Adsorption Properties and Mechanism of Attapulgite to Graphene Oxide in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Adsorption Test
3. Results and Discussion
3.1. Morphology Analysis
3.1.1. SEM and TEM Analysis
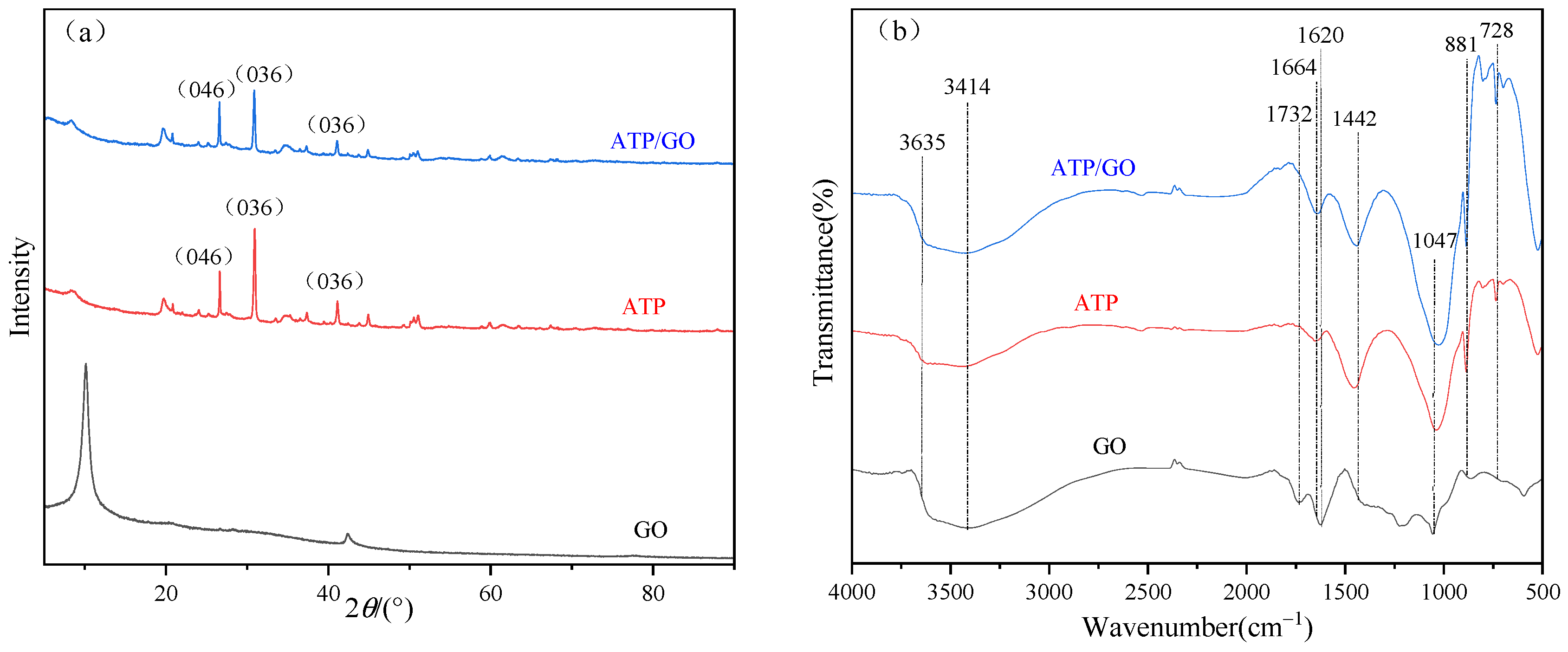
3.1.2. XRD and FTIR Analysis
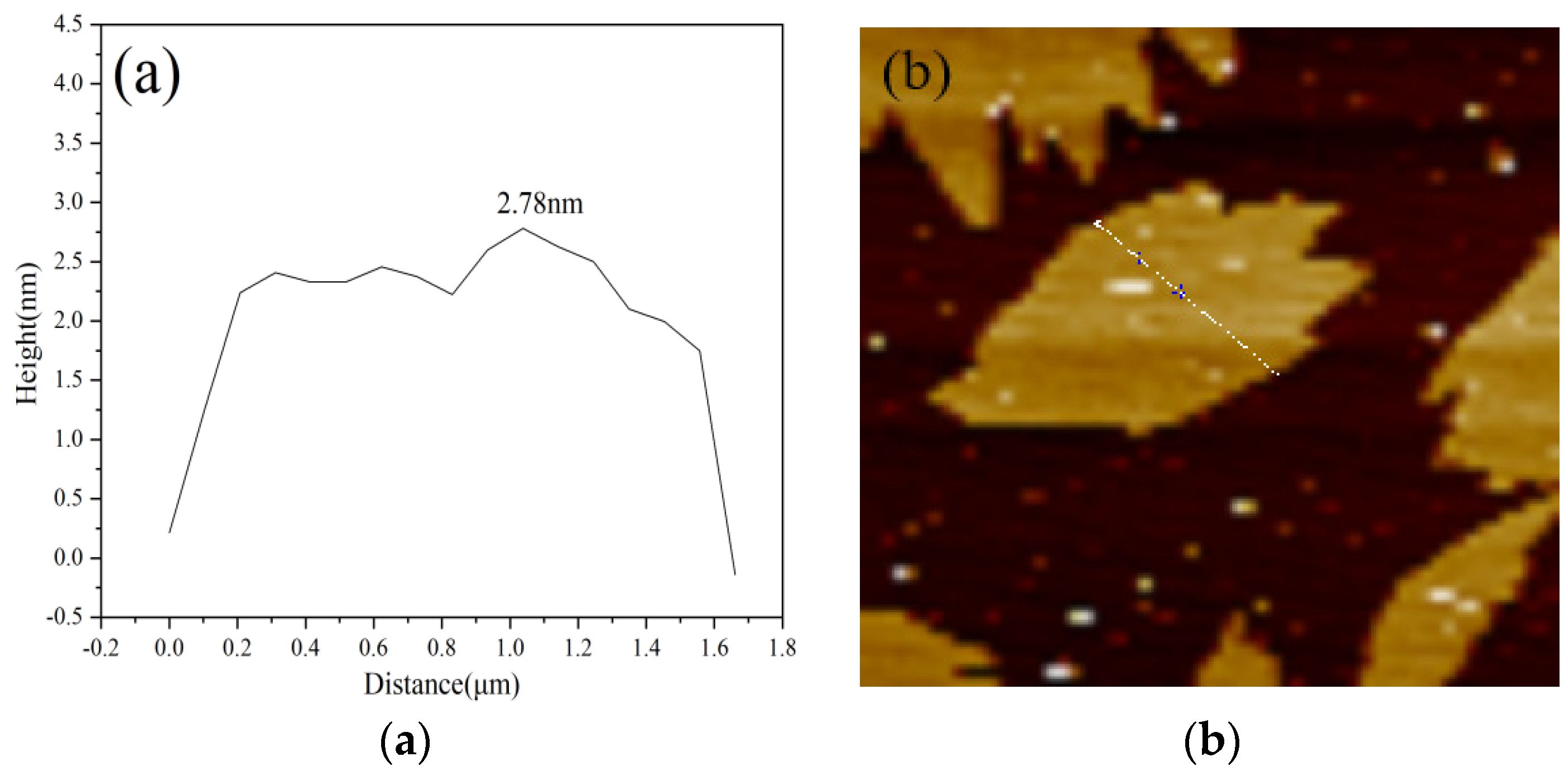
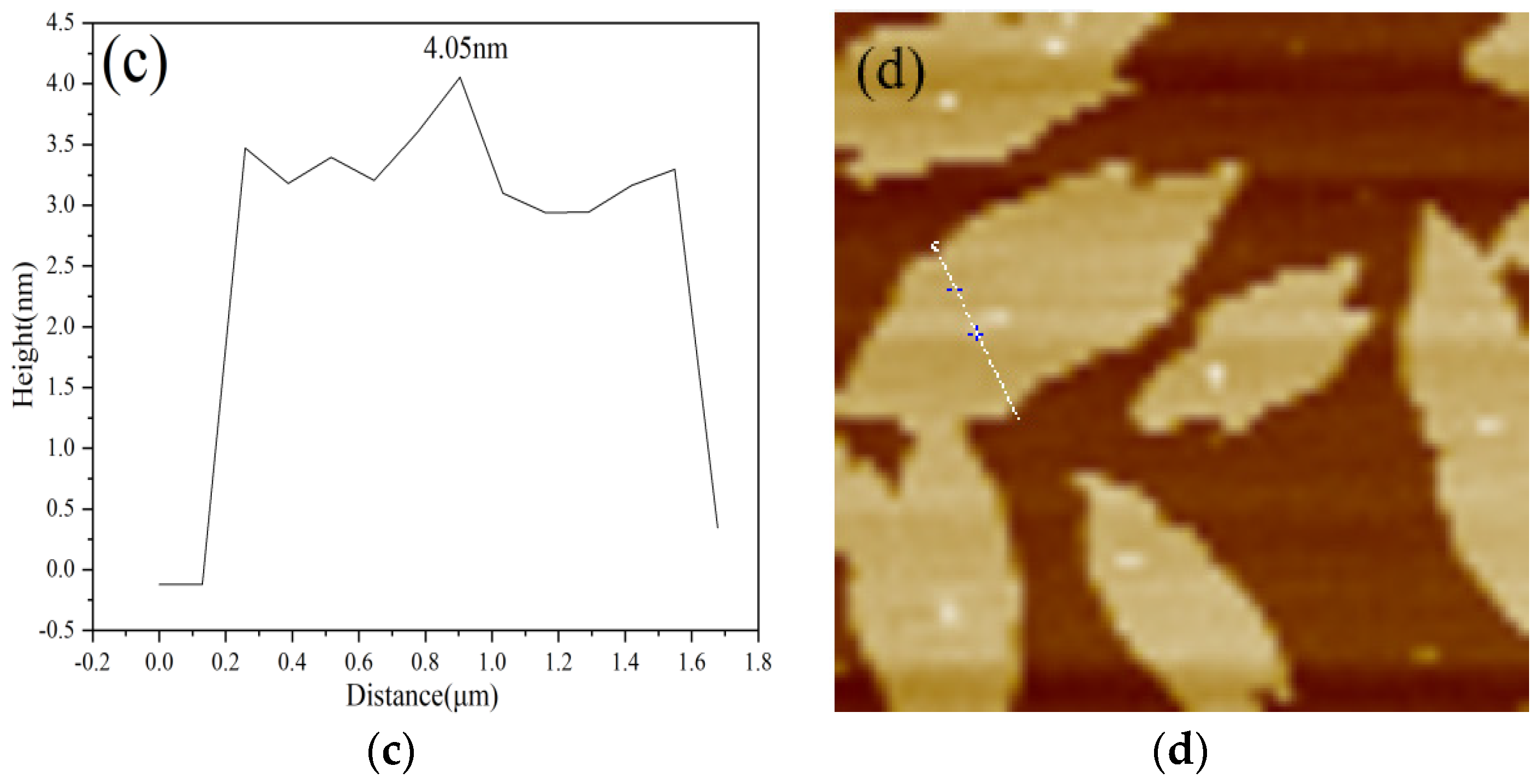
3.1.3. XPS and AFM Analysis
