The Pattern of Hemoglobin A1C Trajectories and Risk of Herpes Zoster Infection: A Follow-Up Study
Abstract
:1. Introduction
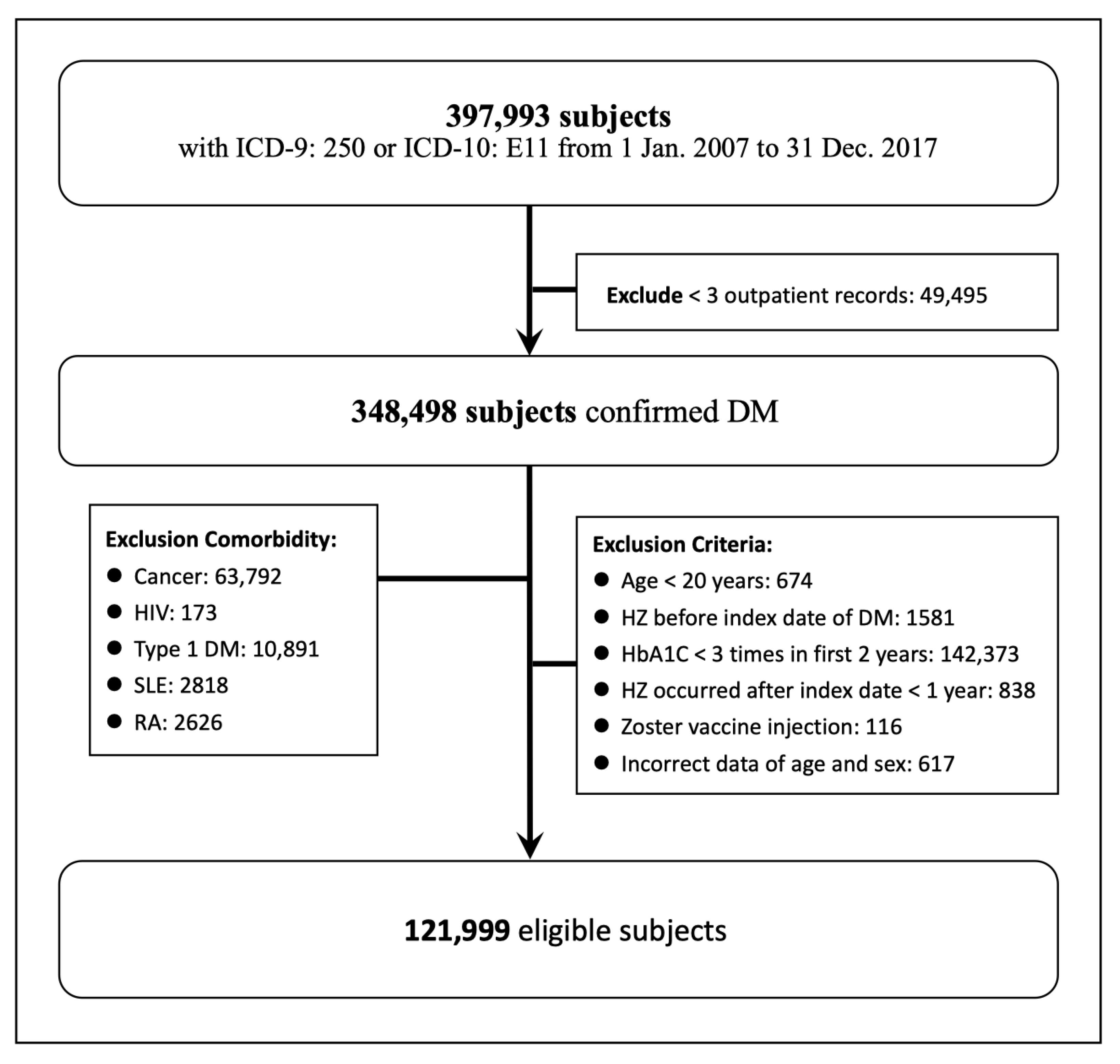
2. Materials and Methods
2.1. Study Design and Population
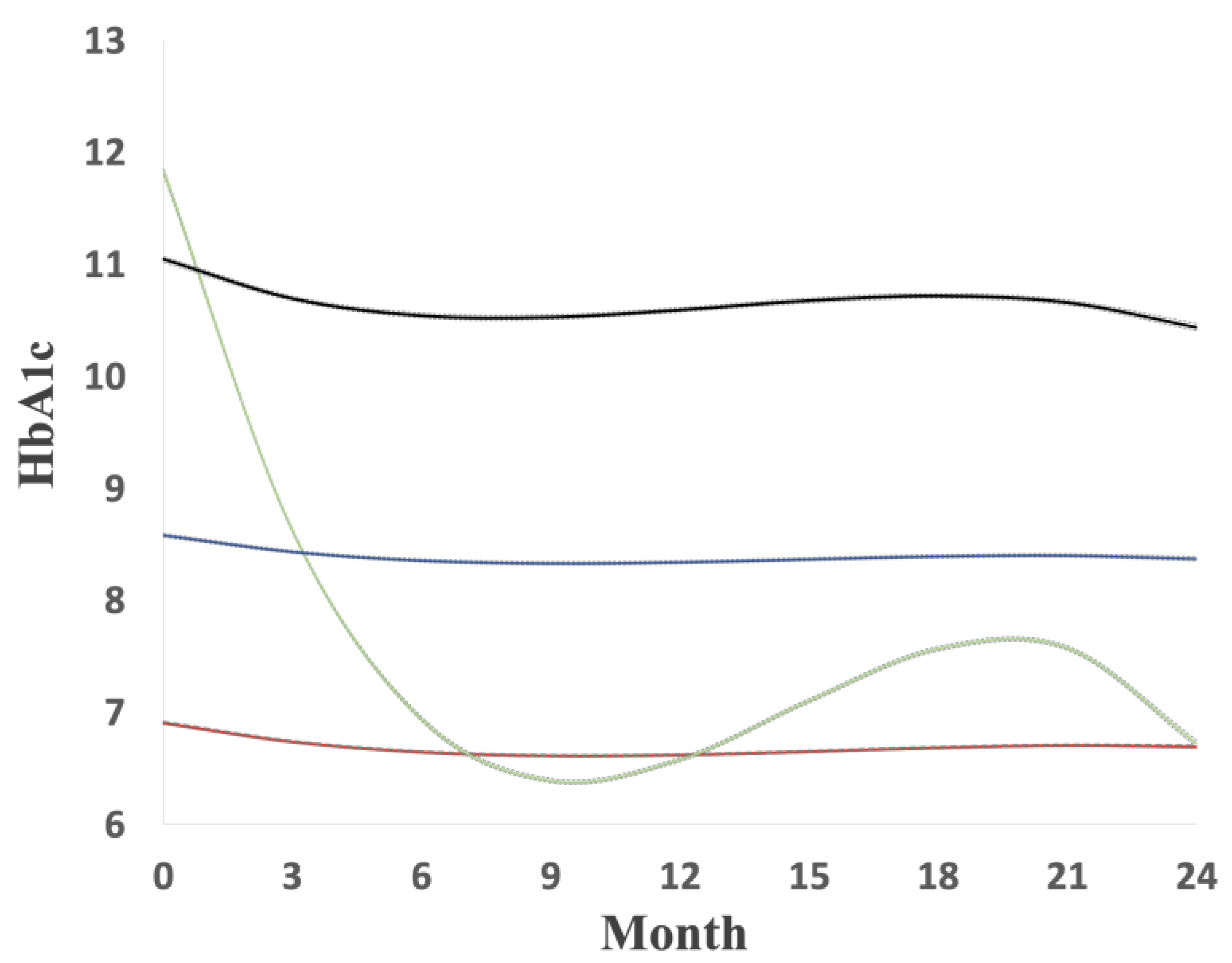
2.2. Glycated Hemoglobin Level
2.3. Outcome
2.4. Major Comorbidities
2.5. Statistical Analysis
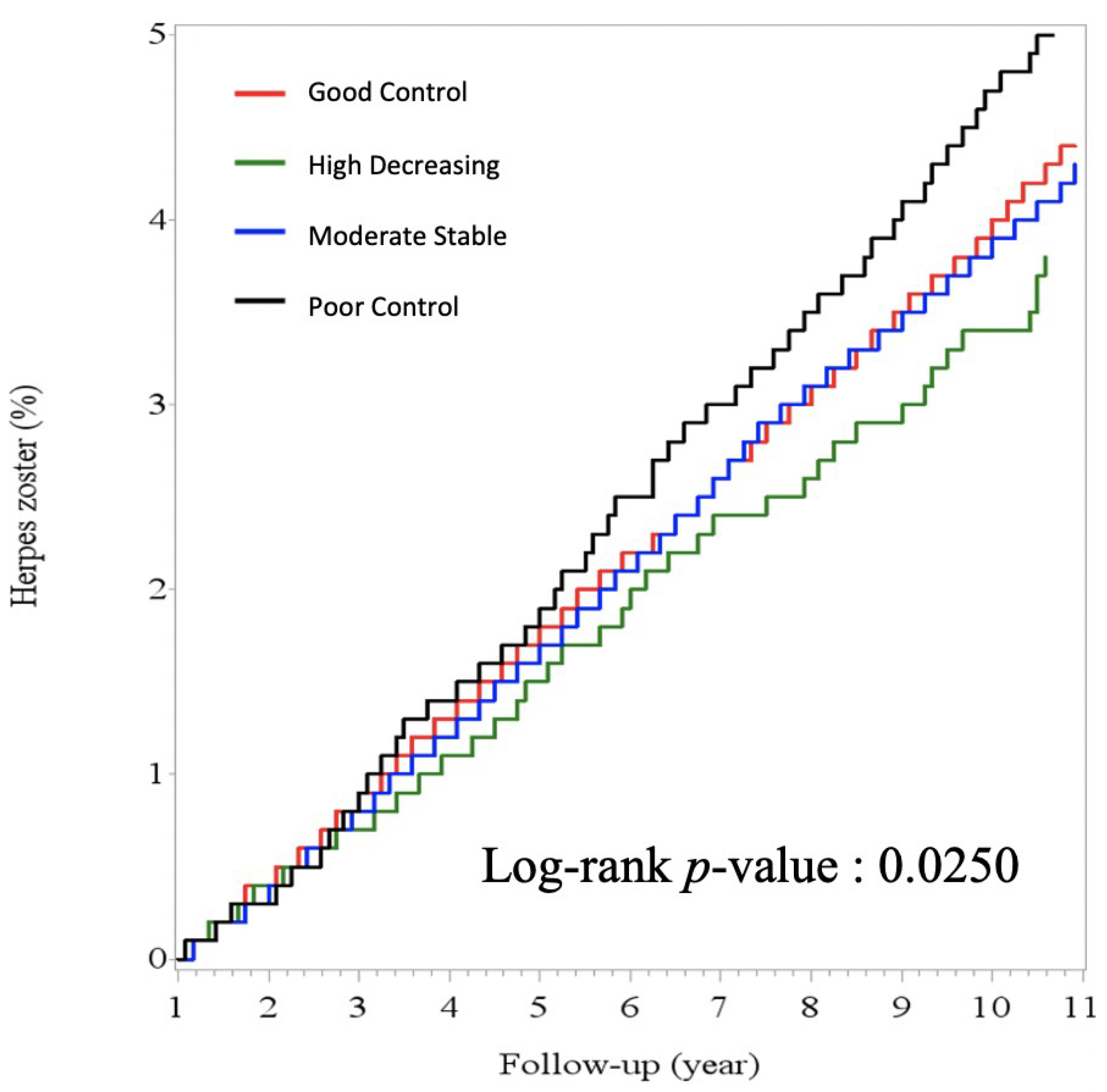
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, J.I. Clinical practice: Herpes zoster. N. Engl. J. Med. 2013, 369, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.M.; Han, J.; Yang, C.M.; Choi, C.Y.; Khoramnia, R.; Chung, T.-Y.; Lim, D.H. Chronic and recurrent herpes zoster ophthalmicus. Medicina 2021, 57, 999. [Google Scholar] [CrossRef] [PubMed]
- Jih, J.S.; Chen, Y.J.; Lin, M.W.; Chen, Y.C.; Chen, T.J.; Huang, Y.L.; Chen, C.C.; Lee, D.D.; Chang, Y.T.; Wang, W.J.; et al. Epidemiological features and costs of herpes zoster in Taiwan: A national study 2000 to 2006. Acta Derm. Venereol. 2009, 89, 612–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, C.C.; Lai, H.C.; Lin, C.H.; Hung, C.J.; Chen, D.Y.; Sheu, W.H.; Lui, P.W. Increased risk of herpes zoster in diabetic patients comorbid with coronary artery disease and microvascular disorders: A population-based study in taiwan. PLoS ONE 2016, 11, e0146750. [Google Scholar] [CrossRef] [Green Version]
- Hata, A.; Kuniyoshi, M.; Ohkusa, Y. Risk of Herpes zoster in patients with underlying diseases: A retrospective hospital-based cohort study. Infection 2011, 39, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, P.G.E.; Gershon, A.A. Clinical features of varicella-zoster virus infection. Viruses 2018, 10, 609. [Google Scholar] [CrossRef] [Green Version]
