Quality of National Disease Surveillance Reporting before and during COVID-19: A Mixed-Method Study in Indonesia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Objectives and Design
2.2. Study Setting and Participants
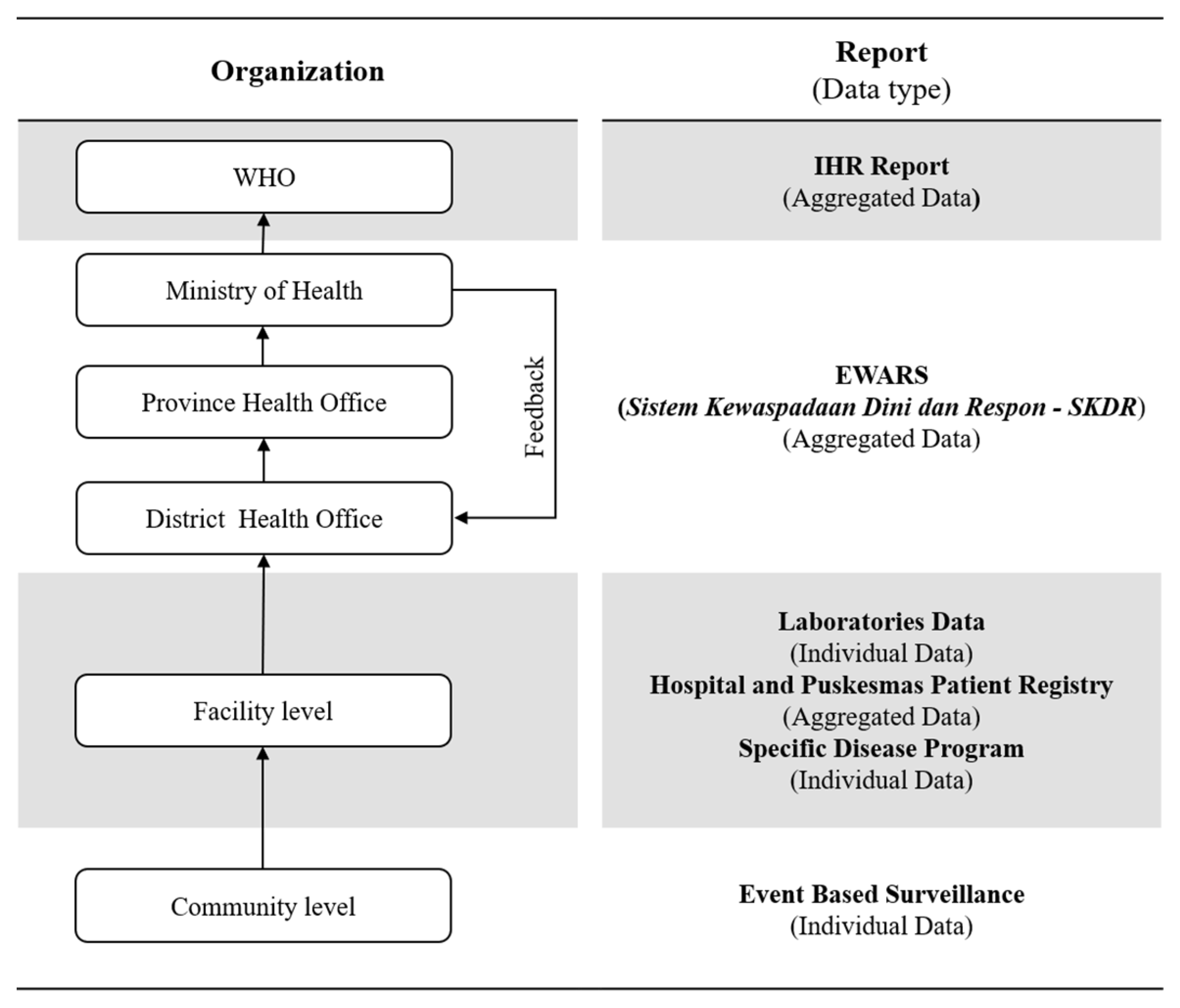
2.3. Measures Quality of Indonesia’s EWARS
2.3.1. Quantitative Stage (Quality of Indonesia’s EWARS)
2.3.2. Qualitative Stage (Burden of Disease Surveillance during COVID-19 Pandemic)
2.4. Data Analysis
2.5. Ethical Approval
3. Results
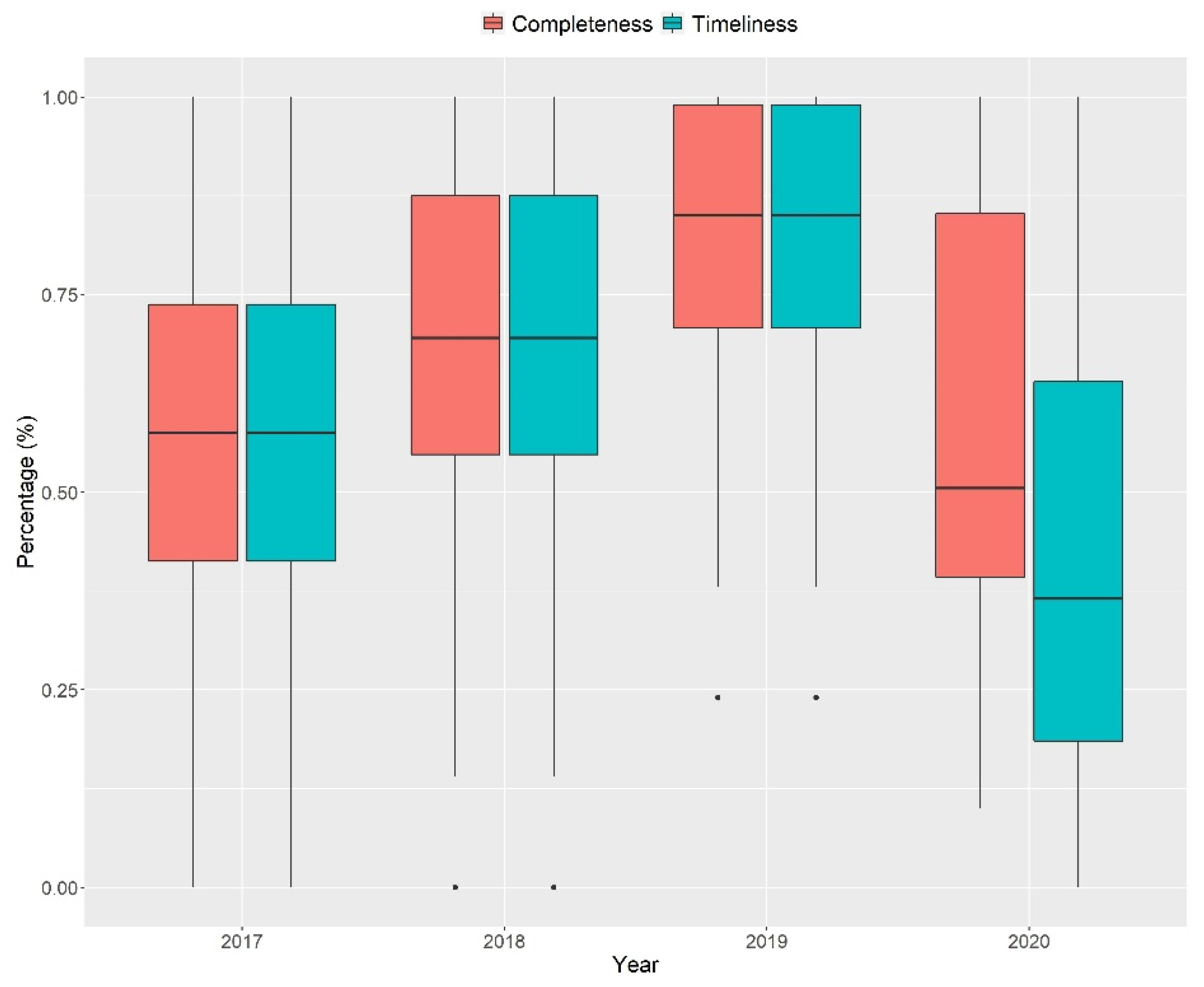
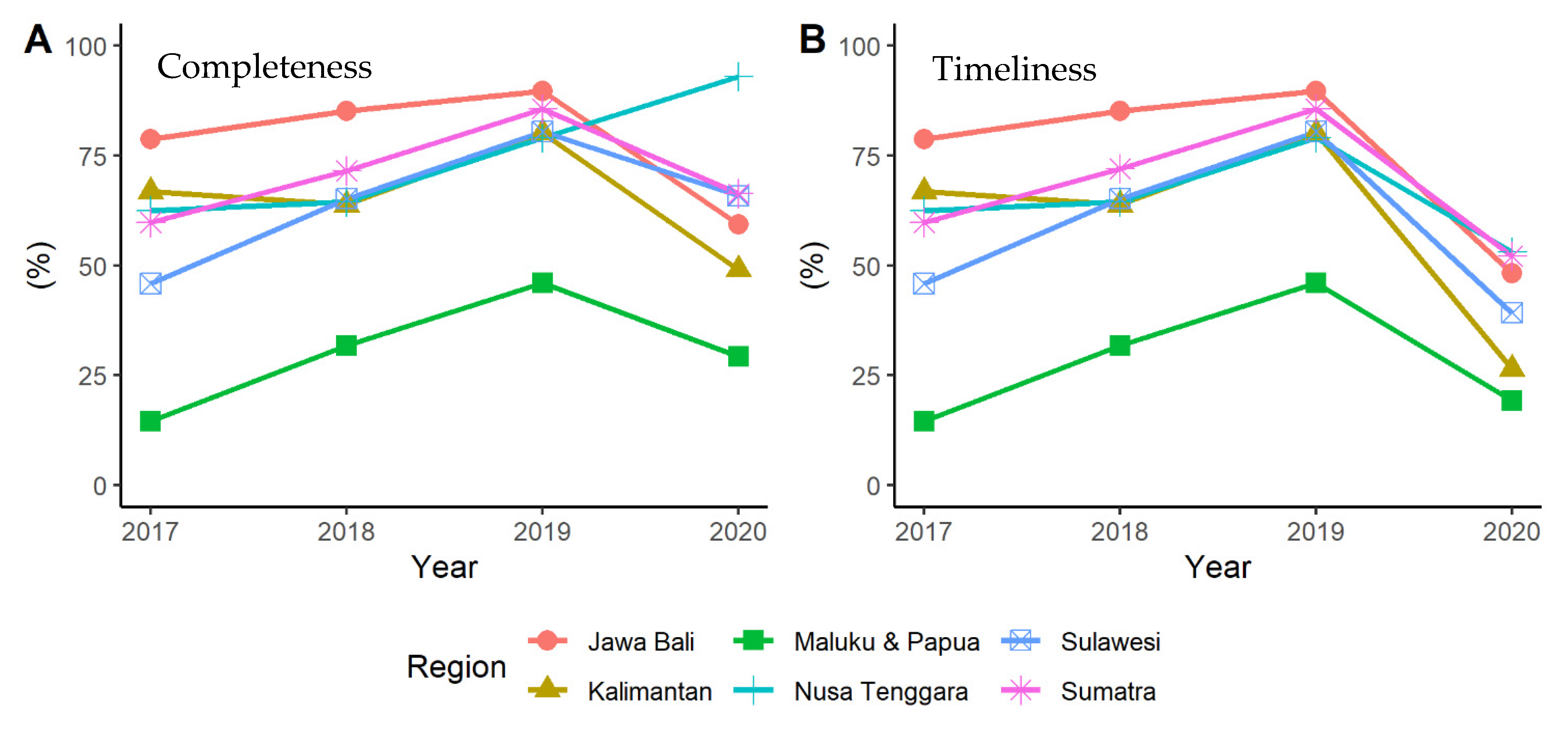
3.1. Quantitative Stage (Quality of Indonesia’s EWARS)
3.2. Qualitative Stage (Burden of Disease Surveillance during COVID-19 Pandemic)
3.2.1. System Description
3.2.2. Outbreak Detection
3.2.3. Implementation Challenges
3.2.4. Improvement Strategy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. COVID-19 Weekly Epidemiological Update, 76th ed.; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Bai, H.M.; Zaid, A.; Catrin, S.; Ahmed, K.; Ahmed, A. The socio-economic implications of the coronavirus pandemic (covid-19): A review. ComFin Res. 2020, 8, 8–17. [Google Scholar] [CrossRef]
- Oppenheim, B.; Gallivan, M.; Madhav, N.K.; Brown, N.; Serhiyenko, V.; Wolfe, N.D.; Ayscue, P. Assessing global preparedness for the next pandemic: Development and application of an epidemic preparedness index. BMJ Glob. Health 2019, 4, e001157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Januraga, P.P.; Harjana, N.P.A. Improving public access to covid-19 pandemic data in indonesia for better public health response. Front. Pub. Health 2020, 8, 825. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, Y.B.; Lequarre, A.-S.; McCourt, J.; Clevestig, P.; Pigazzani, F.; Jeddi, M.Z.; Colosio, C.; Goulart, M. Initial impacts of global risk mitigation measures taken during the combatting of the covid-19 pandemic. Saf. Sci. 2020, 128, 104773. [Google Scholar] [CrossRef]
- Madhav, N.; Oppenheim, B.; Gallivan, M.; Mulembakani, P.; Rubin, E.; Wolfe, N. Pandemics: Risks, Impacts, and Mitigation, 3rd ed.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017. [Google Scholar]
