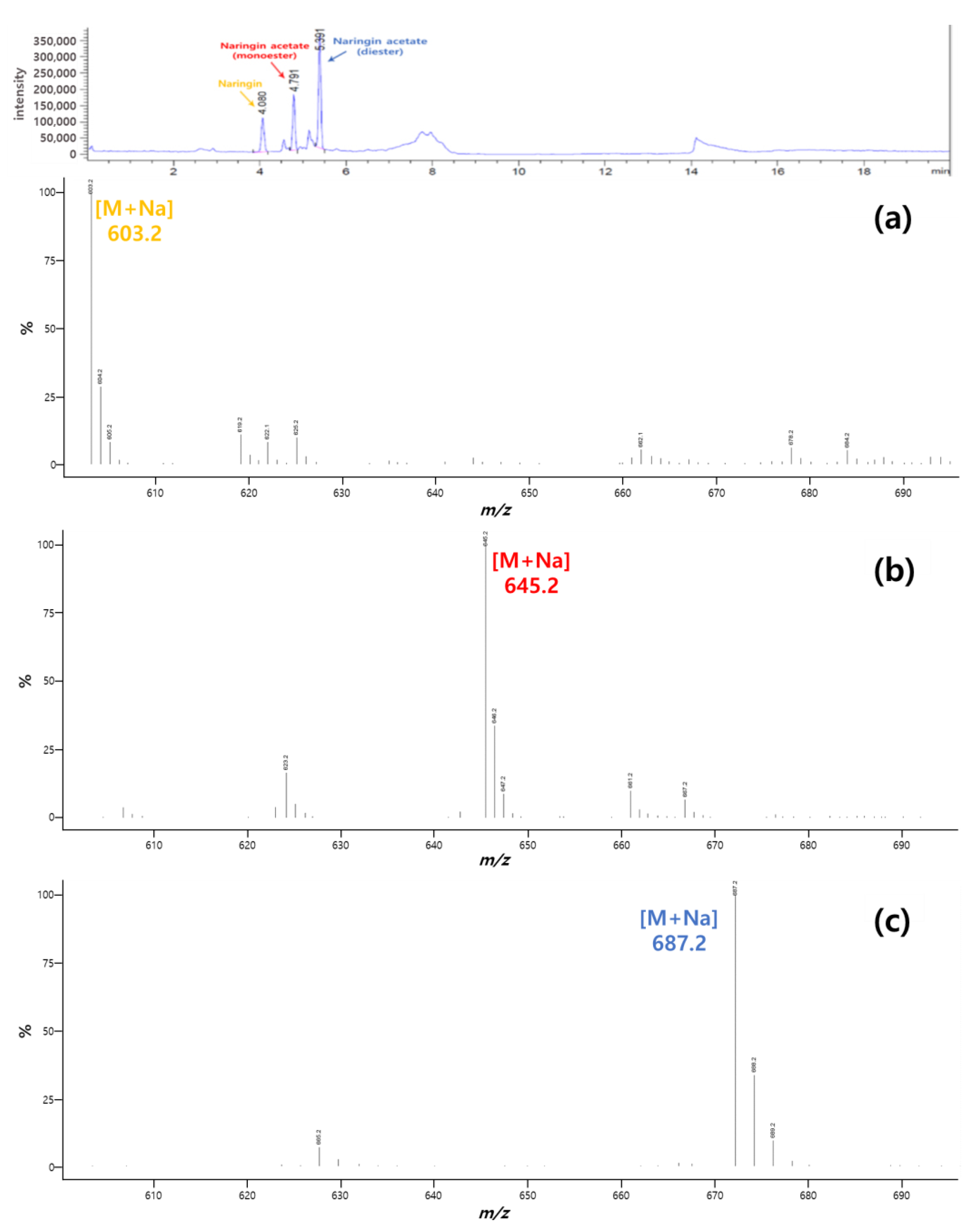
2.4. Analytical Methods
3. Results and Discussion
3.1. Synthesis of Naringin Acetate by Transesterification with Acyl Donors
3.2. Selection of Enzyme
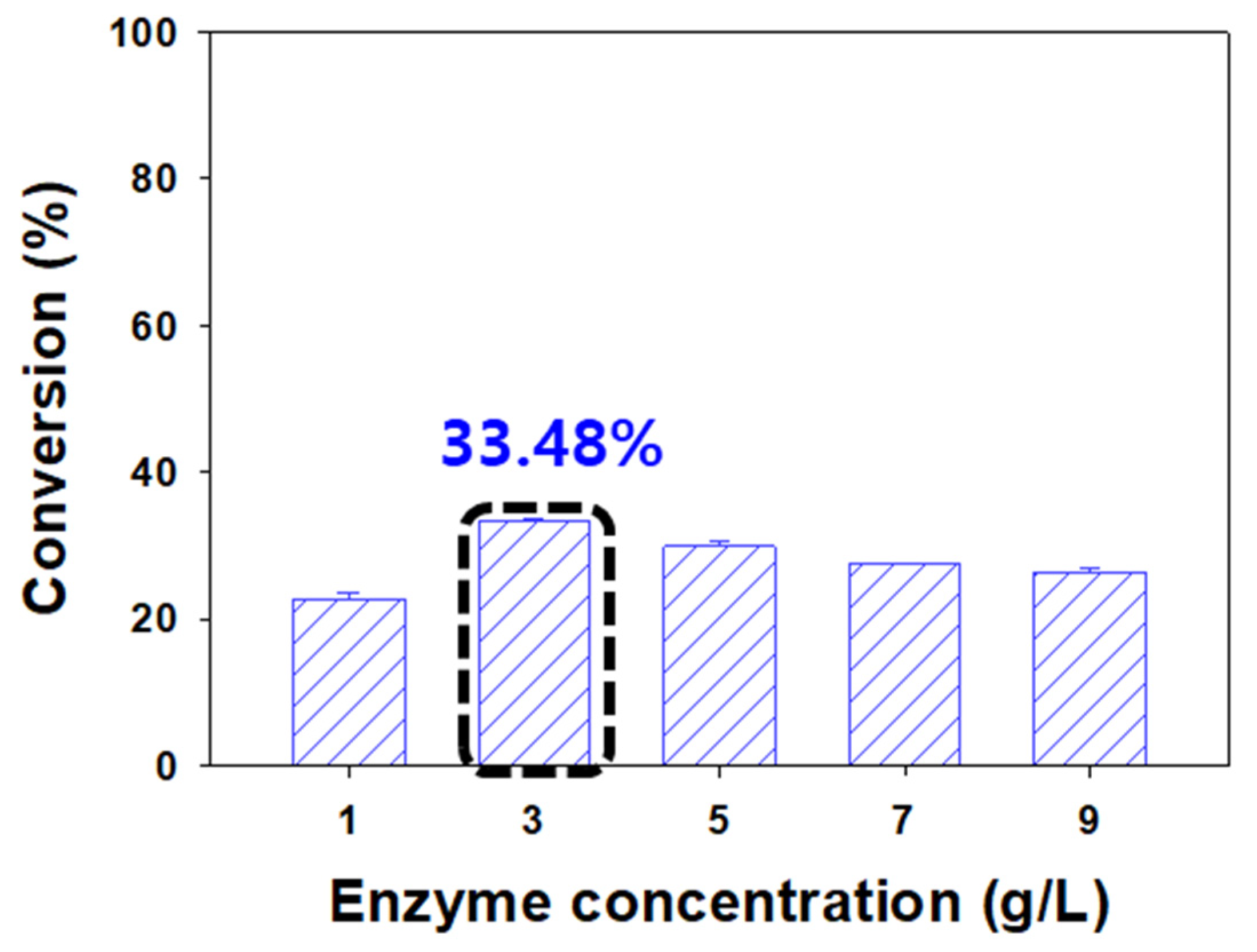
3.3. Effect of Enzyme Concentration on the Conversion of Naringin Acetate
3.4. Effects of Acyl Donors on the Conversion of Naringin Acetate
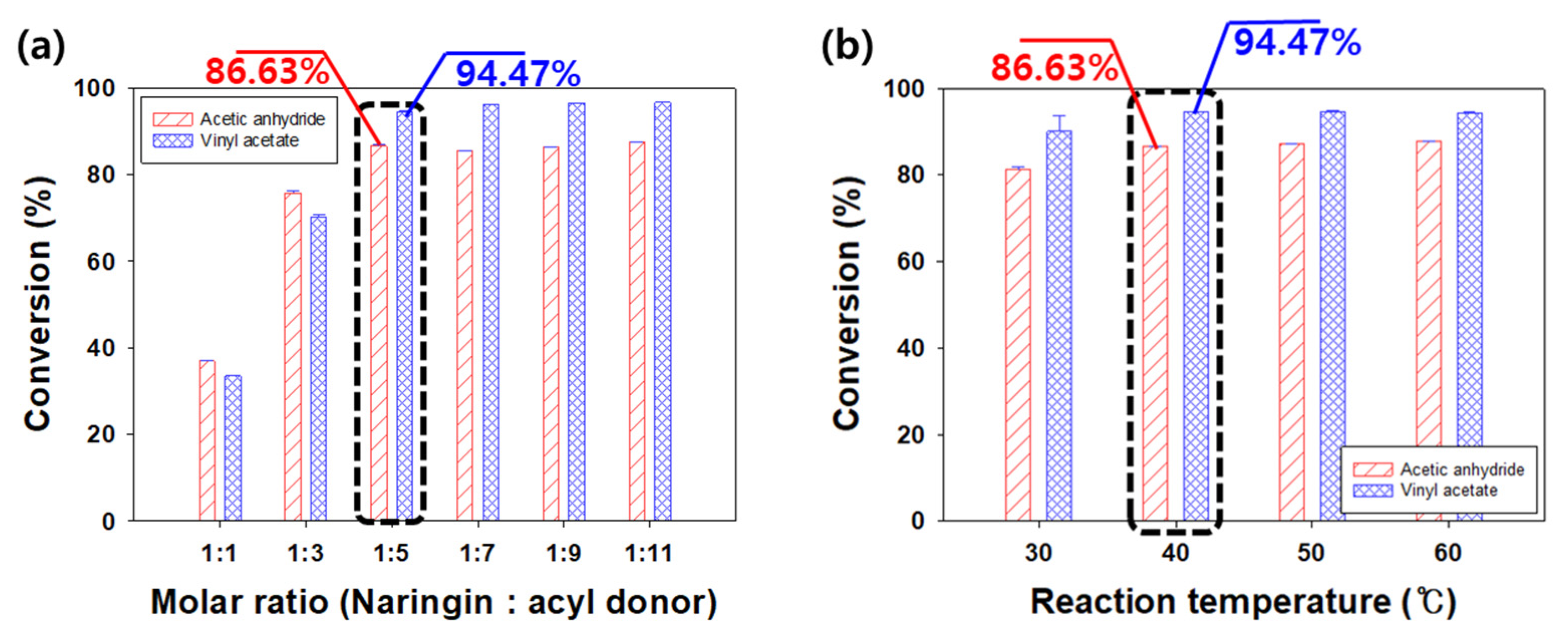
3.5. Effects of Molar Ratio and Reaction Temperature on the Conversion of Naringin Acetate
3.6. Effect of Solvent on the Conversion of Naringin Acetate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, E47. [Google Scholar] [CrossRef] [PubMed]
- Viskupicova, J.; Maliar, T. Rutin fatty acid esters: From synthesis to biological health effects and application. J. Food Nutr. Res. 2017, 56, 232–243. [Google Scholar]
- Kim, W.; Lee, J.K.; Choi, K.Y.; Kim, B.G.; Kim, J. Regioselective biotransformation of phloretin using Streptomyces avermitilis MA4680. Biotechnol. Bioprocess Eng. 2020, 25, 272–278. [Google Scholar] [CrossRef]
- Jung, U.J.; Kim, H.J.; Lee, J.S.; Lee, M.K.; Kim, H.O.; Park, E.J.; Kim, H.K.; Jeong, T.S.; Choi, M.S. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin. Nutr. 2003, 22, 561–568. [Google Scholar] [CrossRef]
- Zhou, Z.; Fu, C. A new flavanone and other constituents from the rhizomes of Cyperus rotundus and their antioxidant activities. Chem. Nat. Compd. 2013, 48, 963–965. [Google Scholar] [CrossRef]
- Cao, R.; Li, X.; Zhou, Z.; Zhao, Z. Synthesis and biophysical analysis of Naringin-Chitooligosaccharide complex. Nat. Prod. Res. 2019, 35, 305–311. [Google Scholar] [CrossRef]
- Szliszka, E.; Kostrzewa-Susłow, E.; Bronikowska, J.; Jaworska, D.; Janeczko, T.; Czuba, Z.P.; Krol, W. Synthetic flavanones augment the anticancer effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Molecules 2012, 17, 11693–11711. [Google Scholar] [CrossRef]
- Fisher, J.F.; Wheaton, T.A. A high-pressure liquid chromatographic method for the resolution and quantitation of naringin and naringenin rutinoside in grapefruit Juice. J. Agric. Food Chem. 1976, 24, 898–899. [Google Scholar] [CrossRef]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Lai, X.; Nong, J.; Zhao, G.; Xiao, X. One-pot biocatalytic synthesis and antioxidant activities of highly lipophilic naringin derivatives by using bi-functional whole-cells. Food Res. Int. 2020, 136, 109291. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Katsoura, M.H.; Polydera, A.C.; Tsironis, L.; Tselepis, A.D.; Stamatis, H. Use of ionic liquids as media for the biocatalytic preparation of flavonoid derivatives with antioxidant potency. J. Biotechnol. 