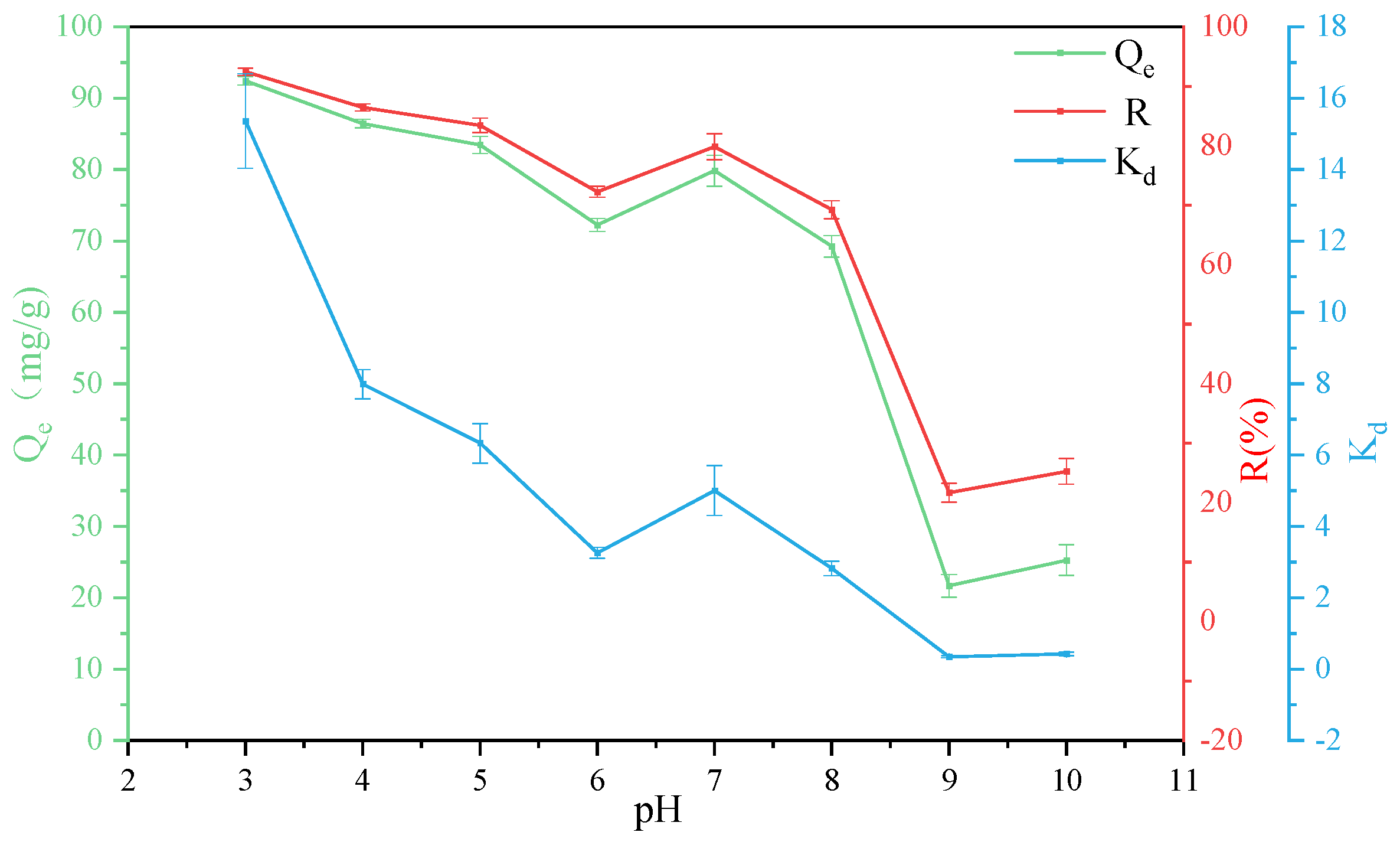
3.2. Effect of PH
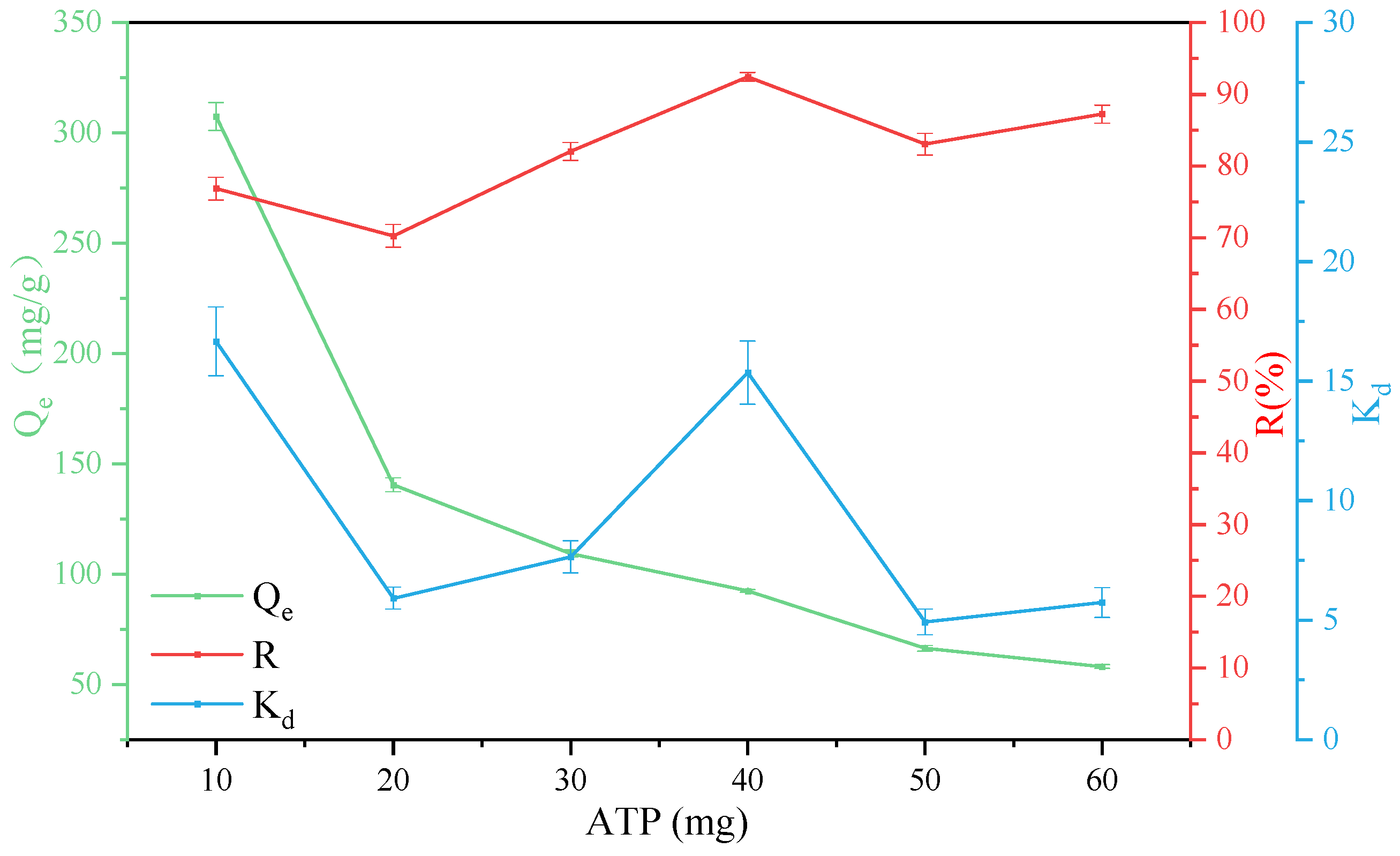
3.3. Effect of Adsorbent Mass
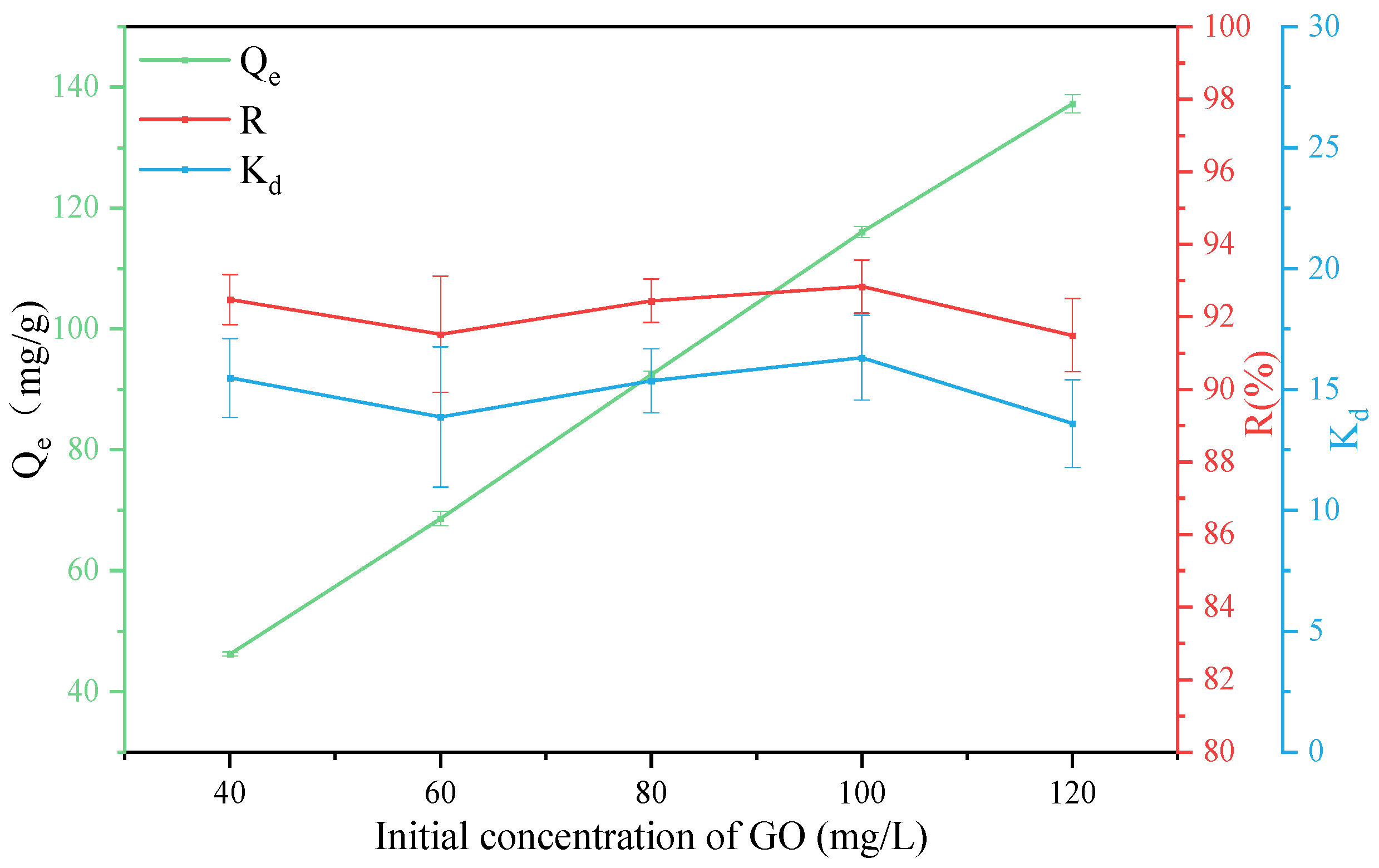
3.4. Effect of GO Concentration
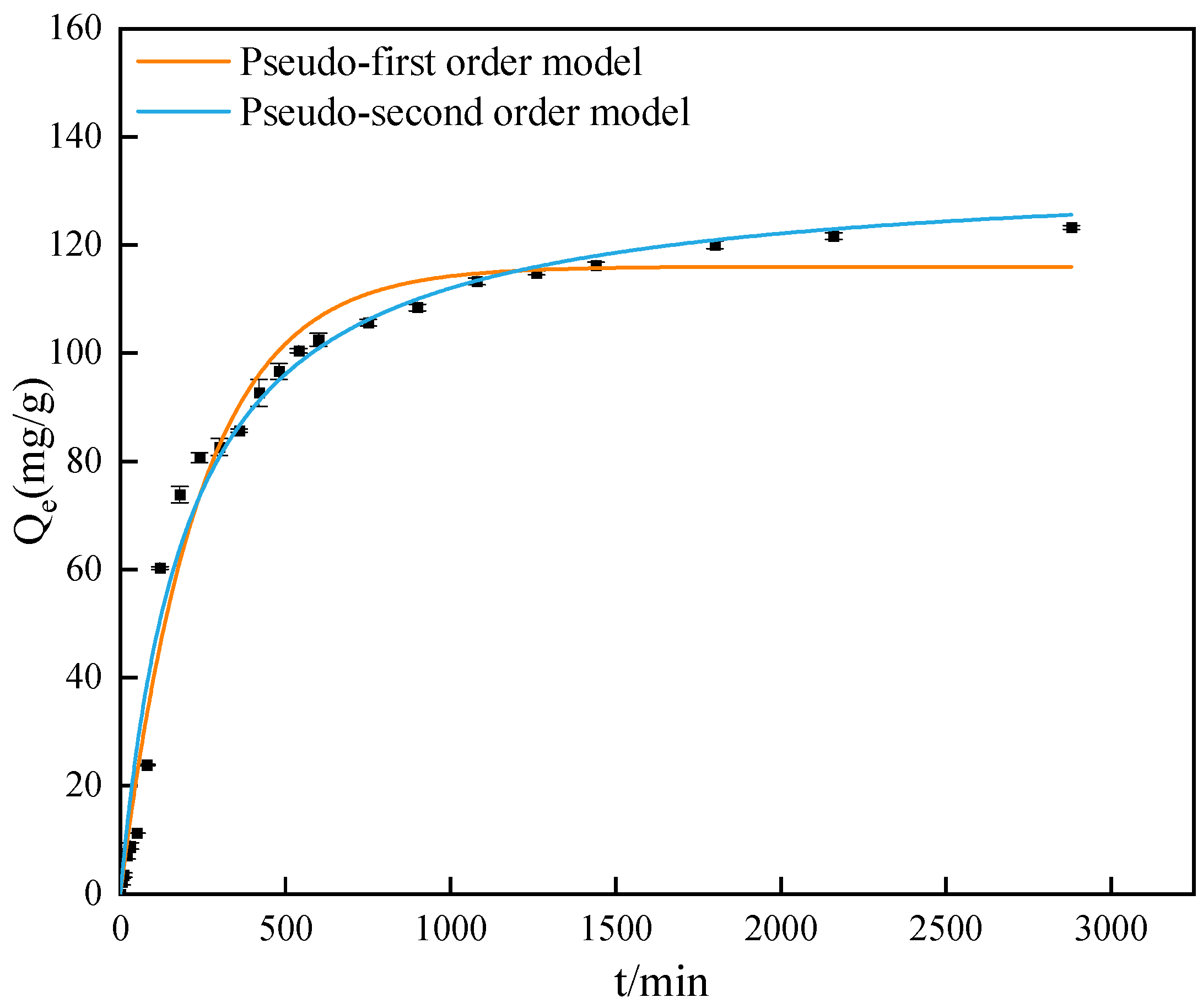
3.5. Adsorption Kinetics
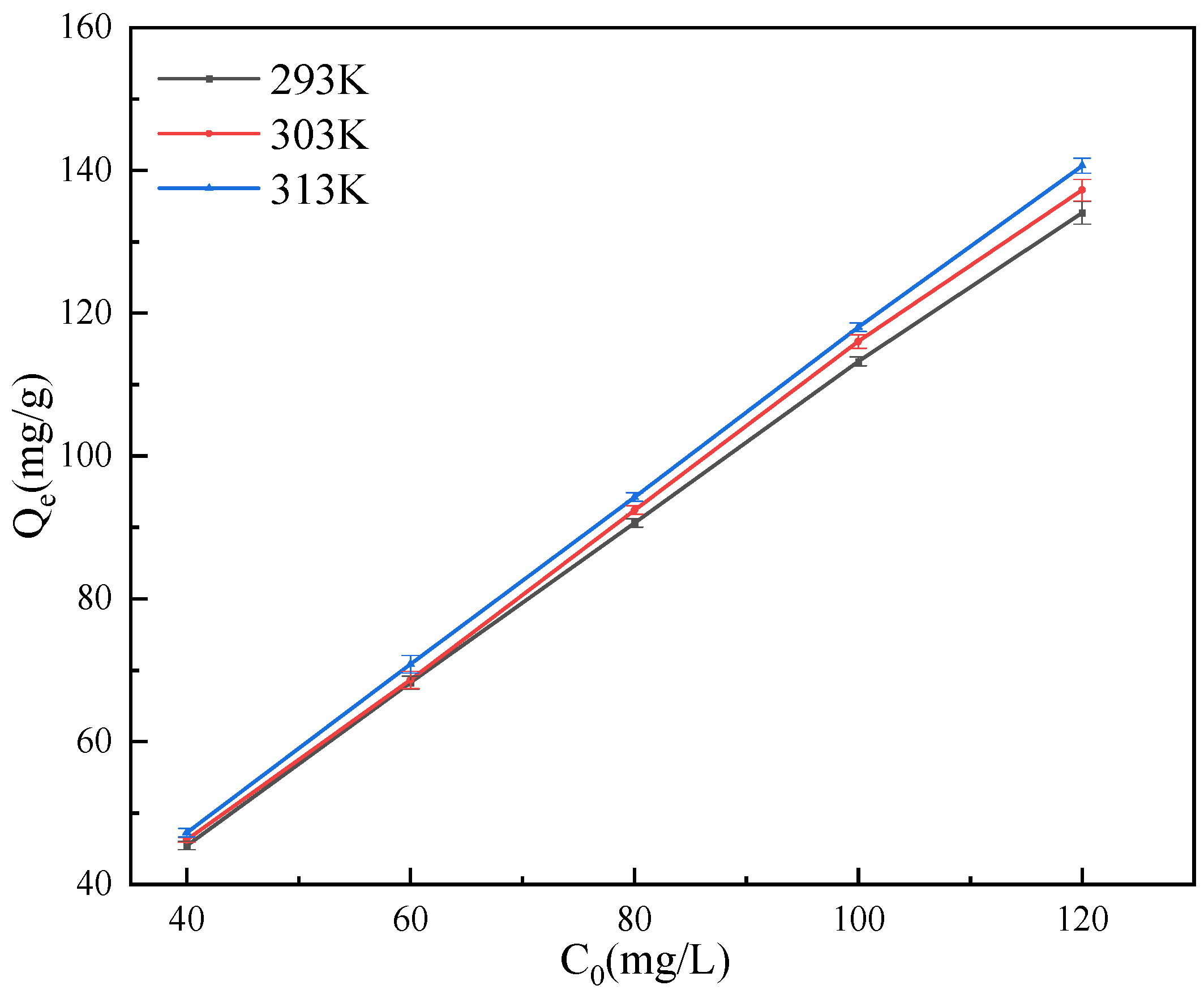
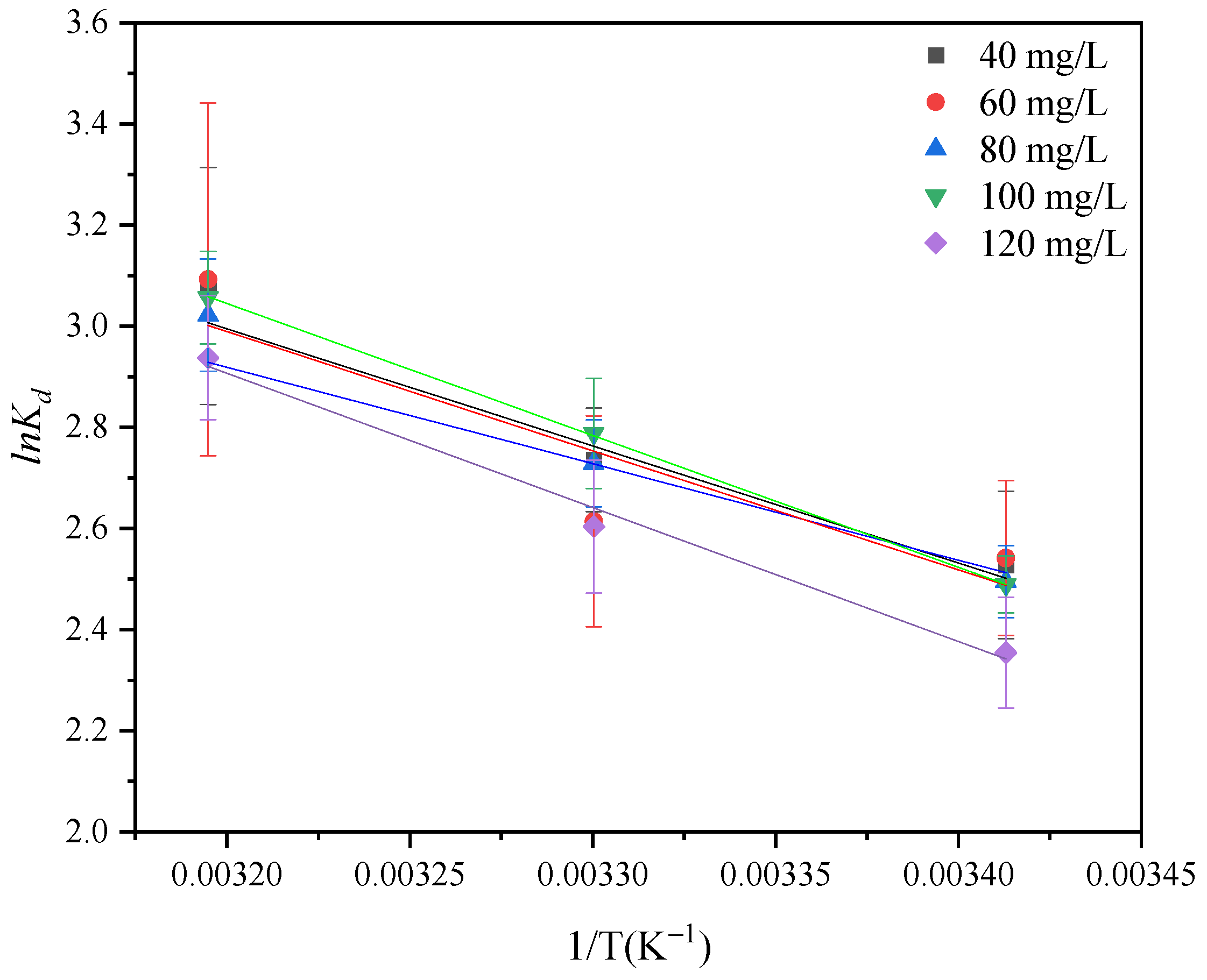
3.6. Adsorption Isotherm and Thermodynamic Analysis
4. Conclusions
- By studying the relationship between pH, adsorbent mass, GO concentration, time and temperature on the adsorption effect, it was found that when T = 303 K, pH = 3, the attapulgite mass is 40 mg, and the GO concentration is 100 mg/L, the adsorption effect is the best, and the removal rate can reach 92.83%. The adsorption effect will increase with the increase of adsorption time. When the adsorption time is 480 min, the increase of adsorption amount tends to be gentle, and the adsorption equilibrium is reached at 2160 min. At the same time, the adsorption capacity of GO by attapulgite increases with the increase of temperature. The higher the concentration is, the more obvious the effect of temperature on the adsorption of GO is.
- By means of SEM, TEM, XRD, AFM, FTIR and XPS, the materials characterization of GO and ATP/GO was conducted. It was found that GO aggregates on the surface of attapulgite, which is not simple accumulation, but is accompanied by the vibration deformation and interaction of functional groups. Further study of XPS spectra shows that the interaction between GO and attapulgite is mainly completed by C-O and O-C=O.