- Marra, F.; Parhar, K.; Huang, B.; Vadlamudi, N. Risk Factors for Herpes Zoster Infection: A Meta-Analysis. Open Forum Infect. Dis. 2020, 7, ofaa005. [Google Scholar] [CrossRef]
- Muller, L.M.; Gorter, K.J.; Hak, E.; Goudzwaard, W.L.; Schellevis, F.G.; Hoepelman, A.I.; Rutten, G.E. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin. Infect. Dis. 2005, 41, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Papagianni, M.; Metallidis, S.; Tziomalos, K. Herpes zoster and diabetes mellitus: A review. Diabetes Ther. 2018, 9, 545–550. [Google Scholar] [CrossRef]
- Heymann, A.D.; Chodick, G.; Karpati, T.; Kamer, L.; Kremer, E.; Green, M.S.; Kokia, E.; Shalev, V. Diabetes as a risk factor for herpes zoster infection: Results of a population-based study in Israel. Infection 2008, 36, 226–230. [Google Scholar] [CrossRef]
- Kawai, K.; Yawn, B.P. Risk Factors for Herpes Zoster: A systematic review and meta-analysis. Mayo Clin. Proc. 2017, 92, 1806–1821. [Google Scholar] [CrossRef] [Green Version]
- Suaya, J.A.; Chen, S.-Y.; Li, Q.; Burstin, S.J.; Levin, M.J. Incidence of herpes zoster and persistent post-zoster pain in adults with or without diabetes in the United States. Open Forum Infect. Dis. 2014, 1, ofu049. [Google Scholar] [CrossRef]
- Saadatian-Elahi, M.; Bauduceau, B.; Del-Signore, C.; Vanhems, P. Diabetes as a risk factor for herpes zoster in adults: A synthetic literature review. Diabetes Res. Clin. Pract. 2020, 159, 107983. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.S.; Lin, M.H.; Lee, C.P.; Yang, Y.H.; Chen, W.C.; Chang, G.H.; Tsai, Y.T.; Chen, P.C.; Tsai, Y.H. Chang gung research database: A multi-institutional database consisting of original medical records. Biomed. J. 2017, 40, 263–269. [Google Scholar] [CrossRef]
