Does Anti-TNF-α Therapy Affect the Bacteriological Profile of Specimens Collected from Perianal Lesions? A Retrospective Analysis in Patients with Crohn’s Disease
Abstract
1. Introduction
2. Materials and Methods
Ethics Statement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Zoeten, E.F.; Pasternak, B.A.; Mattei, P.; Kramer, R.E.; Kader, H.A. Diagnosis and Treatment of Perianal Crohn Disease: NASPGHAN Clinical Report and Consensus Statement. JPGN 2013, 57, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, B.; Feakins, R.M.; Barmias, G.; Coelho, L.; Teixeira, F.V.; Rogler, G.; Scharl, M. Results of the Fifth Scientific Workshop of the ECCO (II): Pathophysiology of Perianal Fistulizing Disease. J. Crohns Colitis 2016, 10, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Varela, E.; Manichanh, C.; Gallart, M.; Torrejón, A.; Borruel, N.; Casellas, F.; Guarner, F.; Antolin, M. Colonisation by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis. Aliment Pharm. 2013, 38, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.K.; Kamm, M.A.; Teo, S.M.; Inouve, M.; Wagner, J.; Kirkwood, C.D. Recent advances in characterizing the gastrointestinal microbiome in Crohn’s disease: A systematic review. Inflamm. Bowel Dis. 2015, 21, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Pascal, V.; Pozuelo, M.; Borruel, N.; Casellas, F.; Campos, D.; Santiago, A.; Martinez, X.; Varela, E.; Sarrabayrouse, G.; Machiels, K.; et al. A microbial signature for Crohn’s disease. Gut 2017, 66, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohn’s Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Gionchetti, P.; Dignass, A.; Danese, S.; Gionchetti, P.; Dignass, A.; Danese, S.; Dias, F.J.M.; Rogler, G.; Lakatos, P.L.; Adamina, M.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 2: Surgical Management and Special Situations. J. Crohn’s Colitis 2017, 11, 135–149. [Google Scholar] [CrossRef]
- Tozer, P.; Raymen, N.; Al-Hassi, O.H.; Murguranathan, A.; Daulatzai, N.; Knight, S.C.; Phillips, R.K.; Whelan, K.; Hart, A.L. The rectal mucosa in patients with crohn’s anal fistulae harbours lower numbers of bifidobacteria, and the fistula tracts are devoid of a microbial ecosystem. Gut 2011, 60, A221. [Google Scholar] [CrossRef][Green Version]
- Seow-Choen, F.; Hay, A.J.; Heard, S.; Phillips, R.K. Bacteriology of anal fistulae. Br. J. Surg. 1992, 79, 27–28. [Google Scholar] [CrossRef]
- De San Pereira, I.; Chimeno, M.; Alvarez, C.F.; Torres, J.; Casal, J.E. Bacteriology of anal fistulae. Rev. Esp. Enferm. Dig. 2002, 94, 533–536. [Google Scholar]
