Patients with Prior Craniectomy or Craniotomy Have No Increased Risk of Acute Hemorrhage after Mild Traumatic Brain Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Treatment Procedures in TBI Patients Admitted to Our Ward
2.2.1. First-Line Therapy
2.2.2. Radiological Assessment and Continued Treatment
2.2.3. Postoperative Treatment Protocol
2.2.4. Follow-Up Examination
2.3. Data Analysis
3. Results
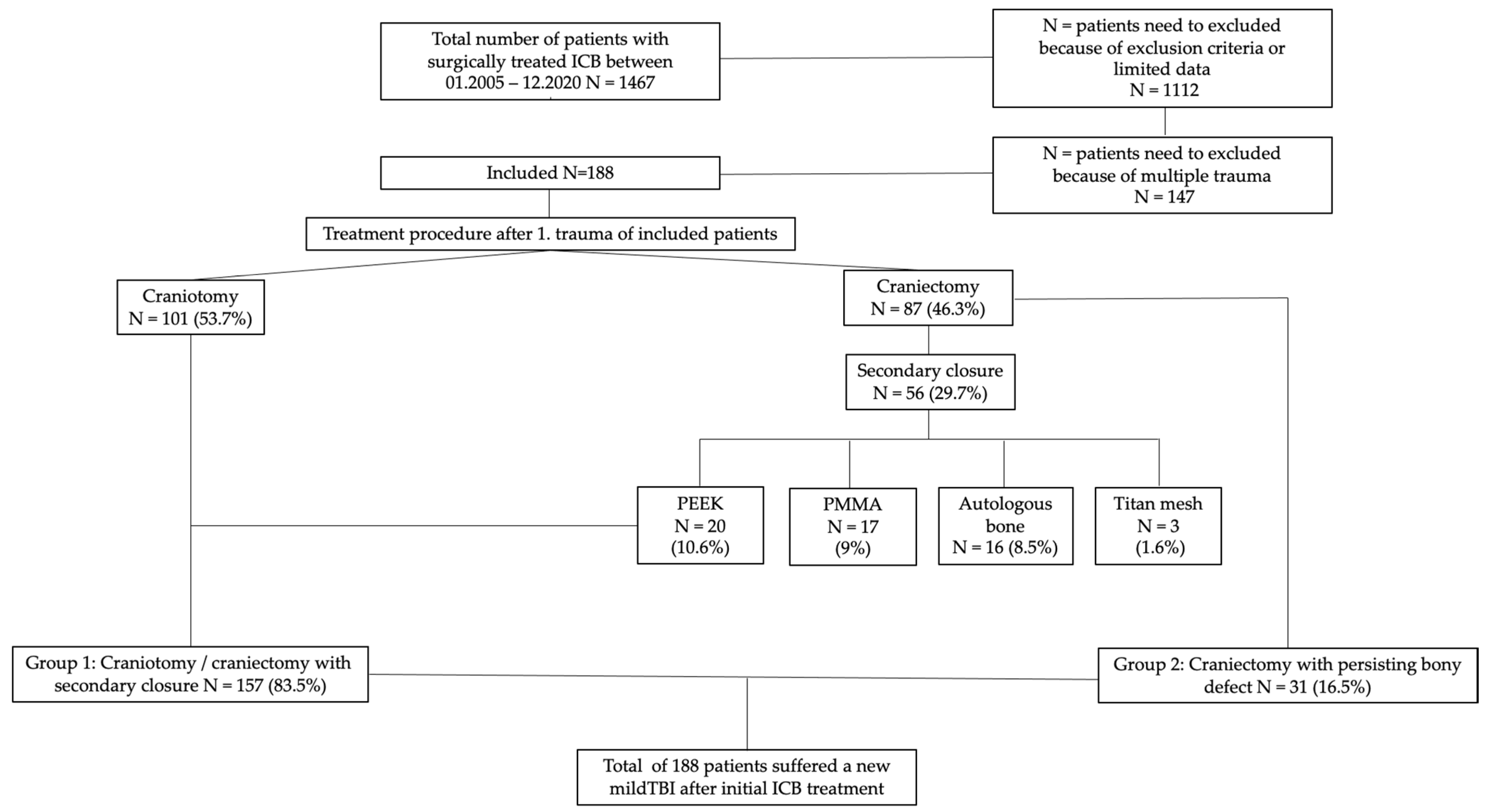
3.1. Patients
3.1.1. Repeat Mild TBI
3.1.2. Treatment Factors in Surgically Managed Patients
3.1.3. Therapeutic Anticoagulation
3.1.4. Bleedings after Repeated TBI
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hyder, A.A.; Wunderlich, C.A.; Puvanachandra, P.; Gururaj, G.; Kobusingye, O.C. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation 2007, 22, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Tagliaferri, F.; Compagnone, C.; Korsic, M.; Servadei, F.; Kraus, J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir. 2006, 148, 255–268, discussion 268. [Google Scholar] [CrossRef] [PubMed]
- McMillan, T.M.; Weir, C.J.; Wainman-Lefley, J. Mortality and morbidity 15 years after hospital admission with mild head injury: A prospective case-controlled population study. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1214–1220. [Google Scholar] [CrossRef]
- Mauritz, W.; Brazinova, A.; Majdan, M.; Leitgeb, J. Epidemiology of traumatic brain injury in Austria. Wien. Klin. Wochenschr. 2014, 126, 42–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niemeier, J.P.; Grafton, L.M.; Chilakamarri, T. Treating persons with traumatic brain injury: History and updates. N. Carol. Med. J. 2015, 76, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackenberg, K.; Unterberg, A. Traumatic brain injury. Nervenarzt 2016, 87, 203–214. [Google Scholar] [CrossRef]
- Thompson, H.J.; McCormick, W.C.; Kagan, S.H. Traumatic brain injury in older adults: Epidemiology, outcomes, and future implications. J. Am. Geriatr. Soc. 2006, 54, 1590–1595. [Google Scholar] [CrossRef]
- Perel, P.; Roberts, I.; Bouamra, O.; Woodford, M.; Mooney, J.; Lecky, F. Intracranial bleeding in patients with traumatic brain injury: A prognostic study. BMC Emerg. Med. 2009, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Alvis-Miranda, H.; Castellar-Leones, S.M.; Moscote-Salazar, L.R. Decompressive Craniectomy and Traumatic Brain Injury: A Review. Bull. Emerg. Trauma 2013, 1, 60–68. [Google Scholar] [PubMed]
- Worm, P.V.; Ferreira, N.P.; Finger, G.; Collares, M.V. Autologous cranial bone graft use for trepanation reconstruction. J Cranio-Maxillofac. Surg. 2015, 43, 1781–1784. [Google Scholar] [CrossRef]
- Anton, J.V.; Winkler, P.A. Dekompressive Kraniektomie in der Neurotraumatologie. J. Neurol. Neurochir. Psychiatr. 2015, 16, 103–110. [Google Scholar]