- Shao, S.C.; Chan, Y.Y.; Kao Yang, Y.H.; Lin, S.J.; Hung, M.J.; Chien, R.N.; Lai, C.C.; Lai, E.C. The chang gung research database—A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol. Drug Saf. 2019, 28, 593–600. [Google Scholar] [CrossRef]
- Diabetes Association of The Republic Of China. Executive summary of the DAROC clinical practice guidelines for diabetes care-2018. J. Formos Med. Assoc. 2020, 119, 577–586. [Google Scholar] [CrossRef]
- Nagin, D.S.; Odgers, C.L. Group-based trajectory modeling in clinical research. Annu. Rev. Clin. Psychol. 2010, 6, 109–138. [Google Scholar] [CrossRef] [Green Version]
- Rathmann, W.; Schwandt, A.; Hermann, J.M.; Kuss, O.; Roden, M.; Laubner, K.; Best, F.; Ebner, S.; Plaumann, M.; Holl, R.W.; et al. Distinct trajectories of HbA1c in newly diagnosed Type 2 diabetes from the DPV registry using a longitudinal group-based modelling approach. Diabet. Med. 2019, 36, 1468–1477. [Google Scholar] [CrossRef]
- Elmer, J.; Jones, B.L.; Nagin, D.S. Using the Beta distribution in group-based trajectory models. BMC Med. Res. Methodol. 2018, 18, 152. [Google Scholar] [CrossRef]
- Jones, B.L.; Nagin, D.S. Advances in Group-Based Trajectory Modeling and an SAS Procedure for Estimating Them. Sociol. Methods Res. 2007, 35, 542–571. [Google Scholar] [CrossRef] [Green Version]
- Laiteerapong, N.; Karter, A.J.; Moffet, H.H.; Cooper, J.M.; Gibbons, R.D.; Liu, J.Y.; Gao, Y.; Huang, E.S. Ten-year hemoglobin A1c trajectories and outcomes in type 2 diabetes mellitus: The Diabetes & Aging Study. J. Diabetes Complicat. 2017, 31, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Vossen, M.T.; Gent, M.R.; Weel, J.F.; de Jong, M.D.; van Lier, R.A.; Kuijpers, T.W. Development of virus-specific CD4+ T cells on reexposure to Varicella-Zoster virus. J. Infect. Dis. 2004, 190, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Pearson-Stuttard, J.; Blundell, S.; Harris, T.; Cook, D.G.; Critchley, J. Diabetes and infection: Assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016, 4, 148–158. [Google Scholar] [CrossRef]