- World Health Organization. Who Resolution Wha74 on Strengthening Who Preparedness for and Response to Health Emergencies; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Merianos, A.; Peiris, M. International health regulations (2005). Lancet 2005, 366, 1249–1251. [Google Scholar] [CrossRef]
- World Health Organization. Who Benchmarks for International Health Regulations Capacities; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization. Ewars—Improving Early Detection and Prompt Response to Acute Public Health Events. 2019. Available online: https://www.afro.who.int/news/ewars-improving-early-detection-and-prompt-response-acute-public-health-events (accessed on 20 December 2021).
- Lestarini, D.; Raflesia, S.P.; Puspita, I.; Liana, P.; Kurnianto, A. Knowledge-Based System for Malaria Prevention and Control: A Conceptual Model. Knowledge-Based System for Malaria Prevention and Control: A Conceptual Model. In Proceedings of the 2018 International Conference on Sustainable Information Engineering and Technology (SIET), Malang, Indonesia, 10–12 November 2018; pp. 16–20. Available online: https://ieeexplore.ieee.org/abstract/document/8693157 (accessed on 20 December 2021). [CrossRef]
- Bravata, D.M.; McDonald, K.M.; Smith, W.M.; Rydzak, C.; Szeto, H.; Buckeridge, D.L.; Haberland, C.; Owens, D.K. Systematic review: Surveillance systems for early detection of bioterrorism-related diseases. Ann. Intern. Med. 2004, 140, 910–922. Available online: https://www.ncbi.nlm.nih.gov/pubmed/15172906 (accessed on 20 December 2021). [CrossRef]
- Christaki, E. New technologies in predicting, preventing and controlling emerging infectious diseases. Virulence 2015, 6, 558–565. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26068569 (accessed on 20 December 2021). [CrossRef] [Green Version]
- Wang, R.; Jiang, Y.; Michael, E.; Zhao, G. How to select a proper early warning threshold to detect infectious disease outbreaks based on the china infectious disease automated alert and response system (cidars). BMC Pub. Health 2017, 17, 570. Available online: https://www.ncbi.nlm.nih.gov/pubmed/28606078 (accessed on 20 December 2021). [CrossRef] [Green Version]