- Park, S.K.; Kim, K.J.; Lee, S.O.; Yang, D.H.; Jung, K.W.; Ye, B.D.; Byeon, J.S.; Myung, S.J.; Yang, S.K.; Kim, J.H.; et al. Ciprofloxacin usage and bacterial resistance patterns in Crohn’s disease patients with abscesses. J. Clin. Gastroenterol. 2014, 48, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Sercia, L.; Branda, J.A.; Burnham, C.-A.D.; Bythrow, M.; Ferraro, M.J.; Garner, O.B.; Ginocchio, C.C.; Jennemann, R.; Lewinski, M.A.; et al. Identification of Enterobacteriaceae by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry using the VITEK MS system. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Siu, G.K.H.; Yeung, A.S.F.; Chen, J.H.K.; Ho, P.L.; Leung, K.W.; Tsang, J.L.Y.; Cheng, V.C.C.; Guo, L.; Yang, J.; et al. Performance of the VITEK MS matrix-assisted laser desorption ionization-time of flight mass spectrometry system for rapid bacterial identification in two diagnostic centres in China. J Med. Microbiol 2015, 64, 18–24. [Google Scholar] [CrossRef]
- Kovaleva, J. Infectious complications in gastrointestinal endoscopy and their prevention. Best Pr. Res. Clin. Gastroenterol. 2016, 30, 689–704. [Google Scholar] [CrossRef] [PubMed]
- Veen, S.Q.; Claas, E.; Kuijper, E.J. High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization–time of flight mass spectrometry in conventional medical microbiology laboratories. J. Clin. Microbiol. 2010, 48, 900–907. [Google Scholar] [CrossRef]
- Jamal, W.; Shahin, M.; Rotimi, V.O. Comparison of two matrix-assisted laser desorption/ionization—Time of flight (MALDI-TOF) mass spectrometry methods and API 20AN for identification of clinically relevant anaerobic bacteria. J. Med. Microbiol. 2013, 62, 540–544. [Google Scholar] [CrossRef]
- Harris, P.; Vinney, J.; Ashhurst-Smith, C.; O’Brien, M.; Graves, S. Comparison of Vitek MS (MALDI-TOF) to standard routine Identification methods: An advance but no panacea. Pathology 2012, 44, 583–585. [Google Scholar] [CrossRef]
- Fang, H.; Ohlsson, A.K.; Ullberg, M.; Özenci, V. Evaluation of species-specific PCR, Bruker MS, VITEK MS and the VITEK 2 system for the identification of clinical Enterococcus isolates. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3073–3077. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekeman, D.J.; Procop, G.W.; Rinaldi, M.G. Multicenter Comparison of the VITEK 2 Antifungal Susceptibility Test with the CLSI Broth Microdilution Reference Method for Testing Amphotericin B, Flucytosine, and Voriconazole against Candida spp. J. Clin. Mikrobiol. 2007, 45, 3522–3528. [Google Scholar] [CrossRef]
- Ulug, M.; Gedik, E.; Girgin, S.; Celen, M.; Braz, C. The evaluation of bacteriology in perianal abscesses of 81 adult patients. J. Infect. Dis. 2010, 14, 225–229. [Google Scholar]
- Brook, I. Section 8 Clinical Microbiology: Bacteria, 184 Anaerobic Bacteria. In Infectious Diseases, 4th ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amasterdam, The Netherlands, 2017; pp. 1628–1644.e2. [Google Scholar]
- Enez, V.E.; Henriquez, C.V. Anal abscess microbiology as an anal fistula predictor. J. Coloproctol. 2020, 40, 129–134. [Google Scholar] [CrossRef]
