Isolated Hypoglossal Nerve Palsy as an Early Symptom of a Granular Cell Tumor
Abstract
1. Introduction
2. Case Report
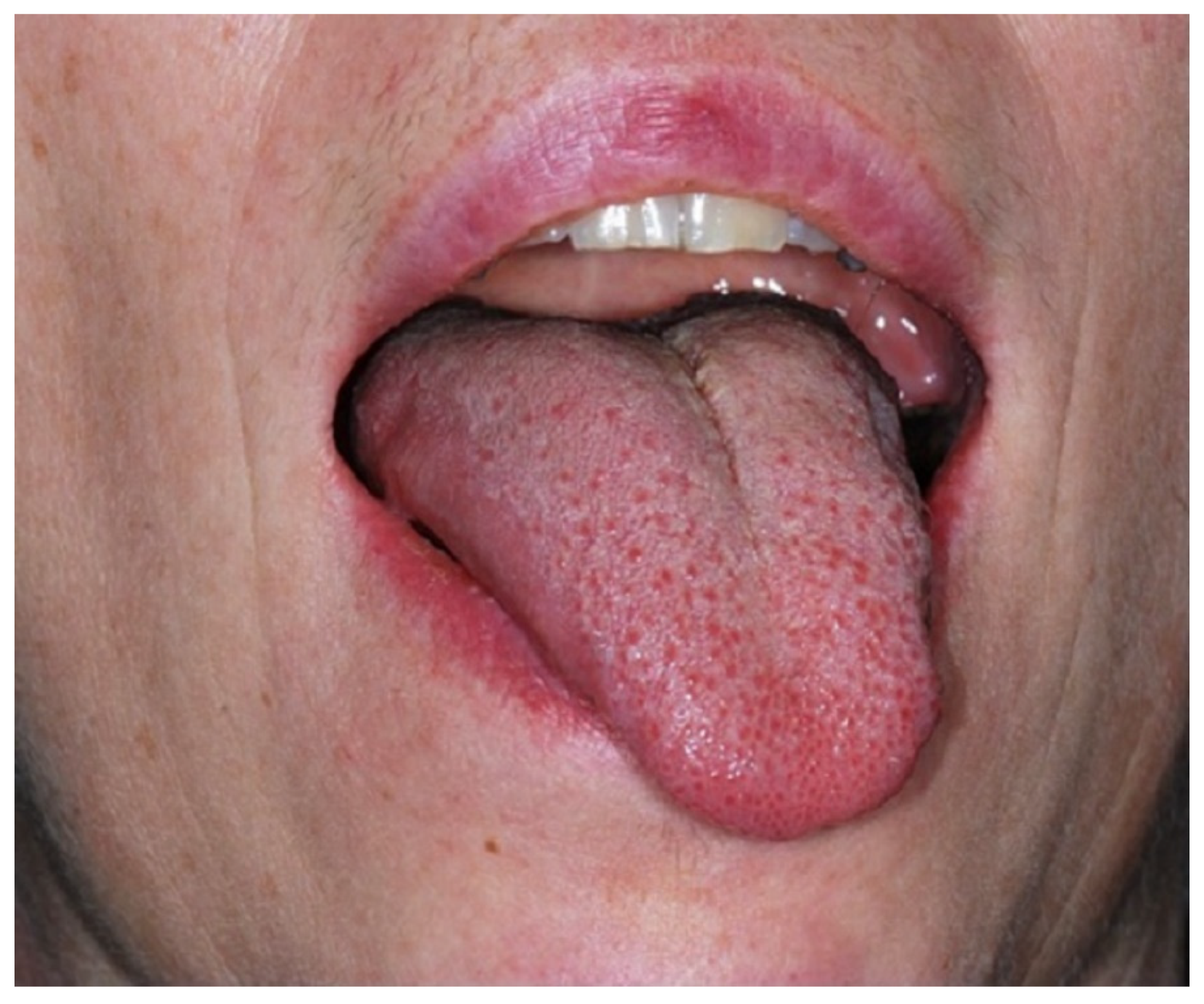
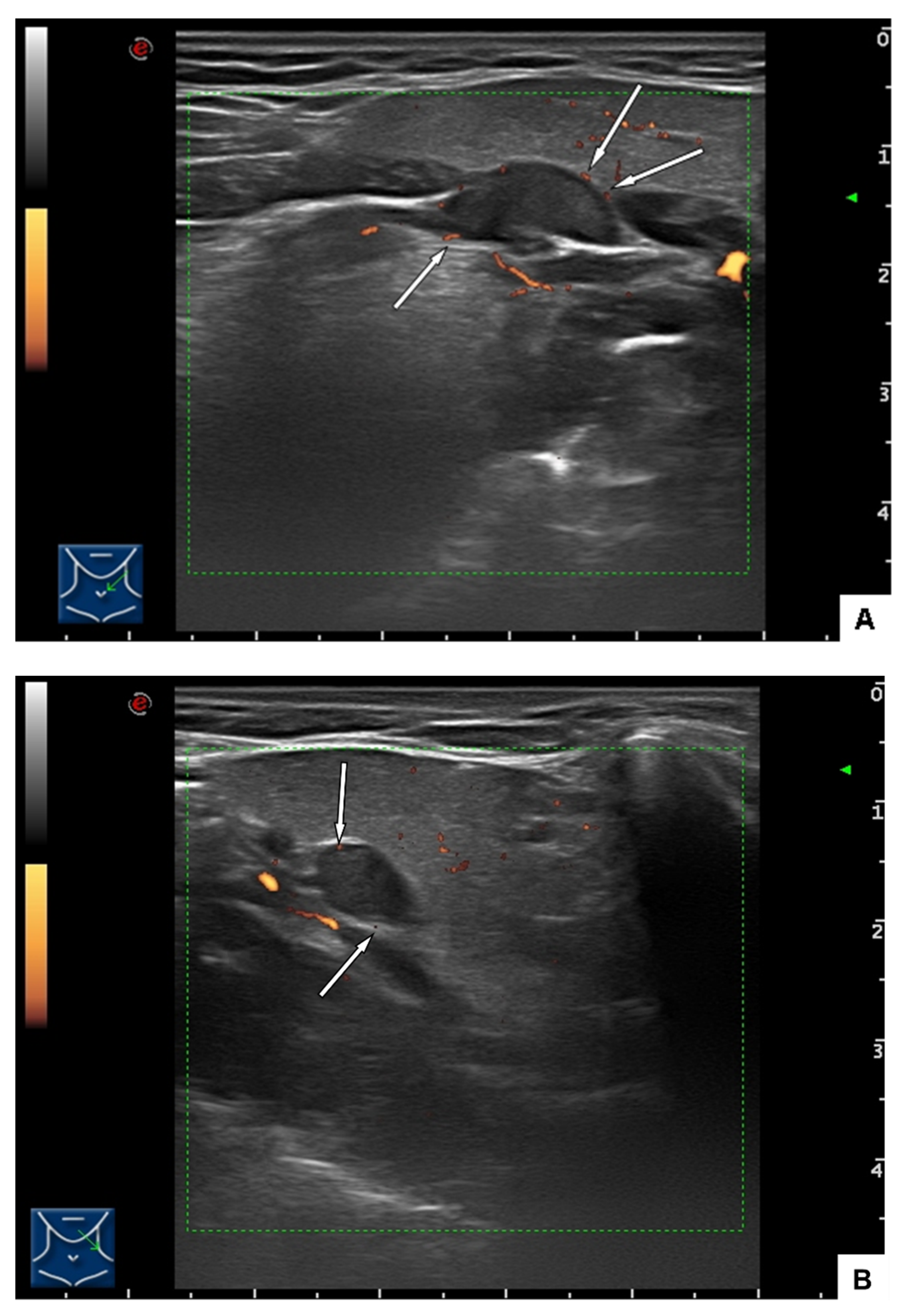
2.1. Clinical Findings and Imaging
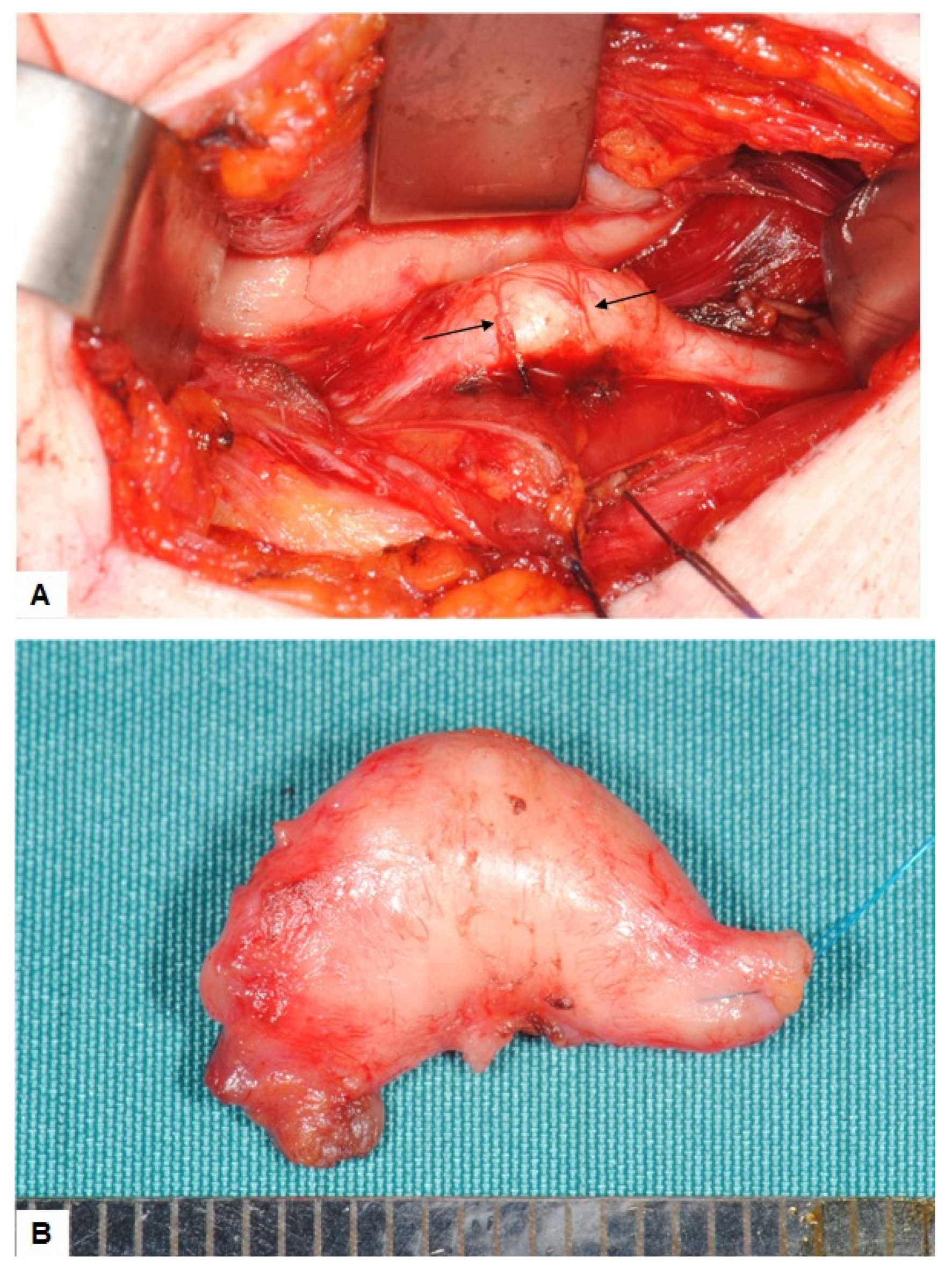
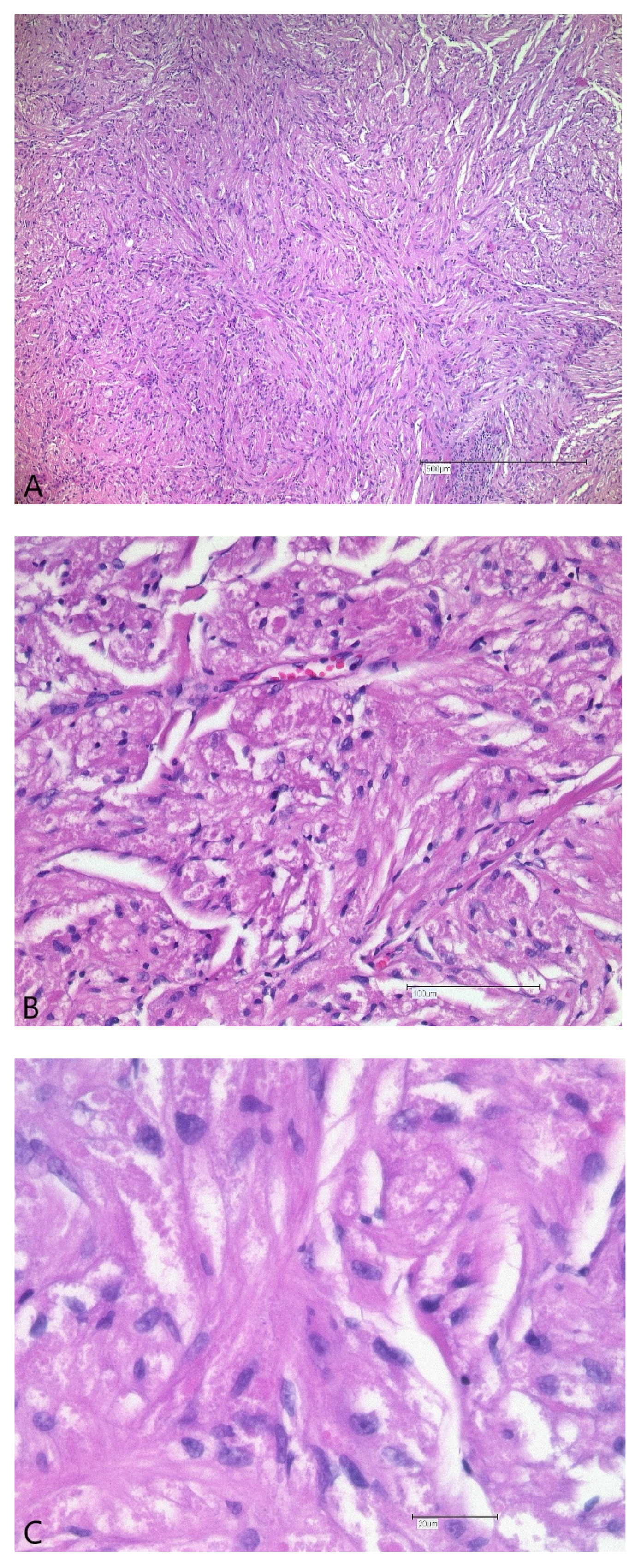
2.2. Surgical Treatment and Outcome
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boban, M.; Brinar, V.V.; Habek, M.; Rados, M. Isolated hypoglossal nerve palsy: A diagnostic challenge. Eur. Neurol. 2007, 58, 177–181. [Google Scholar] [CrossRef]
- Alharbi, F.A.; Lenarz, T.; Stoever, T. A case of unilateral hypoglossus nerve palsy associated with chordoma in the region of clivus. Eur. Arch. Oto-Rhino-Laryngol. 2009, 266, 2001–2003. [Google Scholar] [CrossRef] [PubMed]
- Hewett, R.M.; Stewart, G.E. Neurological picture. Isolated hypoglossal nerve palsy caused by synovial cyst. J. Neurol. Neurosurg. Psychiatry 2011, 82, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Bryer, E.; Henry, D. Isolated hypoglossal nerve palsy as a presenting symptom of metastatic peripheral T-cell lymphoma—Not otherwise specified (PTCL-NOS): A unique case & a review of the literature. Int. J. Hematol. Oncol. 2018, 7, IJH03. [Google Scholar] [CrossRef] [PubMed]
- Cant, A.; Collard, B. Oral medicine: Isolated unilateral hypoglossal nerve palsy. Br. Dent. J. 2018, 225, 95. [Google Scholar] [CrossRef]
- Edizer, D.T.; Mercan, H.; Cansiz, H. Hypoglossal schwannoma presenting only with headache. J. Craniofac. Surg. 2010, 21, 261–262. [Google Scholar] [CrossRef]