2006, 123, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Danihelová, M.; Viskupičová, J.; Šturdík, E. Lipophilization of flavonoids for their food, therapeutic and cosmetic applications. Acta Chim. Slovaca 2012, 5, 59–69. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.; Son, J.; Lee, H.; Song, J.H.; Lee, T.; Jeon, H.; Kim, H.S.; Park, S.J.; Yoo, H.Y.; et al. Improved productivity of naringin oleate with flavonoid and fatty acid by efficient enzymatic esterification. Antioxidants 2022, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kanwar, S.S. Organic solvent tolerant lipases and applications. Sci. World J. 2014, 2014, 625258. [Google Scholar] [CrossRef]
- Franco, Y.; Longato, G.; Carvalho, P.; Messias, M.; de Araújo, M.; Pamphile, J. Biocatalytic synthesis of flavonoid esters by lipases and their biological benefits. Planta Med. 2016, 83, 7–22. [Google Scholar] [CrossRef]
- Rychlicka, M.; Maciejewska, G.; Niezgoda, N.; Gliszczyńska, A. Production of feruloylated lysophospholipids via a one-step enzymatic interesterification. Food Chem. 2020, 323, 126802. [Google Scholar] [CrossRef]
- Okulus, M.; Gliszczyńska, A. Enzymatic synthesis of o-methylated phenophospholipids by lipase-catalyzed acidolysis of egg-yolk phosphatidylcholine with anisic and veratric acids. Catalysts 2020, 10, 538. [Google Scholar] [CrossRef]
- Viskupičová, J.; Ondrejovic, M.; Maliar, T. Enzyme-mediated preparation of flavonoid esters and their applications. Biochemistry 2012, 10, 263–278. [Google Scholar] [CrossRef]
- Shin, M.; Seo, J.; Baek, Y.; Lee, T.; Jang, M.; Park, C. Novel and efficient synthesis of phenethyl formate via enzymatic esterification of formic acid. Biomolecules 2020, 10, 70. [Google Scholar] [CrossRef]
- Baek, Y.; Lee, J.; Son, J.; Lee, T.; Sobhan, A.; Lee, J.; Koo, S.M.; Shin, W.H.; Oh, J.M.; Park, C. Enzymatic synthesis of formate ester through immobilized lipase and its reuse. Polymers 2020, 12, 1802. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Shin, M.; Lee, J.; Lee, T.; Oh, J.M.; Park, C. Novel and highly efficient lipase-catalyzed esterification of formic acid with hexanol for waste gas reutilization. J. Ind. Eng. Chem. 2021, 93, 430–435. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Gao, H.; Bai, X.; Li, Z.J. Preparation and enzymatic activity of Fe3O4-IDA-Ni/NAD kinase magnetic catalyst. Korean J. Chem. Eng. 2020, 37, 475–481. [Google Scholar] [CrossRef]
- Chun, J.; Sang, B.I. Enzymatic esterification under high-pressure CO2 conditions for in situ recovery of butyric acid from anaerobic fermenters. Biotechnol. Bioprocess Eng. 2020, 25, 616–622. [Google Scholar] [CrossRef]