- The results of adsorption kinetic studies show that the adsorption of GO by attapulgite is more in line with a pseudo-second-order kinetic equation, and the adsorption process is accompanied by physical adsorption along with chemical adsorption. The results of the isotherm study show that the adsorption of GO by attapulgite is more consistent with the Langmuir isotherm model. Based on the calculation of thermodynamic parameters, the adsorption of GO by attapulgite is an endothermic process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, X.M.; Liu, L.Z.; Zhou, S.; Zhao, J.J. Physical properties and device applications of graphene oxide. Front. Phys. 2020, 15, 1–70. [Google Scholar] [CrossRef] [Green Version]
- Velusamy, S.; Roy, A.; Sundaram, S.; Mallick, T.K. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef]
- Reynosa-Martinez, A.C.; Tovar, G.N.; Gallegos, W.R.; Rodriguez-Melendez, H.; Torres-Cadena, R.; Mondragon-Solorzano, G.; Barroso-Flores, J.; Alvarez-Lemus, M.A.; Montalvo, V.G.; Lopez-Honorato, E. Effect of the degree of oxidation of graphene oxide on As(III) adsorption. J. Hazard. Mater. 2020, 384, 121440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernke, G.; Shimabuku-Biadola, Q.L.; dos Santos, T.R.T.; Silva, M.F.; Fagundes-Klen, M.R.; Bergamasco, R. Adsorption of cephalexin in aqueous media by graphene oxide: Kinetics, isotherm, and thermodynamics. Environ. Sci. Pollut. Res. 2020, 27, 4725–4736. [Google Scholar] [CrossRef] [PubMed]
- Mondal, N.K.; Chakraborty, S. Adsorption of Cr(VI) from aqueous solution on graphene oxide (GO) prepared from graphite: Equilibrium, kinetic and thermodynamic studies. Appl. Water Sci. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Sun, J.; Xu, X.T.; Alsaedi, A.; Hayat, T.; Li, J.X. Adsorption and desorption of U(VI) on different-size graphene oxide. Chem. Eng. J. 2019, 360, 941–950. [Google Scholar] [CrossRef]
- Zhang, J.F.; Xie, X.D.; Meng, X.G.; Li, Y.; Zhu, W.H. The critical role of oxidative debris in the adsorption and desorption of Pb(II) to graphene oxides under alkaline groundwater conditions. Sci. Total Environ. 2020, 704, 135254. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Roh, H.; Choi, Y.L.; Chang, Y.Y.; Yang, J.K. Adsorption removal of Co(II) from waste-water using graphene oxide. Hydrometallurgy 2016, 165, 90–96. [Google Scholar] [CrossRef]
- Chowdhury, I.; Duch, M.C.; Mansukhani, N.D.; Hersam, M.C.; Bouchard, D. Colloidal Properties and Stability of Graphene Oxide Nanomaterials in the Aquatic Environment. Environ. Sci. Technol. 2013, 47, 6288–6296. [Google Scholar] [CrossRef]
- Cao, X.; Ma, C.; Zhao, J.; Musante, C.; White, J.C.; Wang, Z.; Xing, B. Interaction of graphene oxide with co-existing arsenite and arsenate: Adsorption, transformation and combined toxicity. Environ. Int. 2019, 131, 104992. [Google Scholar] [CrossRef]
- Ren, C.X.; Hu, X.G.; Li, X.Y.; Zhou, Q.X. Ultra-trace graphene oxide in a water environment triggers Parkinson’s disease-like symptoms and metabolic disturbance in zebrafish larvae. Biomaterials 2016, 93, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Yang, Y.; Zhao, L.; Chen, J.; Duan, G.; Yang, Z.; Zhou, R. Graphene oxide toxicity in W1118 flies. Sci. Total Environ. 2022, 805, 150302. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Dou, T.-T.; Chen, J.-Y.; Duan, M.-X.; Zhen, Q.; Wu, H.-Z.; Zhao, Y.-L. Sublethal toxicity of graphene oxide in Caenorhabditis elegans under multi-generational exposure. Ecotoxicol. Environ. Saf. 2022, 229, 113064. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Zhang, Y.; Li, J.; Kong, H.T.; Yan, Q.L.; Zhang, J.C.; Li, W.; Ren, N.; Cui, Y.Z.; Zhang, T.; et al. Blood exposure to graphene oxide may cause anaphylactic death in non-human primates. Nano Today 2020, 35, 100922. [Google Scholar] [CrossRef]
- Ou, L.L.; Lv, X.J.; Wu, Z.X.; Xia, W.B.; Huang, Y.D.; Chen, L.Y.; Sun, W.J.; Qi, Y.; Yang, M.; Qi, L. Oxygen content-related DNA damage of graphene oxide on human retinal pigment epithelium cells. J. Mater. Sci. Mater. Med. 2021, 32, 1–9. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Xu, X.; Sheng, G.; Sun, Y.; Huang, Y.; Alsaedi, A.; Hayat, T.; Li, J. Is the interaction between graphene oxide and minerals reversible? Environ. Pollut. 2019, 249, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yuan, Z.J.; Li, D.Q.; Zheng, M.G.; Nie, X.D.; Liao, Y.S. Effects of soil particle size on the adsorption, distribution, and migration behaviors of heavy metal(loid)s in soil: A review. Environ. Sci. Processes Impacts 2020, 22, 1596–1615. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernandez-Calvino, D.; Nunez-Delgado, A.; Fernandez-Sanjurjo, M.J.; Arias-Estevez, M.; Alvarez-Rodriguez, E. Estimation of adsorption/desorption Freundlich’s affinity coefficients for oxytetracycline and chlortetracycline from soil properties: Experimental data and pedotransfer functions. Ecotoxicol. Environ. Saf. 2020, 196, 110584. [Google Scholar] [CrossRef]
- Jedidi, A.; Kraiem, A.; Dardouri, S.; Marcoux, M.; Sghaier, J. Adsorption of Dye on a Tunisian Unsaturated Layered Soil: Physical and Numerical Modeling. Eurasian Soil Sci. 2020, 53, 1132–1141. [Google Scholar] [CrossRef]
- Otunola, B.O.; Ololade, O.O. A review on the application of clay minerals as heavy metal adsorbents for remediation purposes. Environ. Technol. Innov. 2020, 18, 100692. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Q.; Liang, L.; Ouyang, J. Surface hydroxyls mediated CO2 methanation at ambient pressure over attapulgite-loaded Ni-TiO2 composite catalysts with high activity and reuse ability. J. CO2 Util. 2021, 47, 101489. [Google Scholar] [CrossRef]
- Deng, Q.L.; Chen, C.; Lei, Q.; Liang, J.H.; Zhang, T.H.; Jiang, J.L. Adsorption of aniline from aqueous solution using graphene oxide-modified attapulgite composites. RSC Adv. 2018, 8, 23382–23389. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, J.; Zhong, J.P.; Shen, J.L.; Ren, S.L. Preparation of Graphene Oxide/Attapulgite Composites and Their Demulsification Performance for Oil-in-Water Emulsion. Energ. Fuel 2021, 35, 5172–5180. [Google Scholar] [CrossRef]