- Yang, W.; Li, Z.; Lan, Y.; Ma, J.; Jin, L.; Lai, S.; Liao, Y.; Lv, W.; Sun, Q.; Wang, J. China infectious diseases automated-alert and response system (cidars). Early Warn. Infect. Dis. Outbreak 2017, 133–161. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7149749/ (accessed on 20 December 2021). [CrossRef] [Green Version]
- Vlieg, W.L.; Fanoy, E.B.; van Asten, L.; Liu, X.; Yang, J.; Pilot, E.; Bijkerk, P.; van der Hoek, W.; Krafft, T.; van der Sande, M.A.; et al. Comparing national infectious disease surveillance systems: China and the netherlands. BMC Pub. Health 2017, 17, 415. Available online: https://www.ncbi.nlm.nih.gov/pubmed/28482830 (accessed on 20 December 2021). [CrossRef]
- Keita, M.; Lucaccioni, H.; Ilumbulumbu, M.K.; Polonsky, J.; Nsio-Mbeta, J.; Panda, G.T.; Adikey, P.C.; Ngwama, J.K.; Tosalisana, M.K.; Diallo, B.; et al. Evaluation of early warning, alert and response system for ebola virus disease, democratic republic of the congo, 2018–2020. Emerg. Infect. Dis. 2021, 27, 2988–2998. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8632192/ (accessed on 20 December 2021). [CrossRef] [PubMed]
- Saleh, F.; Kitau, J.; Konradsen, F.; Mboera, L.E.G.; Schioler, K.L. Assessment of the core and support functions of the integrated disease surveillance and response system in zanzibar, tanzania. BMC Pub. Health 2021, 21, 748. Available online: https://www.ncbi.nlm.nih.gov/pubmed/33865347 (accessed on 20 December 2021). [CrossRef] [PubMed]
- Masiira, B.; Nakiire, L.; Kihembo, C.; Katushabe, E.; Natseri, N.; Nabukenya, I.; Komakech, I.; Makumbi, I.; Charles, O.; Adatu, F.; et al. Evaluation of integrated disease surveillance and response (idsr) core and support functions after the revitalisation of idsr in uganda from 2012 to 2016. BMC Pub. Health 2019, 19, 46. Available online: https://www.ncbi.nlm.nih.gov/pubmed/30626358 (accessed on 20 December 2021). [CrossRef] [Green Version]