- Seow-En, I.; Ngu, J. Routine operative swab cultures and post-operative antibiotic use for uncomplicated perianal abscesses are unnecessary. ANZ J. Surg. 2014, 87, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Reuken, P.A.; Kruis, W.; Maaser, C.; Teich, N.; Büning, J.; Preiß, J.C.; Schmelz, R.; Bruns, T.; Fichtner-Feigl, S.; Stallmach, A. Microbial spectrum of intra-abdominal abscesses in perforating Crohn’s disease: Results from a prospective German registry. J. Crohns Colitis 2018, 12, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Penner, J.L. Genus XXIX. Proteus. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Brenner, D.J., Krieg, N.R., Staley, J.T., Garrity, G.M., Eds.; The Proteobacteria: Part B, the Gammaproteobacteria; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; Volume 2, pp. 745–753. [Google Scholar]
- Mondot, S.; Lepage, P.; Seksik, P.; Allez, M.; Treton, X.; Bouhnik, Y.; Colombel, J.F.; Leclerc, M.; Pochart, P.; Dore, J.; et al. GETAID Structural robustness of the gut mucosal microbiota is associated with Crohn’s disease remission after surgery. Gut 2016, 65, 954–962. [Google Scholar] [CrossRef]
- Wright, E.K.; Kamm, M.A.; Wagner, J.; Teo, S.M.; Cruz, P.; Hamilton, A.L.; Ritchie, K.J.; Inouye, M.; Kirkwood, C.D. Microbial factors associated with postoperative Crohn’s disease recurrence. J. Crohns Colitis 2017, 11, 191–203. [Google Scholar] [CrossRef]
- Hamilton, A.L.; Kamm, M.A.; Ng, S.C.; Morrison, M. Proteus spp. as putative gastrointestinal pathogens. Clin. Microbiol. Rev. 2018, 31, e00085-17. [Google Scholar] [CrossRef]
- Liu, K.L.; Lee, T.C.; Lin, M.T.; Chen, S.J. Education and imaging. Gastrointestinal: Abdominal abscess associated with a ventriculoperitoneal shunt. J. Gastroenterol. Hepatol. 2007, 22, 757. [Google Scholar] [CrossRef]
- Segal, R.; Dan, M.; Pogoreliuk, I.; Leibovitz, A. Pathogenic colonization of the stomach in enterally fed elderly patients: Comparing percutaneous endoscopic gastrostomy with nasogastric tube. J. Am. Geriatr. Soc. 2006, 54, 1905–1908. [Google Scholar] [CrossRef]
- Segal, R.; Pogoreliuk, I.; Dan, M.; Baumoehl, Y.; Leibovitz, A. Gastric microbiota in elderly patients fed via nasogastric tubes for prolonged periods. J. Hosp. Infect. 2006, 63, 79–83. [Google Scholar] [CrossRef]
- Vaishnavi, C.; Kapoor, P.; Kochhar, R. Su1148. Bacterial biofilms produced in stents retrieved from patients with biliary and pancreatic diseases. Gastroenterology 2014, 146, S-389. [Google Scholar] [CrossRef]
- Ticac, B.; Ticac, R.; Rukavina, T.; Kesovija, P.G.; Pedisic, D.; Maljevac, B.; Starcevic, R. Microbial colonization of tracheoesophageal voice prostheses (Provox2) following total laryngectomy. Eur. Arch. Otorhinolaryngol. 2010, 267, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.P.; Pimenta, A.T.; Contijo, P.P.; Geocze, S.; Fischman, O. Microbiologic profile of flexible endoscope disinfection in two Brazilian hospitals. Arq. Gastroenterol. 2006, 43, 255–258. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef]