- Milivojević, A.D.; Ćorović, M.M.; Simović, M.B.; Banjanac, K.M.; Blagojević, S.N.; Pjanović, R.V.; Bezbradica, D.I. Novel approach for flavonoid esters production: Statistically optimized enzymatic synthesis using natural oils and application in cosmetics. Ind. Eng. Chem. Res. 2019, 58, 3640–3649. [Google Scholar] [CrossRef]
- Almeida, V.M.; Branco, C.R.C.; Assis, S.A.; Vieira, I.J.C.; Braz-Filho, R.; Branco, A. Synthesis of naringin 6″-ricinoleate using immobilized lipase. Chem. Cent. J. 2012, 6, 41. [Google Scholar] [CrossRef]
- Zheng, M.M.; Wang, L.; Huang, F.H.; Guo, P.M.; Wei, F.; Deng, Q.C.; Zheng, C.; Wan, C.Y. Ultrasound irradiation promoted lipase-catalyzed synthesis of flavonoid esters with unsaturated fatty acids. J. Mol. Catal. B Enzym. 2013, 95, 82–88. [Google Scholar] [CrossRef]
- Sun, C.Q.; Johnson, K.D.; Wong, H.; Foo, L.Y. Biotransformation of flavonoid conjugates with fatty acids and evaluations of their functionalities. Front. Pharmacol. 2017, 8, 759. [Google Scholar] [CrossRef]
- Céliz, G.; Daz, M. Biocatalytic preparation of alkyl esters of citrus flavanone glucoside prunin in organic media. Process Biochem. 2011, 46, 94–100. [Google Scholar] [CrossRef]
- Degenhardt, A.; Ullrich, F.; Hofmann, T.; Stark, T. Flavonoid Sugar Addition Products, Method for Manufacture and Use Thereof. U.S. Patent 8,003,150, 23 August 2011. [Google Scholar]
- Bok, S.-H.; Jeong, T.-S.; Lee, S.-K.; Kim, J.-R.; Moon, S.-S.; Choi, M.-S.; Hyun, B.-H.; Lee, C.-H.; Choi, Y.-K. Flavanone Derivatives and Composition for Preventing or Treating Blood Lipid Level-Related Diseases Comprising Same. U.S. Patent 6,455,577, 24 September 2002. [Google Scholar]
- Mellou, F.; Loutrari, H.; Stamatis, H.; Roussos, C.; Kolisis, F.N. Enzymatic esterification of flavonoids with unsaturated fatty acids: Effect of the novel esters on vascular endothelial growth factor release from K562 cells. Process Biochem. 2006, 41, 2029–2034. [Google Scholar] [CrossRef]
- Hattori, H.; Tsutsuki, H.; Nakazawa, M.; Ueda, M.; Ihara, H.; Sakamoto, T. Naringin lauroyl ester inhibits lipopolysaccharide-induced activation of nuclear factor κB signaling in macrophages. Biosci. Biotechnol. Biochem. 2016, 80, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Moussou, P.; Falcimaigne, A.; Ghoul, M.; Danoux, L.; Pauly, G. Esters of Flavonoids and ω-Substituted C6-C22 Fatty. Acids. Patent 2007/0,184,098, 9 August 2007. [Google Scholar]
- Saija, A.; Tomaino, A.; Trombetta, D.; Pellegrino, M.L.; Tita, B.; Messina, C.; Bonina, F.P.; Rocco, C.; Nicolosi, G.; Castelli, F. “In vitro” antioxidant and photoprotective properties and interaction with model membranes of three new quercetin esters. Eur. J. Pharm. Biopharm. 2003, 56, 167–174. [Google Scholar] [CrossRef]