- Joseph Wu, T.S.; Kagoli, M.; Kaasboll, J.J.; Bjune, G.A. Integrated disease surveillance and response (idsr) in malawi: Implementation gaps and challenges for timely alert. PLoS ONE 2018, 13, e0200858. Available online: https://www.ncbi.nlm.nih.gov/pubmed/30496177 (accessed on 20 December 2021). [CrossRef] [Green Version]
- Nagbe, T.; Yealue, K.; Yeabah, T.; Rude, J.M.; Fallah, M.; Skrip, L.; Agbo, C.; Mouhamoud, N.; Okeibunor, J.C.; Tuopileyi, R.; et al. Integrated disease surveillance and response implementation in liberia, findings from a data quality audit, 2017. Pan. Afr. Med. J. 33 2019, 33 (Suppl. 2), 10. Available online: https://www.ncbi.nlm.nih.gov/pubmed/31402968 (accessed on 20 December 2021). [CrossRef]
- Hapsari, R.B.; Riana, D.A.; Purwanto, E.; Kandel, N.; Setiawaty, V. Early warning alert and response system (ewars) in indonesia: Highlight from the first years of implementation, 2009–2011. Health Sci. J. Indonesia 2017, 8, 81–87. [Google Scholar] [CrossRef]
- Manurung, M.K.; Reo, S.E.; Pardosi, J.F.; Muscatello, D.J. Evaluation of the indonesian early warning alert and response system (ewars) in west papua, indonesia. WHO South East Asia J. Pub. Health 2020, 9, 111–117. Available online: https://www.ncbi.nlm.nih.gov/pubmed/32978343 (accessed on 20 December 2021). [CrossRef]
- Fetters, M.D.; Curry, L.A.; Creswell, J.W. Achieving integration in mixed methods designs-principles and practices. Health Serv. Res. 2013, 48, 2134–2156. Available online: https://www.ncbi.nlm.nih.gov/pubmed/24279835 (accessed on 5 January 2022). [CrossRef] [Green Version]
- Smith, J.L.; Ghimire, P.; Rijal, K.R.; Maglior, A.; Hollis, S.; Andrade-Pacheco, R.; Das Thakur, G.; Adhikari, N.; Thapa Shrestha, U.; Banjara, M.R.; et al. Designing malaria surveillance strategies for mobile and migrant populations in nepal: A mixed-methods study. Malar. J. 2019, 18, 158. Available online: https://www.ncbi.nlm.nih.gov/pubmed/31053075 (accessed on 5 January 2022). [CrossRef]
- Kementerian Kesehatan Republik Indonesia. Investigation and Management Guideline of Outbreaks in Infectious Diseases and Food Poisoning: III revision; Kemenkes RI: Jakarta, Indonesia, 2020.