- Kwon, Y.S.; Yang, K.H.; Lee, Y.H. Craniotomy or Decompressive Craniectomy for Acute Subdural Hematomas: Surgical Selection and Clinical Outcome. Korean J. Neurotrauma 2016, 12, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khellaf, A.; Khan, D.Z.; Helmy, A. Recent advances in traumatic brain injury. J. Neurol. 2019, 266, 2878–2889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikmen, S.S.; Machamer, J.E.; Powell, J.M.; Temkin, N.R. Outcome 3 to 5 years after moderate to severe traumatic brain injury. Arch. Phys. Med. Rehabil. 2003, 84, 1449–1457. [Google Scholar] [CrossRef]
- Albers, C.E.; von Allmen, M.; Evangelopoulos, D.S.; Zisakis, A.K.; Zimmermann, H.; Exadaktylos, A.K. What is the incidence of intracranial bleeding in patients with mild traumatic brain injury? A retrospective study in 3088 Canadian CT head rule patients. BioMed Res. Int. 2013, 2013, 453978. [Google Scholar] [CrossRef]
- Parra, M.W.; Zucker, L.; Johnson, E.S.; Gullett, D.; Avila, C.; Wichner, Z.A.; Kokaram, C.R. Dabigatran bleed risk with closed head injuries: Are we prepared? J. Neurosurg. 2013, 119, 760–765. [Google Scholar] [CrossRef] [Green Version]
- Menditto, V.G.; Lucci, M.; Polonara, S.; Pomponio, G.; Gabrielli, A. Management of minor head injury in patients receiving oral anticoagulant therapy: A prospective study of a 24-hour observation protocol. Ann. Emerg. Med. 2012, 59, 451–455. [Google Scholar] [CrossRef]
- Nishijima, D.K.; Offerman, S.R.; Ballard, D.W.; Vinson, D.R.; Chettipally, U.K.; Rauchwerger, A.S.; Reed, M.E.; Holmes, J.F.; Clinical Research in Emergency Services and Treatment (CREST) Network; Treatment, N. Immediate and delayed traumatic intracranial hemorrhage in patients with head trauma and preinjury warfarin or clopidogrel use. Ann. Emerg. Med. 2012, 59, 460–468.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peck, K.A.; Sise, C.B.; Shackford, S.R.; Sise, M.J.; Calvo, R.Y.; Sack, D.I.; Walker, S.B.; Schechter, M.S. Delayed intracranial hemorrhage after blunt trauma: Are patients on preinjury anticoagulants and prescription antiplatelet agents at risk? J. Trauma 2011, 71, 1600–1604. [Google Scholar] [CrossRef]
- Antoni, A.; Schwendenwein, E.; Binder, H.; Schauperl, M.; Datler, P.; Hajdu, S. Delayed Intracranial Hemorrhage in Patients with Head Trauma and Antithrombotic Therapy. J. Clin. Med. 2019, 8, 1780. [Google Scholar] [CrossRef] [Green Version]
- Jagoda, A.S.; Bazarian, J.J.; Bruns, J.J., Jr.; Cantrill, S.V.; Gean, A.D.; Howard, P.K.; Ghajar, J.; Riggio, S.; Wright, D.W.; Wears, R.L.; et al. Clinical policy: Neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann. Emerg. Med. 2008, 52, 714–748. [Google Scholar] [CrossRef] [PubMed]
- Kuczawski, M.; Stevenson, M.; Goodacre, S.; Teare, M.D.; Ramlakhan, S.; Morris, F.; Mason, S. Should all anticoagulated patients with head injury receive a CT scan? Decision-analysis modelling of an observational cohort. BMJ Open 2016, 6, e013742. [Google Scholar] [CrossRef] [PubMed]