- Chow, A.W.; Taylor, P.R.; Yoshikawa, T.T.; Guze, L.B. A nosocomial outbreak of infections due to multiply resistant Proteus mirabilis: Role of intestinal colonization as a major reservoir. J. Infect. Dis. 1979, 139, 621–627. [Google Scholar] [CrossRef]
- West, R.L.; Van Der Woude, C.J.; Endtz, H.P.H.; Hansen, B.E.; Ouwedijk, M.; Boelens, H.A.M.; Kusters, J.G.; Kuipers, E.J. Perianal Fistulas in Crohn’s Disease Are Predominantly Colonized by Skin Flora: Implications for Antibiotic Treatment? Dig. Dis. Sci. 2005, 50, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Cheng, A.; Huang, S.-Y.; Sheng, W.-H.; Liu, J.-H.; Ko, B.-S.; Yao, M.; Chou, W.-C.; Lin, H.-C.; Chen, Y.-C.; et al. Clinical and Microbiological Characteristics of Perianal Infections in Adult Patients with Acute Leukemia. PLoS ONE 2013, 8, e60624. [Google Scholar] [CrossRef]
- Gordon, P.H. Anorectal abscess and fi stula-in-ano. In Principles and Practice of Surgery of the Colon, Rectum and Anus; Gordon, P.H., Nivatours, S., Eds.; Quality Medical Publishing: St. Louis, MO, USA, 1992. [Google Scholar]
- Lunniss, P.J.; Philips, R.K. Surgical assesment of acute anorectal sepsis is a better predictor of fistula than microbiological analysis. Br. J. Surg. 1994, 81, 368–369. [Google Scholar] [CrossRef]
- Al-Salem, A.H.; Laing, W.; Talwaker, V. Fistula-in-ano in infancy and childhood. J. Pediatr. Surg. 1994, 29, 436–438. [Google Scholar] [CrossRef]
- Brook, I.; Frazier, E.H. The aerobic and anaerobic bacteriology of perirectal abscess. J. Clin. Microbiol. 1997, 35, 2974–2976. [Google Scholar] [CrossRef]
- Toyonaga, T.; Matsushima, M.; Tanaka, Y.; Shimojima, Y.; Matsumura, N.; Kannyama, H.; Nozawa, M.; Hatakeyama, T.; Suzuki, K.; Yanagita, K.; et al. Microbiological analysis and endoanal ultrasonography for diagnosis of anal fi stula in acute anorectal sepsis. Int. J. Colorectal Dis. 2007, 22, 209–213. [Google Scholar] [CrossRef]
- Henrichsen, S.; Christiansen, J. Incidence of fi stula-in-ano complicating anorectal sepsis: A prospective study. Br. J. Surg. 1986, 73, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.; Sundararaman, S.; Varadhan, L.; Rajput, R.; Reay-Jones, N.; Gupta, V. The value of microbiological analysis of pus swabs in perianal abscess. Have they stood the test of time and antibiotic usage? Int. Surg. J. 2015, 2, 175–178. [Google Scholar] [CrossRef]
- Wang, D.; Yang, G.; Qiu, J.; Song, Y.; Wang, L.; Gao, J.; Wang, C. Risk factors for anal fistula: A case–control study. Tech. Coloproctol. 2014, 18, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Haac, B.E.; Palmateer, N.C.; Seaton, M.E.; Peren, R.; Fraser, C.M.; Bafford, A.C. A Distinct Gut Microbiota Exists Within Crohn’s DiseaseeRelated Perianal Fistulae. J. Surg. Res. 2019, 242, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Lalou, l.; Archer, L.; Lim, P.; Kretzmer, L.; Elhassan, A.M.; Awodiya, A.; Seretis, C. Auditing the Routine Microbiological Examination of Pus Swabs from Uncomplicated Perianal Abscesses: Clinical Necessity or Old Habit? Gastroenterol. Res. 2020, 13, 114–116. [Google Scholar] [CrossRef]