- Martinez, P.J.; Mathews, C.; Actor, J.K.; Hwang, S.A.; Brown, E.L.; De Santiago, H.K.; Fisher Hoch, S.P.; McCormick, J.B.; Mirza, S. Impaired CD4+ and T-helper 17 cell memory response to Streptococcus pneumoniae is associated with elevated glucose and percent glycated hemoglobin A1c in Mexican Americans with type 2 diabetes mellitus. Transl. Res. 2014, 163, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Restrepo, B.I.; Twahirwa, M.; Rahbar, M.H.; Schlesinger, L.S. Phagocytosis via complement or Fc-gamma receptors is compromised in monocytes from type 2 diabetes patients with chronic hyperglycemia. PLoS ONE 2014, 9, e92977. [Google Scholar] [CrossRef] [Green Version]
- Stegenga, M.E.; van der Crabben, S.N.; Dessing, M.C.; Pater, J.M.; van den Pangaart, P.S.; de Vos, A.F.; Tanck, M.W.; Roos, D.; Sauerwein, H.P.; van der Poll, T. Effect of acute hyperglycaemia and/or hyperinsulinaemia on proinflammatory gene expression, cytokine production and neutrophil function in humans. Diabet. Med. 2008, 25, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, S.; Hata, A.; Sadaoka, K.; Yamanishi, K.; Mori, Y. Comparison of varicella-zoster virus-specific immunity of patients with diabetes mellitus and healthy individuals. J. Infect. Dis. 2009, 200, 1606–1610. [Google Scholar] [CrossRef] [Green Version]
- Critchley, J.A.; Carey, I.M.; Harris, T.; DeWilde, S.; Hosking, F.J.; Cook, D.G. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care 2018, 41, 2127–2135. [Google Scholar] [CrossRef] [Green Version]