- Viskupicova, J.; Danihelova, M.; Ondrejovic, M.; Liptaj, T.; Sturdik, E. Lipophilic rutin derivatives for antioxidant protection of oil-based foods. Food Chem. 2010, 123, 45–50. [Google Scholar] [CrossRef]
- Viskupicova, J.; Danihelova, M.; Majekova, M.; Liptaj, T.; Sturdik, E. Polyphenol fatty acid esters as serine protease inhibitors: A quantum-chemical QSAR analysis. J. Enzym. Inhib. Med. Chem. 2012, 27, 800–809. [Google Scholar] [CrossRef]
- Razak, N.N.A.; Annuar, M.S.M. Enzymatic synthesis of flavonoid ester: Elucidation of its kinetic mechanism and equilibrium thermodynamic behavior. Ind. Eng. Chem. Res. 2015, 54, 5604–5612. [Google Scholar] [CrossRef]
- Nakajima, N.; Ishihara, K.; Itoh, T.; Furuya, T.; Hamada, H. Lipase-catalyzed direct and regioselective acylation of flavonoid glucoside for mechanistic investigation of stable plant pigments. J. Biosci. Bioeng. 1999, 87, 105–107. [Google Scholar] [CrossRef]
- Kontogianni, A.; Skouridou, V.; Sereti, V.; Stamatis, H.; Kolisis, F.N. Regioselective acylation of flavonoids catalyzed by lipase in low toxicity media. Eur. J. Lipid Sci. Technol. 2001, 103, 655–660. [Google Scholar] [CrossRef]
- Kontogianni, A.; Skouridou, V.; Sereti, V.; Stamatis, H.; Kolisis, F.N. Lipase-catalyzed esterification of rutin and naringin with fatty acids of medium carbon chain. J. Mol. Catal. B Enzym. 2003, 21, 59–62. [Google Scholar] [CrossRef]
- Mellou, F.; Lazari, D.; Skaltsa, H.; Tselepis, A.D.; Kolisis, F.N.; Stamatis, H. Biocatalytic preparation of acylated derivatives of flavonoid glycosides enhances their antioxidant and antimicrobial activity. J. Biotechnol. 2005, 116, 295–304. [Google Scholar] [CrossRef]
- Danieli, B.; De Bellis, P.; Carrea, G.; Riva, S. Enzyme-mediated regioselective acylations of flavonoid disaccharide monoglycosides. Helv. Chim. Acta 1990, 73, 1837–1844. [Google Scholar] [CrossRef]
- Du, L.; Jiang, Z.; Xu, L.; Zhou, N.; Shen, J.; Dong, Z.; Shen, L.; Wang, H.; Luo, X. Microfluidic reactor for lipase-catalyzed regioselective synthesis of neohesperidin ester derivatives and their antimicrobial activity research. Carbohydr. Res. 2018, 455, 32–38. [Google Scholar] [CrossRef]
- Luo, X.P.; Du, L.H.; He, F.; Zhou, C.H. Controllable regioselective acylation of flavonoids catalyzed by lipase in microreactors. J. Carbohydr. Chem. 2013, 32, 450–462. [Google Scholar] [CrossRef]
- Novozymes A/S. Immobilized Lipases for Biocatalysis for Smarter Chemical Synthesis. Available online: https://www.novozymes.com/-/media/Project/Novozymes/Website/website/document-library/Advance-your-business/Pharma/Biocatalysis_brochure_Immobilised_Lipases.pdf (accessed on 20 December 2021).