- Leung, E.; McArdle, K.; Yazbek-Hanna, M. Pus Swabs in Incision and Drainage of Perianal Abscesses: What Is the Point? World J. Surg. 2009, 33, 2448–2451. [Google Scholar] [CrossRef]
- Glenn, J.; Cotton, D.; Wesley, R.; Pizzo, P. Anorectal infections in patients with malignant diseases. Rev. Infect. Dis. 1988, 10, 42–52. [Google Scholar] [CrossRef]
- Fujimoto, K.; Uematsu, S. Vaccine therapy for dysbiosis-related diseases. World J. Gastroenterol. 2020, 26, 2758–2767. [Google Scholar] [CrossRef]
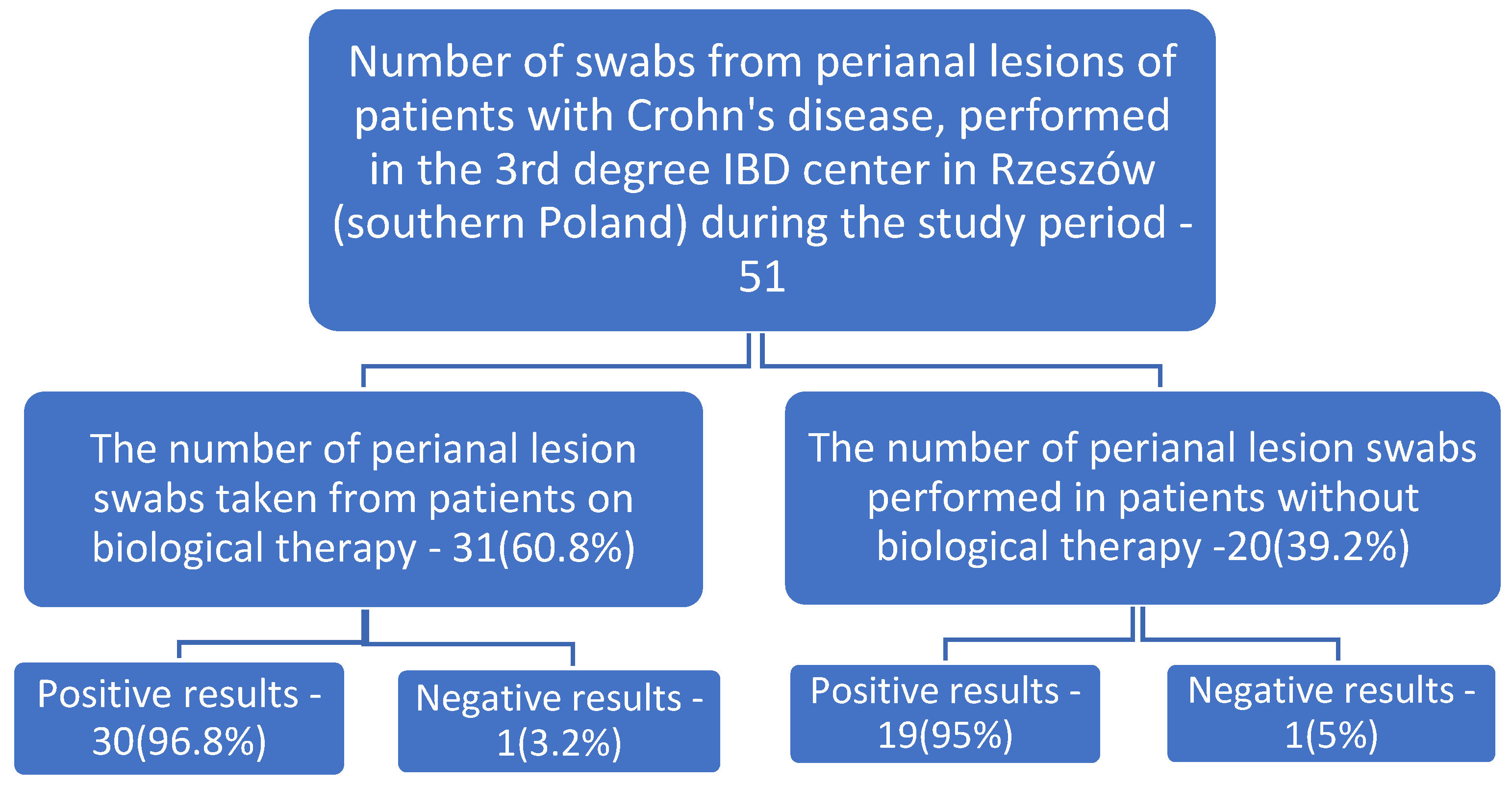
| Number of Tests Ordered n | Positive Results n (%) | Microorganisms Cultured | Number | % in Relation to All Samples Taken | % in Relation to Positive Results | Significance Level p |
|---|---|---|---|---|---|---|
| Summary results | ||||||
| 51 | 49 (96%) | Escherichia coli | 22 | 43.13% | 44.9% | <0.001 |
| Escherichia coli, ESBL | 5 | 9.8% | 10.2% | <0.001 | ||
| Staphylococcus aureus | 6 | 11.8% | 12.2% | <0.001 | ||
| Staphylococcus aureus, MRSA | 1 | 1.96% | 2.% | =0.322 | ||
| Enterococcus faecalis | 5 | 9.8% | 10.2% | <0.001 | ||
| Bacteroides vulgatus | 4 | 7.8% | 8.2% | <0.001 | ||
| Proteus mirabilis | 3 | 5.9% | 6.1% | =0.004 | ||
| Staphylococcus epidermidis, MRCNS | 2 | 3.9% | 4.1% | =0.051 | ||
| Enterobacter cloacae | 2 | 3.9% | 4.1% | =0.051 | ||
| Streptococcus pyogenes | 2 | 3.9% | 4.1% | =0.051 | ||
| Klebsiella pneumoniae, ESBL | 2 | 3.9% | 4.1% | =0.051 | ||
| Klebsiella pneumoniae | 2 | 3.9% | 4.1% | =0.051 | ||
| Streptococcus mitis | 2 | 3.9% | 4.1% | =0.051 | ||
| Pseudomonas aeruginosa | 1 | 1.96% | 2% | =0.322 | ||
| Morganella morganii | 1 | 1.96% | 2% | =0.322 | ||
| Citrobacter freundii | 1 | 1.96% | 2% | =0.322 | ||
| Streptococcus anginosus | 1 | 1.96% | 2% | =0.322 | ||
| Prevotella disiens | 1 | 1.96% | 2% | =0.322 | ||
| Parvimonas micra | 1 | 1.96% | 2% | =0.322 | ||
| Streptococcus constellatus | 1 | 1.96% | 2% | =0.322 | ||
| Results of tests performed on patients during biological therapy | ||||||
| 31 | 30 (96.8%) | Escherichia coli | 14 | 45.2% | 46.7% | <0.001 |
| Escherichia coli, ESBL | 4 | 12.9% | 13.3% | <0.001 | ||
| Staphylococcus aureus | 6 | 19.35% | 20% | <0.001 | ||
| Staphylococcus aureus, MRSA | 1 | 3.2% | 3.3% | =0.325 | ||
| Enterococcus faecalis | 3 | 9.7% | 10% | =0.005 | ||
| Proteus mirabilis | 2 | 6.4% | 6.7% | =0.055 | ||
| Staphylococcus epidermidis, MRCNS | 2 | 6.4% | 6.7% | =0.055 | ||
| Klebsiella pneumoniae | 2 | 6.4% | 6.7% | =0.055 | ||
| Bacteroides vulgatus | 1 | 3.2% | 3.3% | =0.325 | ||
| Enterobacter cloacae | 1 | 3.2% | 3.3% | =0.325 | ||
| Streptococcus mitis | 1 | 3.2% | 3.3% | =0.325 | ||
| Morganella morganii | 1 | 3.2% | 3.3% | =0.325 | ||
| Citrobacter freundii | 1 | 3.2% | 3.3% | =0.325 | ||