- Hine, J.L.; de Lusignan, S.; Burleigh, D.; Pathirannehelage, S.; McGovern, A.; Gatenby, P.; Jones, S.; Jiang, D.; Williams, J.; Elliot, A.J.; et al. Association between glycaemic control and common infections in people with Type 2 diabetes: A cohort study. Diabet. Med. 2017, 34, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Quiles, C.; Lopez-Lacort, M.; Ampudia-Blasco, F.J.; Diez-Domingo, J. Risk and impact of herpes zoster on patients with diabetes: A population-based study, 2009–2014. Hum. Vaccines Immunother. 2017, 13, 2606–2611. [Google Scholar] [CrossRef]
| All | Group 1 | Group 2 | Group 3 | Group 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Good Control | High Decreasing | Moderate Stable | Poor Control | p Value | |||||||
| Total enrollee n. (%) | 121,999 | 71,296 (58.4) | 10,888 (8.9) | 30,602 (25.1) | 9213 (7.6) | ||||||
| Herpes Zoster n. (%) | 2867 | (2.4) | 1621 | (2.3) | 183 | (1.7) | 794 | (2.6) | 269 | (2.9) | <0.0001 |
| Age, years, mean (SD) | 60.4 | (12.6) | 62.1 | (12.4) | 56.1 | (12.7) | 59.6 | (12.1) | 55.6 | (12.6) | <0.0001 |
| Sex, n. (%) | <0.0001 | ||||||||||
| Male | 65,490 | (53.7) | 38,046 | (53.4) | 6659 | (61.2) | 16,000 | (52.3) | 4785 | (52.0) | |
| Female | 56,509 | (46.3) | 33,250 | (46.6) | 4229 | (38.9) | 14,602 | (47.7) | 4428 | (48.1) | |
| Follow date, years, median (IQR) | 6.1 | (3.2–9.8) | 5.8 | (3.0–9.4) | 5.1 | (2.5–8.1) | 7.2 | (3.8–10.8) | 6.9 | (3.4–10.5) | <0.0001 |
| Mean HbA1C, %, mean (SD) | 7.58 | (1.68) | 6.70 | (0.79) | 7.93 | (2.27) | 8.43 | (1.17) | 10.69 | (1.80) | <0.0001 |
| Comorbidity, n. (%) | |||||||||||
| Hypertension | 22,440 | (18.4) | 17,973 | (25.2) | 1359 | (12.5) | 2654 | (8.7) | 454 | (4.9) | <0.0001 |
| COPD | 2214 | (1.8) | 1704 | (2.4) | 154 | (1.4) | 293 | (1.0) | 63 | (0.7) | <0.0001 |