- Hardhantyo, M.; Chuang, Y.C. Urban-rural differences in factors associated with incomplete basic immunization among children in indonesia: A nationwide multilevel study. Pediatr. Neonatol. 2021, 62, 80–89. Available online: https://www.pediatr-neonatol.com/article/S1875-9572(20)30144-3/pdf (accessed on 5 January 2022). [CrossRef]
- Dureab, F.; Ismail, O.; Muller, O.; Jahn, A. Cholera outbreak in yemen: Timeliness of reporting and response in the national electronic disease early warning system. Acta Inform. Med. 2019, 27, 85–88. Available online: https://www.ncbi.nlm.nih.gov/pubmed/31452564 (accessed on 5 January 2022). [CrossRef] [PubMed] [Green Version]
- Jian, S.W.; Chen, C.M.; Lee, C.Y.; Liu, D.P. Real-time surveillance of infectious diseases: Taiwan’s experience. Health Secur. 2017, 15, 144–153. Available online: https://www.ncbi.nlm.nih.gov/pubmed/28418738 (accessed on 5 January 2022). [CrossRef] [PubMed] [Green Version]
- Buehler, J.W.; Hopkins, R.S.; Overhage, J.M.; Sosin, D.M.; Tong, V.; C.D.C. Working Group. Framework for evaluating public health surveillance systems for early detection of outbreaks: Recommendations from the cdc working group. MMWR Recomm. Rep. 2004, 53, 1–11. Available online: https://www.ncbi.nlm.nih.gov/pubmed/15129191 (accessed on 20 December 2021).
- Willis, M.; Duckworth, P.; Coulter, A.; Meyer, E.T.; Osborne, M. The future of health care: Protocol for measuring the potential of task automation grounded in the national health service primary care system. JMIR Res. Protoc. 2019, 8, e11232. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, R.; Kleinman, K.P.; Dashevsky, I.; DeMaria, A.; Platt, R. Using automated medical records for rapid identification of illness syndromes (syndromic surveillance): The example of lower respiratory infection. BMC Pub. Health 2001, 1, 9. Available online: https://www.ncbi.nlm.nih.gov/pubmed/11722798 (accessed on 10 January 2022). [CrossRef] [Green Version]