- Keane, J.R. Twelfth-nerve palsy. Analysis of 100 cases. Arch. Neurol. 1996, 53, 561–566. [Google Scholar] [CrossRef]
- Khoo, S.G.; Ullah, I.; Wallis, F.; Fenton, J.E. Isolated hypoglossal nerve palsy: A harbinger of malignancy. J. Laryngol. Otol. 2007, 121, 803–805. [Google Scholar] [CrossRef]
- Mokri, B.; Silbert, P.L.; Schievink, W.I.; Piepgras, D.G. Cranial nerve palsy in spontaneous dissection of the extracranial internal carotid artery. Neurology 1996, 46, 356–359. [Google Scholar] [CrossRef]
- Binzer, S.; Petkova, V. 12th nerve palsy in a woman with extracranial internal carotid artery aneurysm. Ugeskr. Laeger 2020, 182, 1–3. [Google Scholar]
- Koscielny, S.; Koch, J.; Behrendt, W. Aneurysma der A. carotis interna. Die besondere Differenzialdiagnose einer Parese der kaudalen Hirnnerven. HNO 2003, 51, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Combarros, O.; Alvarez de Arcaya, A.; Quintana, F.; Bèrciano, J. Parálisis del nervio hipogloso como forma de presentación de una fístula arteriovenosa dural de la fosa posterior. Neurologia 1998, 13, 260–262. [Google Scholar] [PubMed]
- Cartwright, M.J.; Eisenberg, M.B.; Page, L.K. Posterior fossa arachnoid cyst presenting with an isolated twelfth nerve paresis. Case report and review of the literature. Clin. Neurol. Neurosurg. 1991, 93, 69–72. [Google Scholar] [CrossRef]