- Deng, Y.H.; Li, Y.N.; Nie, W.J.; Gao, X.; Zhang, L.; Yang, P.L.; Tan, X.C. Fast Removal of Propranolol from Water by Attapulgite/Graphene Oxide Magnetic Ternary Composites. Materials 2019, 12, 924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, B.G.; Cheng, X.B.; Wang, G.; Li, H.; Song, X.S.; Dai, L. Graphene Oxide Adsorption Enhanced by Attapulgite to Remove Pb (II) from Aqueous Solution. Appl. Sci. 2019, 9, 1390. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Xu, X.T.; Sun, J.; Alsaedi, A.; Hayat, T.; Li, J.X.; Wang, X.K. Insight into the impact of interaction between attapulgite and graphene oxide on the adsorption of U(VI). Chem. Eng. J. 2018, 343, 217–224. [Google Scholar] [CrossRef]
- Abba, M.U.; Che Man, H.; Azis, S.; Idris, A.I.; Hazwan Hamzah, M.; Abdulsalam, M. Synthesis of nano-magnetite from industrial mill chips for the application of boron removal: Characterization and adsorption efficacy. Int. J. Environ. Res. Public Health 2021, 18, 1400. [Google Scholar] [CrossRef]
- Piaskowski, K.; Zarzycki, P.K. Carbon-based nanomaterials as promising material for wastewater treatment processes. Int. J. Environ. Res. Public Health 2020, 17, 5862. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Xing, L.; Wang, D.; Wang, L.; Xiao, H.; Ke, J. Magnetic Fe3O4/attapulgite hybrids for Cd (II) adsorption: Performance, mechanism and recovery. J. Hazard. Mater. 2021, 412, 125237. [Google Scholar] [CrossRef]
- Huang, R.L.; Lin, Q.T.; Zhong, Q.F.; Zhang, X.F.; Wen, X.Q.; Luo, H.Y. Removal of Cd(II) and Pb(II) from aqueous solution by modified attapulgite clay. Arab. J. Chem. 2020, 13, 4994–5008. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Neolaka, Y.A.B.; Lawa, Y.; Naat, J.N.; Riwu, A.A.P.; Iqbal, M.; Darmokoesoemo, H.; Kusuma, H.S. The adsorption of Cr(VI) from water samples using graphene oxide-magnetic (GO-Fe3O4) synthesized from natural cellulose-based graphite (kusambi wood or Schleichera oleosa): Study of kinetics, isotherms and thermodynamics. J. Mater. Res. Technol. 2020, 9, 6544–6556. [Google Scholar] [CrossRef]
- Fernandes, E.P.; Silva, T.S.; Carvalho, C.M.; Selvasembian, R.; Chaukura, N.; Oliveira, L.M.; Meneghetti, S.M.P.; Meili, L. Efficient adsorption of dyes by γ-alumina synthesized from aluminum wastes: Kinetics, isotherms, thermodynamics and toxicity assessment. J. Environ. Chem. Eng. 2021, 9, 106198. [Google Scholar] [CrossRef]
- Abou Taleb, M.F.; Abou El Fadl, F.I.; Albalwi, H. Adsorption of Toxic dye in wastewater onto Magnetic NVP/CS Nanocomposite hydrogels synthesized using gamma radiation. Sep. Purif. Technol. 2021, 266, 118551. [Google Scholar] [CrossRef]
- Cui, M.K.; Mu, P.; Shen, Y.Q.; Zhu, G.R.; Luo, L.; Li, J. Three-dimensional attapulgite with sandwich-like architecture used for multifunctional water remediation. Sep. Purif. Technol. 2020, 235, 116210. [Google Scholar] [CrossRef]
- Hoor, Y.Q.; Au, P.I.; Mubarak, N.M.; Khalid, M.; Jagadish, P.; Walvekar, R.; Abdullah, E.C. Surface force arising from Adsorbed graphene oxide in kaolinite suspensions. Colloid Surf. A 2020, 592, 124592. [Google Scholar] [CrossRef]
- Wang, M.; Guo, Y.; Fu, X.; Cui, H.; Sun, T.; Tang, Y.; Liu, Q. Facile synthesis of novel Zn3 (OH) 2V2O7· 2H2O nanocables with excellent adsorption properties. Mater. Lett. 2021, 283, 128710. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Liu, X.; Wu, J.; Zhu, X.; Bai, Z.; Yu, Z. Biointerfaces, S.B. Preparation of β-cyclodextrin/graphene oxide and its adsorption properties for methylene blue. Colloid Surf. B Biointerfaces 2021, 200, 111605. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, S.; Wang, Y. Mechanism of Oxytetracycline Removal by Coconut Shell Biochar Loaded with Nano-Zero-Valent Iron. Int. J. Env. Res. Pub. Heal. 2021, 18, 13107. [Google Scholar] [CrossRef]
- Khalil, W.F.; El-Sayyad, G.S.; El Rouby, W.M.A.; Sadek, M.A.; Farghali, A.A.; El-Batal, A.I. Graphene oxide-based nanocomposites (GO-chitosan and GO-EDTA) for outstanding antimicrobial potential against some Candida species and pathogenic bacteria. Int. J. Biol. Macromol. 2020, 164, 1370–1383. [Google Scholar] [CrossRef]
- Boukhoubza, I.; Khenfouch, M.; Achehboune, M.; Leontie, L.; Galca, A.C.; Enculescu, M.; Carlescu, A.; Guerboub, M.; Mothudi, B.M.; Jorio, A.; et al. Graphene Oxide Concentration Effect on the Optoelectronic Properties of ZnO/GO Nanocomposites. Nanomaterials 2020, 10, 1532. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.J.; Ye, Y.P.; Wan, H.Q.; Chen, L.; Zhou, H.D.; Chen, J.M. Chemical modification of graphene oxide to reinforce the corrosion protection performance of UV-curable polyurethane acrylate coating. Prog. Org. Coat. 2020, 141, 105547. [Google Scholar] [CrossRef]
- Wu, Q.; He, J.Q.; Wang, F.; Yang, X.; Zhu, J.F. Comparative study on effects of covalent-covalent, covalent-ionic and ionicionic bonding of carbon fibers with polyether amine/GO on the interfacial adhesion of epoxy composites. Appl. Surf. Sci. 2020, 532, 147359. [Google Scholar] [CrossRef]
- Meng, F.Y.; Song, M.; Chen, Y.Y.; Wei, Y.X.; Song, B.; Cao, Q.Q. Promoting adsorption of organic pollutants via tailoring surface physicochemical properties of biomass-derived carbon-attapulgite. Environ. Sci. Pollut. Res. 2021, 28, 11106–11118. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, Z.; Zhang, J.; Jiao, C.Z.; Ding, L.; Yang, S.R. Synthesis and Adsorption Properties of Novel Bacterial Cellulose/Graphene Oxide/Attapulgite Materials for Cu and Pb Ions in Aqueous Solutions. Materials 2020, 13, 3703. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zeng, W.J.; Jiang, T.T.; Chen, X.; Zhang, X.L. Incorporating attapulgite nanorods into graphene oxide nanofiltration membranes for efficient dyes wastewater treatment. Sep. Purif. Technol. 2019, 214, 21–30. [Google Scholar] [CrossRef]
- Wang, R.-s.; Li, Y.; Shuai, X.-x.; Liang, R.-h.; Chen, J.; Liu, C.M. Pectin/activated carbon-based porous microsphere for Pb2+ adsorption: Characterization and adsorption behaviour. Polymers 2021, 13, 2453. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Chimonyo, W.; Fletcher, B.; Peng, Y.J.M.E. Adsorption and morphology of oxidized starches on graphite. Miner. Eng. 2021, 168, 106936. [Google Scholar] [CrossRef]