- Hernández-Martín, E.; Otero, C. Different enzyme requirements for the synthesis of biodiesel: Novozym® 435 and Lipozyme® TL IM. Bioresour. Technol. 2008, 99, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Awang, R.; Basri, M.; Ahmad, S.; Salleh, A.B. Thermomyces lanuginosus lipase-catalyzed esterification of 9, 10-dihydroxystearic acid and monohydric alcohol. J. Oleo Sci. 2005, 54, 305–309. [Google Scholar] [CrossRef][Green Version]
- Romero, M.D.; Calvo, L.; Alba, C.; Daneshfar, A.; Ghaziaskar, H.S. Enzymatic synthesis of isoamyl acetate with immobilized Candida antarctica lipase in n-hexane. Enzym. Microb. Technol. 2005, 37, 42–48. [Google Scholar] [CrossRef]
- Hari Krishna, S.; Divakar, S.; Prapulla, S.G.; Karanth, N.G.K. Enzymatic synthesis of isoamyl acetate using immobilized lipase from Rhizomucor miehei. J. Biotechnol. 2001, 87, 193–201. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Chiang, S.-H.; Ju, H.-Y.; Chen, Y.-M.; Liao, M.-Y.; Liu, Y.-C.; Shieh, C.-J. Enzymatic synthesis of rose aromatic ester (2-phenylethyl acetate) by lipase. J. Sci. Food Agric. 2012, 92, 2141–2147. [Google Scholar] [CrossRef]
- He, W.S.; Li, J.J.; Pan, X.X.; Zhou, Y.; Jia, C.S.; Zhang, X.M.; Feng, B. Lipase-mediated synthesis of water-soluble plant stanol derivatives in tert-butanol. Bioresour. Technol. 2012, 114, 1–5. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.H.; Chen, N.; Zhi, G.Y. Synthesis of benzyl cinnamate by enzymatic esterification of cinnamic acid. Bioresour. Technol. 2015, 198, 256–261. [Google Scholar] [CrossRef]
- Yadav, G.D.; Devendran, S. Lipase catalyzed synthesis of cinnamyl acetate via transesterification in non-aqueous medium. Process Biochem. 2012, 47, 496–502. [Google Scholar] [CrossRef]
- Lee, S.B.; Ryu, D.D.Y. External diffusion effects on the kinetic constants of immobilized enzyme systems. J. Theor. Biol. 1980, 84, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, C. Enzymatic synthesis of phenethyl ester from phenethyl alcohol with acyl donors. Enzym. Microb. Technol. 2017, 100, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.D.; Calvo, L.; Alba, C.; Daneshfar, A. A kinetic study of isoamyl acetate synthesis by immobilized lipase-catalyzed acetylation in n-hexane. J. Biotechnol. 2007, 127, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Danieli, B.; Luisetti, M.; Sampognaro, G.; Carrea, G.; Riva, S. Regioselective acylation of polyhydroxylated natural compounds catalyzed by Candida Antarctica lipase B (Novozym 435) in organic solvents. J. Mol. Catal. B Enzym. 1997, 3, 193–201. [Google Scholar] [CrossRef]