- Tarantino, R.; Marruzzo, D.; Colistra, D.; Mancarella, C.; Delfini, R. Twelfth nerve paresis induced by an unusual posterior fossa arachnoid cyst: Case report and literature review. Br. J. Neurosurg. 2014, 28, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Mujic, A.; Hunn, A.; Liddell, J.; Taylor, B.; Havlat, M.; Beasley, T. Isolated unilateral hypoglossal nerve paralysis caused by an atlanto-occipital joint synovial cyst. J. Clin. Neurosci. 2003, 10, 492–495. [Google Scholar] [CrossRef]
- Gassie, K.; Grewal, S.; Chen, S.G. Atlantooccipital Synovial Cyst with Isolated Hypoglossal Nerve Palsy: Case Report of Nonfusion Surgical Approach and Review of Literature. World Neurosurg. 2019, 126, 434–438. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, J.Y. Isolated unilateral paralysis of the hypoglossal nerve after transoral intubation for general anesthesia. Dysphagia 2009, 24, 354–356. [Google Scholar] [CrossRef]
- Rubio-Nazábal, E.; Marey-Lopez, J.; Lopez-Facal, S.; Alvarez-Perez, P.; Martinez-Figueroa, A.; Del Rey Corral, P. Isolated bilateral paralysis of the hypoglossal nerve after transoral intubation for general anesthesia. Anesthesiology 2002, 96, 245–247. [Google Scholar] [CrossRef]
- Schmidt, T.; Philipsen, B.B.; Manhoobi, Y.; Bruun Christiansen, E.L. Vagus and hypoglossus palsy after nasotracheal intubation and throat packing. Ugeskr. Laeger 2018, 180, 2–3. [Google Scholar]
- Ulusoy, H.; Besir, A.; Cekic, B.; Kosucu, M.; Geze, S. Transient unilateral combined paresis of the hypoglossal nerve and lingual nerve following intubation anesthesia. Bras. J. Anesthesiol. 2014, 64, 124–127. [Google Scholar] [CrossRef]
- King, C.; Street, M.K. Twelfth cranial nerve paralysis following use of a laryngeal mask airway. Anaesthesia 1994, 49, 786–787. [Google Scholar] [CrossRef] [PubMed]