- Unden, J.; Ingebrigtsen, T.; Romner, B.; Scandinavian Neurotrauma, C. Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: An evidence and consensus-based update. BMC Med. 2013, 11, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Craniectomy with Bony Defect (n = 31) | Craniotomy/Craniectomy with Secondary Closure (n = 157) | p-Values | |
|---|---|---|---|
| Age in years | 0.211 | ||
| 59.02 ± 17.81 | 56.03 ± 10.7 | ||
| Sex | 0.836 | ||
| male: 20 (64.5%) | male: 105 (66.9%) | ||
| female: 11 (35.5%) | female: 52 (33.1%) | ||
| Anticoagulation | 0.794 | ||
| yes: 10 (32.3%) | yes: 35 (22.3%) | ||
| no: 21 (67.7) | no: 122 (77.7%) | ||
| Trauma mechanism | |||
| Mild TBI: 31 (100%) | Mild TBI: 157 (100%) | ||
| Bleedings | 0.216 | ||
| 6 (19.4%) | 16 (10.2%) | ||
| SDB: 2 (6.5%) | SDB: 6 (3.8%) | ||
| EDH: 1 (3.2%) | EDH: 6 (3.8%) | ||
| ICB: 4 (12.9%) | ICB: 8 (5.1%) | ||
| SABL: 4 (12.9%) | SABL: 3 (1.9%) | ||
| GCS | 0.042 | ||
| 3–8: 4 (12.9%) | 3–8: 5 (3.2%) | ||
| 13–15: 27 (87.1%) | 13–15: 152 (96.8%) | ||
| Treatment necessary | 6 (100%) | 16 (100%) | |
| Surgical treatment | 1.000 | ||
| 2 (33.3%) | 5 (31.3%) | ||
| Conservative treatment | |||
| 4 (66.6%) | 11 (68.7%) | ||
| Type of Anticoagulation | Frequency | Percentage |
|---|---|---|
| T-Ass, Plavix, (Brillique) | 28 | 14.9 |
| Ivor, Fragmin, Lovenox | 12 | 6.4 |
| Marcoumar, Sintrom | 7 | 3.7 |
| Xarelto, Pradaxa, Lixiana | 7 | 3.7 |
| Total | 54 | 28.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binder, H.; Schallmeiner, D.; Tiefenboeck, T.M.; Payr, S.; Winnisch, M.; Kdolsky, R.; Hajdu, S.; Schwarz, G.M.; Hofbauer, M. Patients with Prior Craniectomy or Craniotomy Have No Increased Risk of Acute Hemorrhage after Mild Traumatic Brain Injury. Int. J. Environ. Res. Public Health 2022, 19, 2684. https://doi.org/10.3390/ijerph19052684
Binder H, Schallmeiner D, Tiefenboeck TM, Payr S, Winnisch M, Kdolsky R, Hajdu S, Schwarz GM, Hofbauer M. Patients with Prior Craniectomy or Craniotomy Have No Increased Risk of Acute Hemorrhage after Mild Traumatic Brain Injury. International Journal of Environmental Research and Public Health. 2022; 19(5):2684. https://doi.org/10.3390/ijerph19052684
Chicago/Turabian StyleBinder, Harald, Daniel Schallmeiner, Thomas M. Tiefenboeck, Stephan Payr, Markus Winnisch, Richard Kdolsky, Stefan Hajdu, Gilbert Manuel Schwarz, and Marcus Hofbauer. 2022. "Patients with Prior Craniectomy or Craniotomy Have No Increased Risk of Acute Hemorrhage after Mild Traumatic Brain Injury" International Journal of Environmental Research and Public Health 19, no. 5: 2684. https://doi.org/10.3390/ijerph19052684
APA StyleBinder, H., Schallmeiner, D., Tiefenboeck, T. M., Payr, S., Winnisch, M., Kdolsky, R., Hajdu, S., Schwarz, G. M., & Hofbauer, M. (2022). Patients with Prior Craniectomy or Craniotomy Have No Increased Risk of Acute Hemorrhage after Mild Traumatic Brain Injury. International Journal of Environmental Research and Public Health, 19(5), 2684. https://doi.org/10.3390/ijerph19052684