- Chebil, L.; Anthoni, J.; Humeau, C.; Gerardin, C.; Engasser, J.M.; Ghoul, M. Enzymatic acylation of flavonoids: Effect of the nature of the substrate, origin of lipase, and operating conditions on conversion yield and regioselectivity. J. Agric. Food Chem. 2007, 55, 9496–9502. [Google Scholar] [CrossRef]
- Stergiou, P.Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef]
- Polynomials, R. Substrate inhibition and mixed dead-end and product inhibition. In Comprehensive Enzyme Kinetics; Springer: Boston, MA, USA, 2004; pp. 191–207. [Google Scholar] [CrossRef]
- Chebil, L.; Humeau, C.; Falcimaigne, A.; Engasser, J.M.; Ghoul, M. Enzymatic acylation of flavonoids. Process Biochem. 2006, 41, 2237–2251. [Google Scholar] [CrossRef]
- Yadav, G.D.; Dhoot, S.B. Immobilized lipase-catalysed synthesis of cinnamyl laurate in non-aqueous media. J. Mol. Catal. B Enzym. 2009, 57, 34–39. [Google Scholar] [CrossRef]
- Khor, G.K.; Sim, J.H.; Kamaruddin, A.H.; Uzir, M.H. Thermodynamics and inhibition studies of lipozyme TL IM in biodiesel production via enzymatic transesterification. Bioresour. Technol. 2010, 101, 6558–6561. [Google Scholar] [CrossRef]
- Qian, J.; Gou, L.; Chen, Y.; Ding, J.; Xu, J.; Guo, H. Enzymatic acylation of flavone isolated from extractive of bamboo leaves with oleic acid and antioxidant activity of acylated product. Eng. Life Sci. 2019, 19, 66–72. [Google Scholar] [CrossRef]
- Chang, S.W.; Shaw, J.F. Biocatalysis for the production of carbohydrate esters. New Biotechnol. 2009, 26, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Laane, C.; Boeren, S.; Vos, K.; Veeger, C. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 1987, 30, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Milisavljevic, A.; Stojanovic, M.; Carevic, M.; Mihailovic, M.; Milosavic, N.; Bezbradica, D. Lipase-catalyzed esterification of phloridzin: Acyl donor effect on enzymatic affinity and antioxidant properties of esters. Ind. Eng. Chem. Res. 2014, 53, 16644–16651. [Google Scholar] [CrossRef]
- Hazarika, S.; Goswami, P.; Dutta, N.N. Lipase catalysed transesterification of 2-o-benzylglycerol with vinyl acetate: Solvent effect. Chem. Eng. J. 2003, 94, 1–10. [Google Scholar] [CrossRef]
- Mbatia, B.; Kaki, S.S.; Mattiasson, B.; Mulaa, F.; Adlercreutz, P. Enzymatic synthesis of lipophilic rutin and vanillyl esters from fish byproducts. J. Agric. Food Chem. 2011, 59, 7021–7027. [Google Scholar] [CrossRef]
- Vaisali, C.; Belur, P.D.; Regupathi, I. Lipase mediated synthesis of rutin fatty ester: Study of its process parameters and solvent polarity. Food Chem. 2017, 232, 278–285. [Google Scholar] [CrossRef]
| Reactant | Reaction Type | Reaction Time | Conversion | Ref. | |
|---|---|---|---|---|---|