- Tham, L.Y.; Beh, Z.Y.; Shariffuddin, I.I.; Wang, C.Y. Unilateral hypoglossal nerve palsy after the use of laryngeal mask airway (LMA) Protector. Korean J. Anesthesiol. 2019, 72, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Lao, I.W.; Ji, Q. Granular cell tumor of the hypoglossal nerve presenting with tonque atrophy and deviation: A case report. Int. J. Clin. Exp. Pathol. 2016, 9, 2504–2507. [Google Scholar]
- Abrikossoff, A. Über Myome. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 1926, 260, 215–233. [Google Scholar] [CrossRef]
- Papachristou, D.J.; Palekar, A.; Surti, U.; Cieply, K.; McGough, R.L.; Rao, U.N.M. Malignant granular cell tumor of the ulnar nerve with novel cytogenetic and molecular genetic findings. Cancer Genet. Cytogenet. 2009, 191, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, M.; Sadamoto, A.; Murakami, S. Malignant granular cell tumour of the cervical sympathetic nerve trunk. J. Laryngol. Otol. 2001, 115, 833–835. [Google Scholar] [CrossRef]
- Tsuchida, T.; Okada, K.; Itoi, E.; Sato, T.; Sato, K. Intramuscular malignant granular cell tumor. Skelet. Radiol. 1997, 26, 116–121. [Google Scholar] [CrossRef]
- Hurrell, M.A.; McLean, C.; Desmond, P.; Tress, B.M.; Kaye, A. Malignant granular cell tumour of the sciatic nerve. Australas. Radiol. 1995, 39, 86–89. [Google Scholar] [CrossRef]
- Mirza, F.N.; Tuggle, C.T.; Zogg, C.K.; Mirza, H.N.; Narayan, D. Epidemiology of malignant cutaneous granular cell tumors: A US population-based cohort analysis using the Surveillance, Epidemiology, and End Results (SEER) database. J. Am. Acad. Dermatol. 2018, 78, 490–497.e1. [Google Scholar] [CrossRef]