- Belhaj, A.F.; Elraies, K.A.; Mahmood, S.M.; Zulkifli, N.N.; Akbari, S.; Hussien, O.S. The effect of surfactant concentration, salinity, temperature, and pH on surfactant adsorption for chemical enhanced oil recovery: A review. J. Pet. Explor. Prod. Technol. 2020, 10, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Shih, C.J.; Lin, S.C.; Sharma, R.; Strano, M.S.; Blankschtein, D. Understanding the pH-Dependent Behavior of Graphene Oxide Aqueous Solutions: A Comparative Experimental and Molecular Dynamics Simulation Study. Langmuir 2012, 28, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhou, L.; Wang, W.; Li, N.; Zhang, F. Performance and mechanisms of fly ash for graphene oxide removal from aqueous solution. Environ. Sci. Pollut. Res. 2022, 29, 3773–3783. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, S.Y.; Huang, T.L.; Cui, F.Y.; Xing, B.S. Effects of pH and electrolytes on the sheet-to-sheet aggregation mode of graphene oxide in aqueous solutions. Environ. Sci.-Nano 2020, 7, 984–995. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, S.-T.; Feng, S.; Ma, Q.; Peng, X.; Wu, D. Preparation and application of carboxylated graphene oxide sponge in dye removal. Int. J. Env. Res. Public Health 2017, 14, 1301. [Google Scholar] [CrossRef] [Green Version]
- Nan, F.; Liu, C.B.; Pu, J.B. Anticorrosive performance of waterborne epoxy coatings containing attapulgite/graphene nanocomposites. Surf. Topogr. Metrol. Prop. 2019, 7, 024002. [Google Scholar] [CrossRef]
- Benjelloun, M.; Miyah, Y.; Evrendilek, G.A.; Zerrouq, F.; Lairini, S. Recent Advances in Adsorption Kinetic Models: Their Application to Dye Types. Arab. J. Chem. 2021, 14, 103031. [Google Scholar] [CrossRef]
- Salis, A.; Meloni, D.; Ligas, S.; Casula, M.F.; Monduzzi, M.; Solinas, V.; Dumitriu, E. Physical and chemical adsorption of Mucor javanicus lipase on SBA-15 mesoporous silica. Synthesis, structural characterization, and activity performance. Langmuir 2005, 21, 5511–5516. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, G.; Kumar, A.; AlGarni, T.S.; Naushad, M.; ALOthman, Z.A.; Stadler, F.J. Adsorption of cationic dyes onto carrageenan and itaconic acid-based superabsorbent hydrogel: Synthesis, characterization and isotherm analysis. J. Hazard. Mater. 2022, 421, 126729. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Jia, Y.N.; Song, W.K.; Li, X.Q.; Xu, K.; Wang, Z. Optimization of boron adsorption from desalinated seawater onto UiO-66-NH2/GO composite adsorbent using response surface methodology. J. Clean. Prod. 2021, 300, 126974. [Google Scholar] [CrossRef]
- Han, Y.; Wang, L.; Guo, X.; Jiao, T.; Ding, H. Enhanced adsorption efficiency of graphene oxide by electrostatic field for Hg (II) removal from water. J. Mol. Liq. 2021, 341, 117410. [Google Scholar] [CrossRef]
- Kong, Q.; Preis, S.; Li, L.; Luo, P.; Wei, C.; Li, Z.; Hu, Y.; Wei, C. Relations between metal ion characteristics and adsorption performance of graphene oxide: A comprehensive experimental and theoretical study. Sep. Purif. Technol. 2020, 232, 115956. [Google Scholar] [CrossRef]
- Kumar, V.; Bahadur, N.; Sachdev, D.; Gupta, S.; Reddy, G.; Pasricha, R. Restructural confirmation and photocatalytic applications of graphene oxide–gold composites synthesized by Langmuir–Blodgett method. Carbon 2014, 80, 290–304. [Google Scholar] [CrossRef]
- Kang, H.; Gu, J.; Liu, G.; Li, B.; Wang, W. Performance and mechanism of layered double hydroxide to remove graphene oxide in aqueous solution. Nat. Environ. Pollut. Technol. 2021, 20, 55–62. [Google Scholar] [CrossRef]
- Lv, B.; Yu, W.; Luo, J.; Qian, B.; Asefa, M.B.; Li, N. Study on the Adsorption Mechanism of Graphene Oxide by Calcareous Sand in South China Sea. Adsorpt. Sci. Technol. 2021, 2021, 227570. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, L.; Wang, Y.; Zhao, W.; Gu, J. Study on the adsorption properties of iron tailings for GO. Coatings 2021, 11, 768. [Google Scholar] [CrossRef]



| Element | C | O | H | S |
|---|---|---|---|---|
| Content/% | 41.70 | 51.49 | 2.41 | 2.00 |
| Chemical Composition | SiO2 | MgO | CaO | Al2O3 |
|---|---|---|---|---|
| Content/% | 58.05 | 11.03 | 1.18 | 9.55 |
| pH | Temperature(K) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| Qe (mg/g) | k1 g/(mg·min) | R2 | Qe (mg/g) | k2 g/(mg·min) | R2 | ||
| 3 | 303 k | 113.664 | 0.004 | 0.980 | 130.634 | 3.758 × 10−5 | 0.983 |
| C0 (mg/L) | pH | Temperature(K) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | KL (L/mg) | R2 | KF (mg/g) | n | R2 | |||
| 100 | 3 | 313 | 311.944 | 0.063 | 0.993 | 23.999 | 1.138 | 0.987 |
| 303 | 302.852 | 0.042 | 0.984 | 18.415 | 1.156 | 0.978 | ||
| 293 | 258.396 | 0.037 | 0.996 | 14.938 | 1.161 | 0.986 | ||
| C0 (mg/L) | ΔG0/(kJ·mol−1) | ΔH0/(kJ·mol−1) | ΔS0/(J·mol−1·K−1) | ||
|---|---|---|---|---|---|
| 293 K | 303 K | 313 K | |||
| 40 | −6.159 | −6.892 | −8.014 | 19.248 | 86.499 |
| 60 | −6.192 | −6.586 | −8.048 | 15.883 | 75.100 |
| 80 | −6.079 | −6.875 | −7.864 | 19.598 | 87.571 |
| 100 | −6.064 | −7.024 | −7.955 | 21.681 | 94.696 |
| 120 | −5.736 | −6.559 | −7.644 | 22.056 | 94.746 |
| Adsorbent | Layered Double Hydroxide | Calcareous Sand | Iron Tailings | Attapulgite |
|---|---|---|---|---|
| Adsorbent dosage (mg) | 5, 10, 15, 20, 25 | 30, 40, 50, 60, 70 | 30, 40, 50, 60, 70 | 10, 20, 30, 40, 50, 60 |
| GO initial concentration (mg/L) | 20, 40, 60, 80, 100, 120 | 80, 100, 120, 140, 160 | 40, 60, 80, 100 | 40, 60, 80, 100, 120, 140, 160 |
| pH effect | Alkaline environment inhibits adsorption | Alkaline environment inhibits adsorption | pH = 7 is the best | pH = 3 is the best |
| Optimal removal rate | 92% | 91.5% | 85.92% | 92.83% |
| Equilibrium time (min) | 360 min | 300 min | 1680 min | 2160 min |
| References | [63] | [64] | [65] | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Fang, J.; Jiang, P.; Li, C.; Kang, H.; Wang, W. Adsorption Properties and Mechanism of Attapulgite to Graphene Oxide in Aqueous Solution. Int. J. Environ. Res. Public Health 2022, 19, 2793. https://doi.org/10.3390/ijerph19052793
Li N, Fang J, Jiang P, Li C, Kang H, Wang W. Adsorption Properties and Mechanism of Attapulgite to Graphene Oxide in Aqueous Solution. International Journal of Environmental Research and Public Health. 2022; 19(5):2793. https://doi.org/10.3390/ijerph19052793
Chicago/Turabian StyleLi, Na, Jiyuan Fang, Ping Jiang, Cuihong Li, Haibo Kang, and Wei Wang. 2022. "Adsorption Properties and Mechanism of Attapulgite to Graphene Oxide in Aqueous Solution" International Journal of Environmental Research and Public Health 19, no. 5: 2793. https://doi.org/10.3390/ijerph19052793
APA StyleLi, N., Fang, J., Jiang, P., Li, C., Kang, H., & Wang, W. (2022). Adsorption Properties and Mechanism of Attapulgite to Graphene Oxide in Aqueous Solution. International Journal of Environmental Research and Public Health, 19(5), 2793. https://doi.org/10.3390/ijerph19052793