| Naringin | Oleic acid | Esterification | 48 h | 93.10% | [14] |
| Naringin | Coconut oil | Esterification | 90 h | 75.43% | [25] |
| Linseed oil | 76.70% | ||||
| Sunflower oil | 85.08% | ||||
| Naringin | Ricinoleic acid | Esterification | 120 h | 33% | [26] |
| Naringin | Oleic acid | Esterification | 96 h | 78.4% | [27] |
| Linoleic acid | 77.6% | ||||
| Linolenic acid | 86.6% | ||||
| Naringin | Oleic acid | Esterification | 96 h | 80–90% | [28] |
| Lauric acid | |||||
| Linolenic acid | |||||
| Naringin | Vinyl acetate | Trans-esterification | 96 h | 41.03% | [10] |
| Vinyl octanoate | 91.40% | ||||
| Naringin | Vinyl butyrate | Trans-esterification | 144 h | 90% | [12] |
| Naringin | Vinyl laurate | Trans-esterification | 8 h | 50% | [29] |
| Immobilized Lipase (Regioselectivity) | Source | Support | Conversion (%) |
|---|---|---|---|
| Novozym 435 (Nonspecific) | Candida antarctica lipase B | Acrylic resin | 17.06 ± 0.72 |
| Lipozyme TL IM (1,3-specific) | Thermomyces lanuginosus | Silica resin | 29.89 ± 0.07 |
| Lipozyme RM IM (1,3-specific) | Rhizomucor miehei | Anion-exchange resin | 19.91 ± 0.20 |
| Acyl Donor | Molecular Formula | Structure | Molecular Weight (g/mol) | Conversion (%) |
|---|---|---|---|---|
| Acetic acid | C2H4O2 | 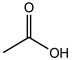 | 60.05 | 15.60 ± 0.24 |
| Acetic anhydride | C4H6O3 | 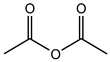 | 102.09 | 36.91 ± 0.35 |
| Methyl acetate | C3H6O2 | 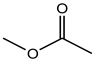 | 74.08 | 20.67 ± 0.90 |
| Vinyl acetate | C4H6O2 | 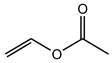 | 86.09 | 33.48 ± 0.28 |
| Ethyl acetate | C4H8O2 | 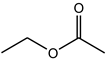 | 88.11 | 17.07 ± 1.04 |
| Propyl acetate | C5H10O2 | 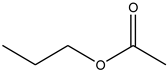 | 102.13 | 17.19 ± 0.60 |
| Butyl acetate | C6H12O2 | 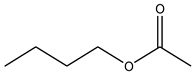 | 116.16 | 18.91 ± 0.62 |
| Organic Solvent | Log p | Dielectric Constant | Conversion (%) a | Conversion (%) b |
|---|---|---|---|---|
| Acetonitrile | −0.33 | 37.5 | 98.51 ± 0.01 | 98.49 ± 0.01 |
| 1,4-Dioxane | −0.27 | 2.25 | 89.96 ± 0.19 | 78.36 ± 1.52 |
| Acetone | −0.16 | 20.7 | 98.37 ± 0.03 | 75.67 ± 0.04 |
| THF | 0.49 | 7.5 | 98.20 ± 0.02 | 88.30 ± 3.99 |
| tert-Butyl alcohol | 0.58 | 10.9 | 81.11 ± 0.46 | 91.16 ± 0.23 |
| tert-Amyl alcohol | 1.09 | 5.78 | 86.63 ± 0.27 | 94.47 ± 0.02 |
| 1,2-Dichloroethane | 1.48 | 10.4 | 16.01 ± 2.85 | 37.32 ± 3.21 |
| Acyl Donor | Enzyme | Enzyme Conc., Molar Ratio | Solvent | Reaction | Conversion | Ref. |
|---|---|---|---|---|---|---|
| (Naringin: Acyl Donor) | ||||||
| Vinyl acetate | Whole-cell catalyst | Whole-cell 50 mg/mL, 1:50 | Organic solvent | 50 °C, 96 h | 41.03%, | [10] |