- Marolleau, F.; Baert, F.; Mertens, V.; Ghillebert, G. Abrikossoff cell tumor of the oesophagus: A case report and review of the literature. Acta Clin. Belg. 2008, 63, 273–276. [Google Scholar] [CrossRef]
- Ordóñez, N.G.; Mackay, B. Granular cell tumor: A review of the pathology and histogenesis. Ultrastruct. Pathol. 1999, 23, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Dogramaci, Y.; Kalaci, A.; Sevinc, T.T.; Hakverdi, S.; Canda, S.; Yanat, A.N. Granular cell tumor of the posterior tibial nerve as a rare cause of heel pain: A case report. J. Am. Podiatr. Med. Assoc. 2009, 99, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Brannon, R.B.; Anand, P.M. Oral granular cell tumors: An analysis of 10 new pediatric and adolescent cases and a review of the literature. J. Clin. Pediatr. Dent. 2004, 29, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Lack, E.E.; Worsham, G.F.; Callihan, M.D.; Crawford, B.E.; Klappenbach, S.; Rowden, G.; Chun, B. Granular cell tumor: A clinicopathologic study of 110 patients. J. Surg. Oncol. 1980, 13, 301–316. [Google Scholar] [CrossRef]
- Charles, N.C.; Fox, D.M.; Glasberg, S.S.; Sawicki, J. Epibulbar granular cell tumor. Report of a case and review of the literature. Ophthalmology 1997, 104, 1454–1456. [Google Scholar] [CrossRef]
- Butler, J.D.; Brown, K.M. Granular cell tumor of the extrahepatic biliary tract. Am. Surg. 1998, 64, 1033–1036. [Google Scholar]
- Billeret Lebranchu, V. La tumeur à cellules granuleuses. Epidémiologie de 263 cas. Arch. Anat. Cytol. Pathol. 1999, 47, 26–30. [Google Scholar]