| Heart failure | 2701 | (2.2) | 2030 | (2.9) | 153 | (1.4) | 419 | (1.4) | 99 | (1.1) | <0.0001 |
| Ischemic heart disease | 7382 | (6.1) | 5747 | (8.1) | 382 | (3.5) | 1052 | (3.4) | 201 | (2.2) | <0.0001 |
| Stroke | 2958 | (2.4) | 2344 | (3.3) | 185 | (1.7) | 364 | (1.2) | 65 | (0.7) | <0.0001 |
| Chronic kidney disease | 2206 | (1.8) | 1676 | (2.4) | 75 | (0.7) | 373 | (1.2) | 83 | (0.9) | <0.0001 |
| Group | Crude HR (95% Cl) | Model 1 † HR (95% Cl) | Model 2 ‡ HR (95% Cl) | |||
|---|---|---|---|---|---|---|
| HbA1C trajectories | ||||||
| Group 1 good control | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| Group 2 high decreasing | 0.87 | (0.74–1.01) | 1.04 | (0.89–1.21) | 1.06 | (0.90–1.23) |
| Group 3 moderate stable | 0.98 | (0.90–1.06) | 1.06 | (0.97–1.15) | 1.08 | (0.99–1.18) |
| Group 4 poor control | 1.15 | (1.01–1.30) | 1.41 | (1.23–1.60) | 1.44 | (1.26–1.64) |
| Age | 1.03 | (1.03–1.04) | 1.03 | (1.03–1.04) | 1.03 | (1.03–1.04) |
| Sex | ||||||
| Female | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| Male | 0.81 | (0.75–0.87) | 0.91 | (0.85–0.98) | 0.90 | (0.83–0.97) |
| Hypertension | 1.16 | (1.05–1.29) | 1.01 | (0.90–1.14) | ||
| COPD | 1.94 | (1.52–2.49) | 1.63 | (1.26–2.09) | ||
| Heart failure | 1.18 | (0.89–1.56) | 0.89 | (0.67–1.19) | ||
| Ischemic heart disease | 1.26 | (1.07–1.48) | 1.10 | (0.92–1.31) | ||
| Stroke | 1.37 | (1.09–1.74) | 1.10 | (0.86–1.40) | ||
| Chronic kidney disease | 2.99 | (2.42–3.68) | 2.80 | (2.26–3.46) | ||
| Herpes Zoster n (%) | HbA1C %, Mean (SD) | ANOVA | Post Hoc Tests by Scheffe’s Method | Difference between Means (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Trajectory group | p < 0.0001 * | |||||||