- Van-Dijk, A.; Aramini, J.; Edge, G.; Moore, K.M. Real-time surveillance for respiratory disease outbreaks, ontario, canada. Emerg. Infect. Dis. 2009, 15, 799–801. Available online: https://www.ncbi.nlm.nih.gov/pubmed/19402974 (accessed on 10 January 2022). [CrossRef]
- Jung, J.; Im, J.H.; Ko, Y.-J.; Huh, K.; Yoon, C.-g.; Rhee, C.; Kim, Y.-E.; Go, D.-S.; Kim, A.; Jung, Y. Complementing conventional infectious disease surveillance with national health insurance claims data in the republic of korea. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Erlangga, D.; Ali, S.; Bloor, K. The impact of public health insurance on healthcare utilisation in indonesia: Evidence from panel data. Int. J. Pub. Health 2019, 64, 603–613. Available online: https://www.ncbi.nlm.nih.gov/pubmed/30737522 (accessed on 10 January 2022). [CrossRef] [Green Version]
- Blackman, I.R.; Mannix, T.; Sinclair, P.M. Developing renal nurses’ buttonhole cannulation skills using e-learning. J. Ren. Care 2014, 40, 55–63. Available online: https://www.ncbi.nlm.nih.gov/pubmed/24479841 (accessed on 10 January 2022). [CrossRef]
- Shen, D.; Cho, M.-H.; Tsai, C.-L.; Marra, R. Unpacking online learning experiences: Online learning self-efficacy and learning satisfaction. Internet High. Educ. 2013, 19, 10–17. [Google Scholar] [CrossRef]
- Bennett, P.N.; Jaeschke, S.; Sinclair, P.M.; Kerr, P.G.; Holt, S.; Schoch, M.; Fortnum, D.; Ockerby, C.; Kent, B. Increasing home dialysis knowledge through a web-based e-learning program. Nephrology 2014, 19, 345–351. [Google Scholar] [CrossRef]
- Estrella, M.M.; Sisson, S.D.; Roth, J.; Choi, M.J. Efficacy of an internet-based tool for improving physician knowledge of chronic kidney disease: An observational study. BMC Nephrol. 2012, 13, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Larsen, R.; Zahner, S.J. The impact of web-delivered education on preceptor role self-efficacy and knowledge in public health nurses. Pub. Health Nurs. 2011, 28, 349–356. [Google Scholar] [CrossRef]
- Cook, D.A.; Levinson, A.J.; Garside, S.; Dupras, D.M.; Erwin, P.J.; Montori, V.M. Internet-based learning in the health professions: A meta-analysis. Jama 2008, 300, 1181–1196. [Google Scholar] [CrossRef]