| Prevotella disiens | 1 | 3.2% | 3.3% | =0.325 | ||
| Parvimonas micra | 1 | 3.2% | 3.3% | =0.325 | ||
| Results of tests performed on patients without biological therapy | ||||||
| 20 | 19 (95%) | Escherichia coli | 8 | 40% | 42.1% | <0.001 |
| Escherichia coli, ESBL | 1 | 5% | 5.3% | =0.329 | ||
| Bacteroides vulgatus | 3 | 15% | 15.8% | <0.007 | ||
| Enterococcus faecalis | 2 | 10% | 10.5% | =0.059 | ||
| Streptococcus pyogenes | 2 | 10% | 10.5% | =0.059 | ||
| Klebsiella pneumoniae, ESBL | 2 | 10% | 10.5% | =0.059 | ||
| Proteus mirabilis | 1 | 5% | 5.3% | =0.329 | ||
| Enterobacter cloacae | 1 | 5% | 5.3% | =0.329 | ||
| Streptococcus mitis | 1 | 5% | 5.3% | =0.329 | ||
| Pseudomonas aeruginosa | 1 | 5% | 5.3% | =0.329 | ||
| Streptococcus anginosus | 1 | 5% | 5.3% | =0.329 | ||
| Streptococcus constellatus | 1 | 5% | 5.3% | =0.329 | ||
| Characteristics of the Patient | CD (n = 51) | |||
|---|---|---|---|---|
| Patients during Biological Therapy | Patients without Biological Therapy | |||
| Women (n = 6) | Men (n = 25) | Women (n = 9) | Men (n = 11) | |
| Age, years | 18–33 | 24–57 | 26–50 | 2165 |
| Age, mean (standard deviation) | 24.33 (7.203) | 37.95 (9) | 34.667 (8.994) | 39.412 (15.069) |
| Length of hospital stay, days | 2–35 | |||
| Length of hospital stay, mean (standard deviation) | 11.6 (7.1) | |||
| Onset of symptoms prior to admission to hospital, weeks | 1–8 | |||
| Onset of symptoms prior to admission to hospital, mean (standard deviation) | 3.863 (6.103) | |||
| Taking samples for research | All samples were taken during hospitalization | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruszecka, J.; Filip, R. Does Anti-TNF-α Therapy Affect the Bacteriological Profile of Specimens Collected from Perianal Lesions? A Retrospective Analysis in Patients with Crohn’s Disease. Int. J. Environ. Res. Public Health 2022, 19, 2892. https://doi.org/10.3390/ijerph19052892
Gruszecka J, Filip R. Does Anti-TNF-α Therapy Affect the Bacteriological Profile of Specimens Collected from Perianal Lesions? A Retrospective Analysis in Patients with Crohn’s Disease. International Journal of Environmental Research and Public Health. 2022; 19(5):2892. https://doi.org/10.3390/ijerph19052892
Chicago/Turabian StyleGruszecka, Jolanta, and Rafał Filip. 2022. "Does Anti-TNF-α Therapy Affect the Bacteriological Profile of Specimens Collected from Perianal Lesions? A Retrospective Analysis in Patients with Crohn’s Disease" International Journal of Environmental Research and Public Health 19, no. 5: 2892. https://doi.org/10.3390/ijerph19052892
APA StyleGruszecka, J., & Filip, R. (2022). Does Anti-TNF-α Therapy Affect the Bacteriological Profile of Specimens Collected from Perianal Lesions? A Retrospective Analysis in Patients with Crohn’s Disease. International Journal of Environmental Research and Public Health, 19(5), 2892. https://doi.org/10.3390/ijerph19052892