| Vinyl octanoate | Aspergillus oryzae | 91.40% | ||||
| Vinyl butyrate | Novozym 435 | Enzyme 80 g/L, 1:10 | Acetone | 60 °C, 144 h | 90% | [12] |
| Oleic acid | Lipozyme TL IM | Enzyme 10 g/L, 1:20 | Acetonitrile | 40 °C, 48 h | 93.10% | [14] |
| 40 °C, 24 h | 92.17% | |||||
| Coconut oil | Novozym 435 | Enzyme 5 g/L, 1:6 | Acetonitrile | 65 °C, 90 h | 75.43%, | [25] |
| Linseed oil | 76.70%, | |||||
| Sunflower oil | 85.08% | |||||
| Ricinoleic acid | Novozym 435 | Enzyme 20 g/L, 1:3 | Acetone | 50 °C, 120 h | 33% | [26] |
| Oleic acid | Novozym 435 | Enzyme 15 g/L, 1:4 | Acetone: tert-amyl alcohol (2:1) | 50 °C, 96 h | 78.4%, | [27] |
| Linoleic acid | 77.6%, | |||||
| Linolenic acid | 86.6% | |||||
| Lauric acid | Novozym 435 | Enzyme 12 g/L, 1:5 | Acetone | 45 °C, 96 h | 80–90% | [28] |
| Oleic acid | ||||||
| Linolenic acid | ||||||
| Vinyl laurate | Novozym 435 | Enzyme 17 g/L, 1:10 | Acetonitrile | 50 °C, 8 h | 50% | [29] |
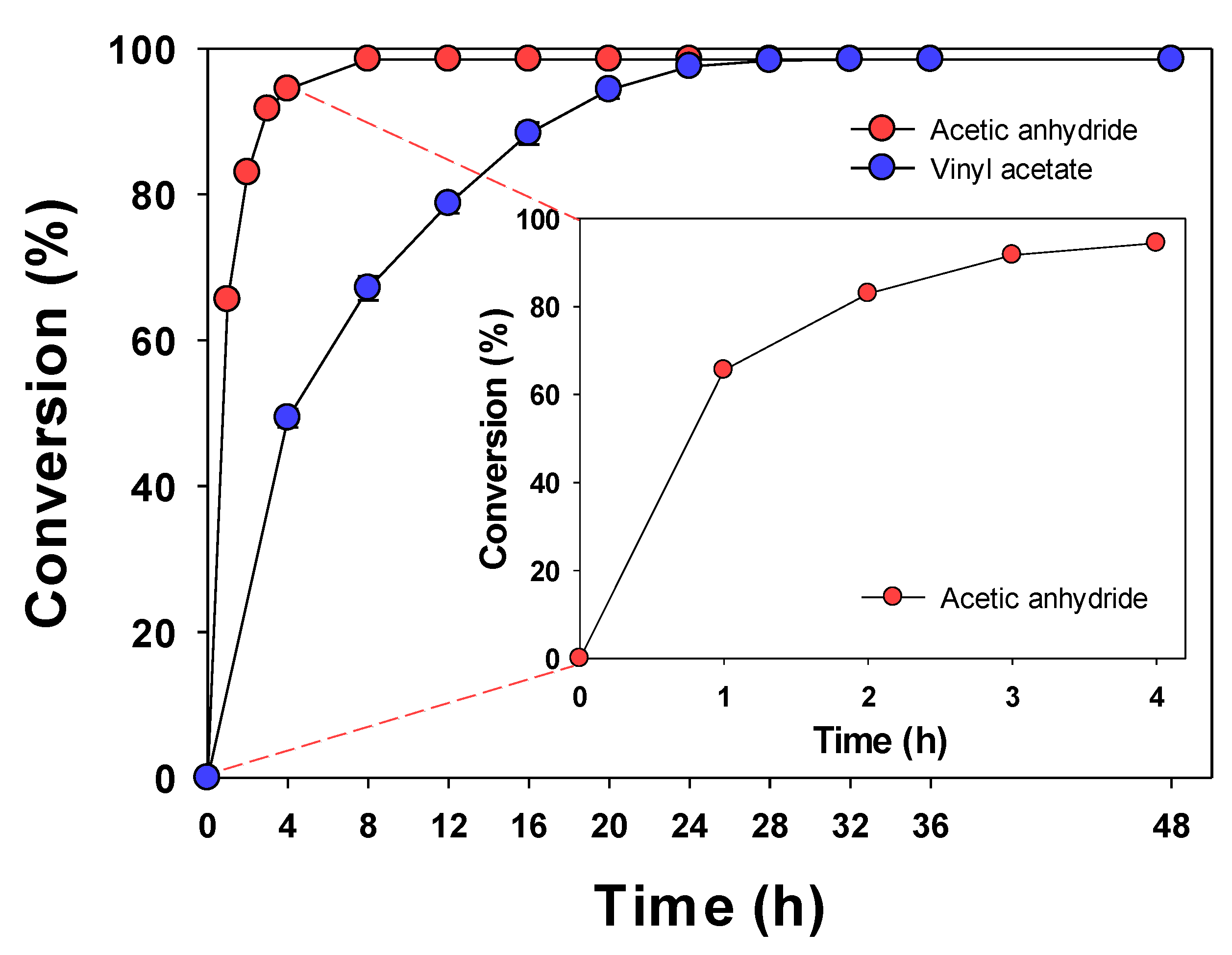
| Acetic anhydride | Lipozyme TL IM | Enzyme 3 g/L, 1:5 | Acetonitrile | 40 °C, 8 h | 98.51% | This study |
| Vinyl acetate | Lipozyme TL IM | Enzyme 3 g/L, 1:5 | Acetonitrile | 40 °C, 24 h | 97.54% | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, Y.; Lee, S.; Son, J.; Lee, T.; Oh, J.-M.; Lee, S.H.; Kim, H.U.; Seo, S.W.; Park, S.J.; Yoo, H.Y.; et al. Efficient Production of Naringin Acetate with Different Acyl Donors via Enzymatic Transesterification by Lipases. Int. J. Environ. Res. Public Health 2022, 19, 2972. https://doi.org/10.3390/ijerph19052972
Baek Y, Lee S, Son J, Lee T, Oh J-M, Lee SH, Kim HU, Seo SW, Park SJ, Yoo HY, et al. Efficient Production of Naringin Acetate with Different Acyl Donors via Enzymatic Transesterification by Lipases. International Journal of Environmental Research and Public Health. 2022; 19(5):2972. https://doi.org/10.3390/ijerph19052972
Chicago/Turabian StyleBaek, Yesol, Seungmee Lee, Jemin Son, Taek Lee, Jong-Min Oh, Sang Hun Lee, Hyun Uk Kim, Sang Woo Seo, Si Jae Park, Hah Young Yoo, and et al. 2022. "Efficient Production of Naringin Acetate with Different Acyl Donors via Enzymatic Transesterification by Lipases" International Journal of Environmental Research and Public Health 19, no. 5: 2972. https://doi.org/10.3390/ijerph19052972
APA StyleBaek, Y., Lee, S., Son, J., Lee, T., Oh, J.-M., Lee, S. H., Kim, H. U., Seo, S. W., Park, S. J., Yoo, H. Y., & Park, C. (2022). Efficient Production of Naringin Acetate with Different Acyl Donors via Enzymatic Transesterification by Lipases. International Journal of Environmental Research and Public Health, 19(5), 2972. https://doi.org/10.3390/ijerph19052972