- Victoria, L.V.; Hoffman, H.T.; Robinson, R.A. Granular cell tumour of the larynx. J. Laryngol. Otol. 1998, 112, 373–376. [Google Scholar] [CrossRef]
- Abdulhamid, I.; Rabah, R. Granular cell tumor of the bronchus. Pediatr. Pulmonol. 2000, 30, 425–428. [Google Scholar] [CrossRef][Green Version]
- Adeniran, A.; Al-Ahmadie, H.; Mahoney, M.C.; Robinson-Smith, T.M. Granular cell tumor of the breast: A series of 17 cases and review of the literature. Breast J. 2004, 10, 528–531. [Google Scholar] [CrossRef]
- Daentzer, D.; Schmidinger, A.; Böker, D.-K. Atypical granular cell tumour of the sural nerve mimicking a Schwannoma. Acta Neurochir. 2003, 145, 1019–1020. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, L.B.; Lorentzen, M.; Besjakov, J.; Lundborg, G. Granular cell tumour of the ulnar nerve in a young adult. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2002, 36, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, I.; Bray, P.; Ghazarian, D. Plexiform intraneural granular cell tumour of a digital cutaneous sensory nerve. J. Clin. Pathol. 2007, 60, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Bue, P.; Holck, S.; Holst-Nielsen, F. Granulaercelletumor lokaliseret til en fingernerve. Ugeskr. Laeger 1984, 146, 2319–2320. [Google Scholar]
- Toker, C. Neural origin of granular cell tumor. Mt. Sinai J. Med. 1974, 41, 655–657. [Google Scholar]
- López-Jornet, P. Granular cell tumor of the tongue. N. Y. State Dent. J. 2008, 74, 71–72. [Google Scholar]