- Liaw, S.-S. Investigating students’ perceived satisfaction, behavioral intention, and effectiveness of e-learning: A case study of the blackboard system. Comput. Educ. 2008, 51, 864–873. [Google Scholar] [CrossRef]
- Sun, P.-C.; Tsai, R.J.; Finger, G.; Chen, Y.-Y.; Yeh, D. What drives a successful e-learning? An empirical investigation of the critical factors influencing learner satisfaction. Comput. Educ. 2008, 50, 1183–1202. [Google Scholar] [CrossRef]
| (a) | ||||
|---|---|---|---|---|
| Region | Completeness (%) | |||
| 2017 | 2018 | 2019 | 2020 | |
| Sumatra | 59.86 ± 22.5 | 71.60 ± 24.9 | 85.75 ± 19.9 | 66.36 ± 30.7 |
| Java and Bali | 78.77 ± 21.8 | 85.18 ± 15.0 | 89.67 ± 10.5 | 59.33 ± 28.0 |
| Kalimantan | 66.81 ± 16.3 | 63.85 ± 17.4 | 80.07 ± 18.4 | 48.99 ± 15.5 |
| Sulawesi | 46.03 ± 14.7 | 65.15 ± 13.0 | 80.52 ± 11.5 | 65.93 ± 29.0 |
| Nusa Tenggara | 62.27 ± 10.9 | 64.55 ± 7.71 | 79.09 ± 15.4 | 93.18 ± 9.6 |
| Maluku and Papua | 14.73 ± 20.9 | 31.63 ± 32.0 | 46.10 ± 24.8 | 29.00 ± 19.0 |
| (b) | ||||
| Region | Timeliness (%) | |||
| 2017 | 2018 | 2019 | 2020 | |
| Sumatra | 59.86 ± 22.5 | 72.04 ± 24.1 | 85.75 ± 19.9 | 52.04 ± 34.1 |
| Java and Bali | 78.77 ± 21.8 | 85.18 ± 15.0 | 89.67 ± 10.5 | 48.15 ± 29.6 |
| Kalimantan | 66.81 ± 16.3 | 63.85 ± 17.4 | 80.07 ± 18.4 | 26.37 ± 20.1 |
| Sulawesi | 46.03 ± 14.7 | 65.15 ± 13.0 | 80.52 ± 11.5 | 38.94 ± 30.3 |
| Nusa Tenggara | 62.27 ± 10.9 | 64.55 ± 7.7 | 79.09 ± 15.4 | 53.18 ± 23.7 |
| Maluku and Papua | 14.73 ± 20.9 | 31.63 ± 32.0 | 46.10 ± 24.8 | 19.21 ± 24.3 |
| (a) | |||
|---|---|---|---|
| Year | 2017 | 2018 | 2019 |
| 2018 | 0.28 | ||
| 2019 | <0.001 * | 0.08 | |
| 2020 | 0.98 | 0.38 | 0.03 * |
| (b) | |||
| Year | 2017 | 2018 | 2019 |
| 2018 | 0.09 | ||
| 2019 | <0.001 * | 0.05 | |
| 2020 | 0.05 | <0.001 * | <0.001 * |
| Themes | Sub-Themes | Selected Quote |
|---|---|---|
| System Description | Mandatory program at all health office |
|
| Surveillance on potentially outbreak and vaccine preventable diseases |
| |
| Alert and response |
| |
| Outbreak Detection | Aggregate data reporting |
|