| Poor control | 269 | (2.9) | 9.73 | (1.11) | Poor control vs. moderate stable | 1.53 | (1.38–1.68) * | |
| vs. high decreasing | 1.99 | (1.78–2.20) * | ||||||
| vs. good control | 2.96 | (2.82–3.10) * | ||||||
| Moderate stable | 794 | (2.6) | 8.20 | (0.75) | Moderate stable vs. high decreasing | 0.46 | (0.29–0.64) * | |
| vs. good control | 1.43 | (1.34–1.52) * | ||||||
| High decreasing | 183 | (1.7) | 7.74 | (0.86) | High decreasing vs. good control | 0.96 | (0.80–1.13) * | |
| Good control | 1621 | (2.3) | 6.77 | (0.69) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, B.-L.; Chou, C.-P.; Huang, K.-S.; Bin, P.-J.; Luo, K.-H.; Chuang, H.-Y. The Pattern of Hemoglobin A1C Trajectories and Risk of Herpes Zoster Infection: A Follow-Up Study. Int. J. Environ. Res. Public Health 2022, 19, 2646. https://doi.org/10.3390/ijerph19052646
Pan B-L, Chou C-P, Huang K-S, Bin P-J, Luo K-H, Chuang H-Y. The Pattern of Hemoglobin A1C Trajectories and Risk of Herpes Zoster Infection: A Follow-Up Study. International Journal of Environmental Research and Public Health. 2022; 19(5):2646. https://doi.org/10.3390/ijerph19052646
Chicago/Turabian StylePan, Bo-Lin, Chia-Pei Chou, Kun-Siang Huang, Pin-Jie Bin, Kuei-Hau Luo, and Hung-Yi Chuang. 2022. "The Pattern of Hemoglobin A1C Trajectories and Risk of Herpes Zoster Infection: A Follow-Up Study" International Journal of Environmental Research and Public Health 19, no. 5: 2646. https://doi.org/10.3390/ijerph19052646
APA StylePan, B.-L., Chou, C.-P., Huang, K.-S., Bin, P.-J., Luo, K.-H., & Chuang, H.-Y. (2022). The Pattern of Hemoglobin A1C Trajectories and Risk of Herpes Zoster Infection: A Follow-Up Study. International Journal of Environmental Research and Public Health, 19(5), 2646. https://doi.org/10.3390/ijerph19052646