- Mackenzie, D.J.; Klapper, E.; Gordon, L.A.; Silberman, A.W. Granular cell tumor of the biliary system. Med. Pediatr. Oncol. 1994, 23, 50–56. [Google Scholar] [CrossRef]
- Sobel, H.J.; Marquet, E.; Avrin, E.; Schwarz, R. Granular cell myoblastoma. An electron microscopic and cytochemical study illustrating the genesis of granules and aging of myoblastoma cells. Am. J. Pathol. 1971, 65, 59–78. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemound, J.; Papadimas, D.; Skodda, S.; Tannapfel, A.; Alekseyev, A.; Kunkel, M. Isolated Hypoglossal Nerve Palsy as an Early Symptom of a Granular Cell Tumor. Int. J. Environ. Res. Public Health 2022, 19, 2690. https://doi.org/10.3390/ijerph19052690
Lemound J, Papadimas D, Skodda S, Tannapfel A, Alekseyev A, Kunkel M. Isolated Hypoglossal Nerve Palsy as an Early Symptom of a Granular Cell Tumor. International Journal of Environmental Research and Public Health. 2022; 19(5):2690. https://doi.org/10.3390/ijerph19052690
Chicago/Turabian StyleLemound, Juliana, Dimitrios Papadimas, Sabine Skodda, Andrea Tannapfel, Anriy Alekseyev, and Martin Kunkel. 2022. "Isolated Hypoglossal Nerve Palsy as an Early Symptom of a Granular Cell Tumor" International Journal of Environmental Research and Public Health 19, no. 5: 2690. https://doi.org/10.3390/ijerph19052690
APA StyleLemound, J., Papadimas, D., Skodda, S., Tannapfel, A., Alekseyev, A., & Kunkel, M. (2022). Isolated Hypoglossal Nerve Palsy as an Early Symptom of a Granular Cell Tumor. International Journal of Environmental Research and Public Health, 19(5), 2690. https://doi.org/10.3390/ijerph19052690