| Online collaboration |
| |
| Implementation Challenges | Human resource limitation |
|
| ||
| Increasing workload |
| |
| ||
| ||
| Management shifting |
| |
| ||
| Improvement Strategy | Additional epidemiologists at primary health care level |
|
| ||
| Interoperability with laboratory | “I hope SKDR is not only limited to monitoring for suspects but also being able to link with the laboratory…” |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardhantyo, M.; Djasri, H.; Nursetyo, A.A.; Yulianti, A.; Adipradipta, B.R.; Hawley, W.; Mika, J.; Praptiningsih, C.Y.; Mangiri, A.; Prasetyowati, E.B.; et al. Quality of National Disease Surveillance Reporting before and during COVID-19: A Mixed-Method Study in Indonesia. Int. J. Environ. Res. Public Health 2022, 19, 2728. https://doi.org/10.3390/ijerph19052728
Hardhantyo M, Djasri H, Nursetyo AA, Yulianti A, Adipradipta BR, Hawley W, Mika J, Praptiningsih CY, Mangiri A, Prasetyowati EB, et al. Quality of National Disease Surveillance Reporting before and during COVID-19: A Mixed-Method Study in Indonesia. International Journal of Environmental Research and Public Health. 2022; 19(5):2728. https://doi.org/10.3390/ijerph19052728
Chicago/Turabian StyleHardhantyo, Muhammad, Hanevi Djasri, Aldilas Achmad Nursetyo, Andriani Yulianti, Bernadeta Rachela Adipradipta, William Hawley, Jennifer Mika, Catharina Yekti Praptiningsih, Amalya Mangiri, Endang Burni Prasetyowati, and et al. 2022. "Quality of National Disease Surveillance Reporting before and during COVID-19: A Mixed-Method Study in Indonesia" International Journal of Environmental Research and Public Health 19, no. 5: 2728. https://doi.org/10.3390/ijerph19052728
APA StyleHardhantyo, M., Djasri, H., Nursetyo, A. A., Yulianti, A., Adipradipta, B. R., Hawley, W., Mika, J., Praptiningsih, C. Y., Mangiri, A., Prasetyowati, E. B., & Brye, L. (2022). Quality of National Disease Surveillance Reporting before and during COVID-19: A Mixed-Method Study in Indonesia. International Journal of Environmental Research and Public Health, 19(5), 2728. https://doi.org/10.3390/ijerph19052728







