A Structural Model of Quality of Life in Patients after Colorectal Cancer Surgery
Abstract
1. Introduction
Research Aims
2. Materials and Methods
2.1. Research Design
2.2. Criteria for Selection
2.3. Study Design and Sample
2.4. Instruments and Measures
2.4.1. Social Support
2.4.2. Self-Efficacy
2.4.3. Symptom Experience
2.4.4. Health Promoting Life Style Profile
2.4.5. Quality of Life
2.5. Ethical Considerations
2.6. Statistical Analysis
- The general characteristics of the subjects and descriptive statistics for each variable were analyzed as descriptive statistics.
- Correlation between major variables was analyzed using the Pearson Correlation Coefficient.
- The reliability of the measurement tool was analyzed with Cronbach’s α value.
- Structural model analysis is a two-step approach, first estimating the measurement model and then measuring the structural model. To verify the validity of the measurement model, confirmatory factor analysis was performed.
- The maximum likelihood method was used to test the fit of the model. The fitness indices such as χ2, TLI, CFI, NFI, IFI, RMSEA, and SRMR were used for the model fit criteria.
- Bootstrapping was used to verify the significance of the indirect and total effects of the research model.
- Based on the theoretical basis, a modified model was developed by fixing or liberalizing the path while referring to the parameter estimates and fitness statistics of the hypothetical model, and the final model was presented.
3. Results
3.1. Patient Characteristics
3.2. Descriptive Statistics and Multicollinearity Test of Measured Variables
3.3. Validity and Reliability of the Measurement Model
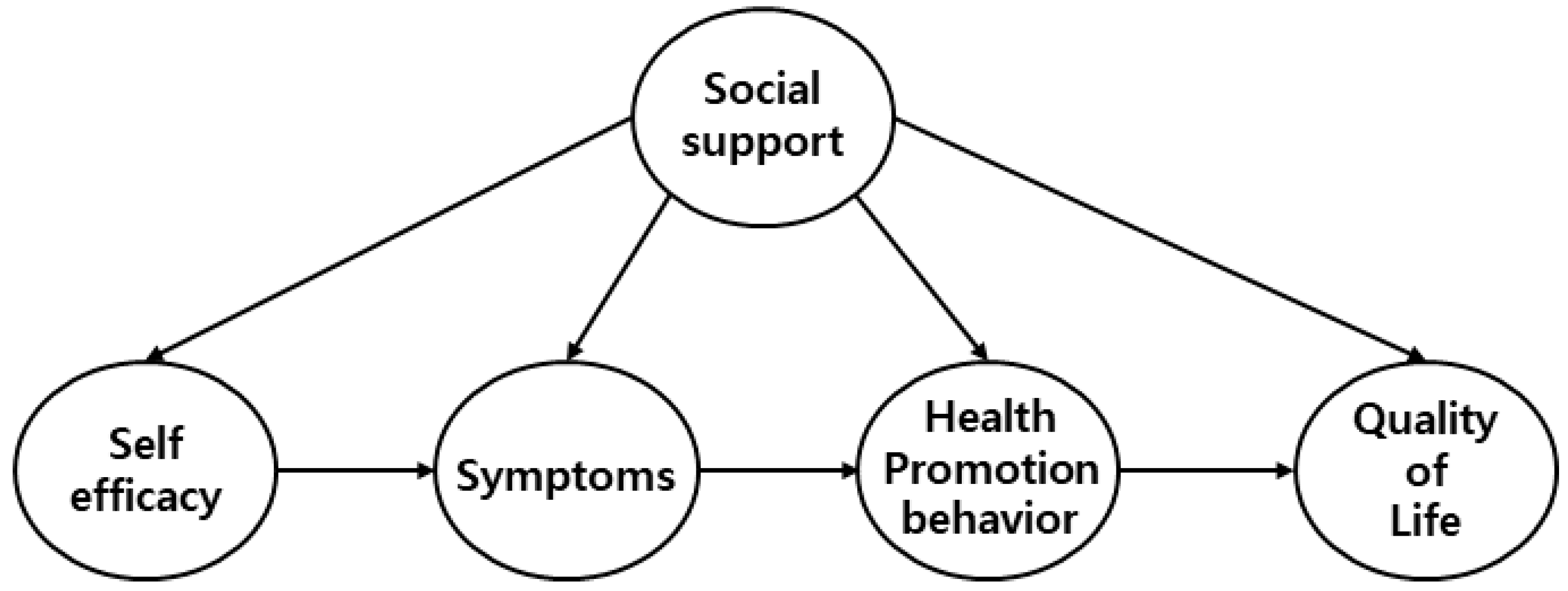
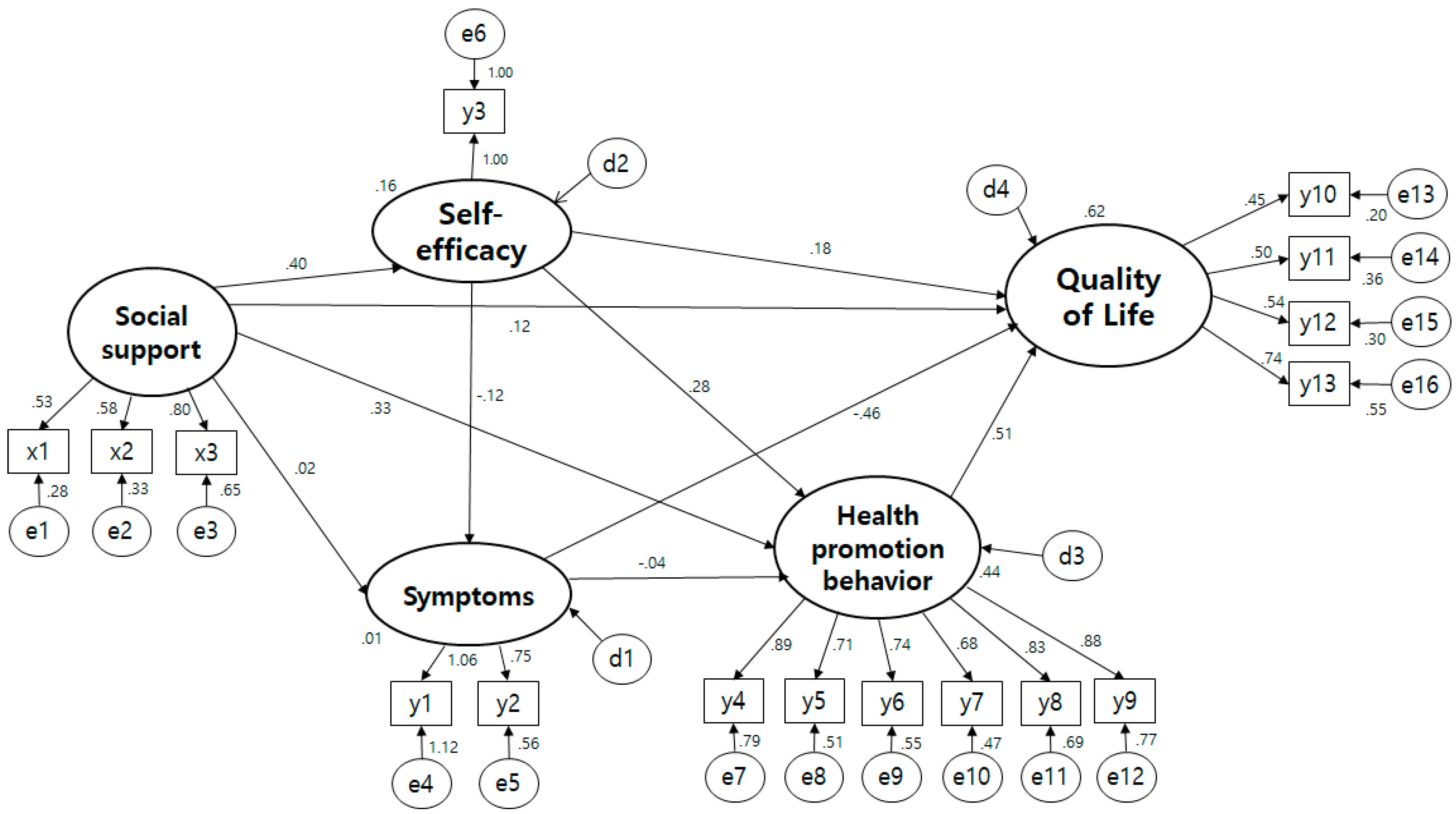
3.4. Testing the Hypothetical Model
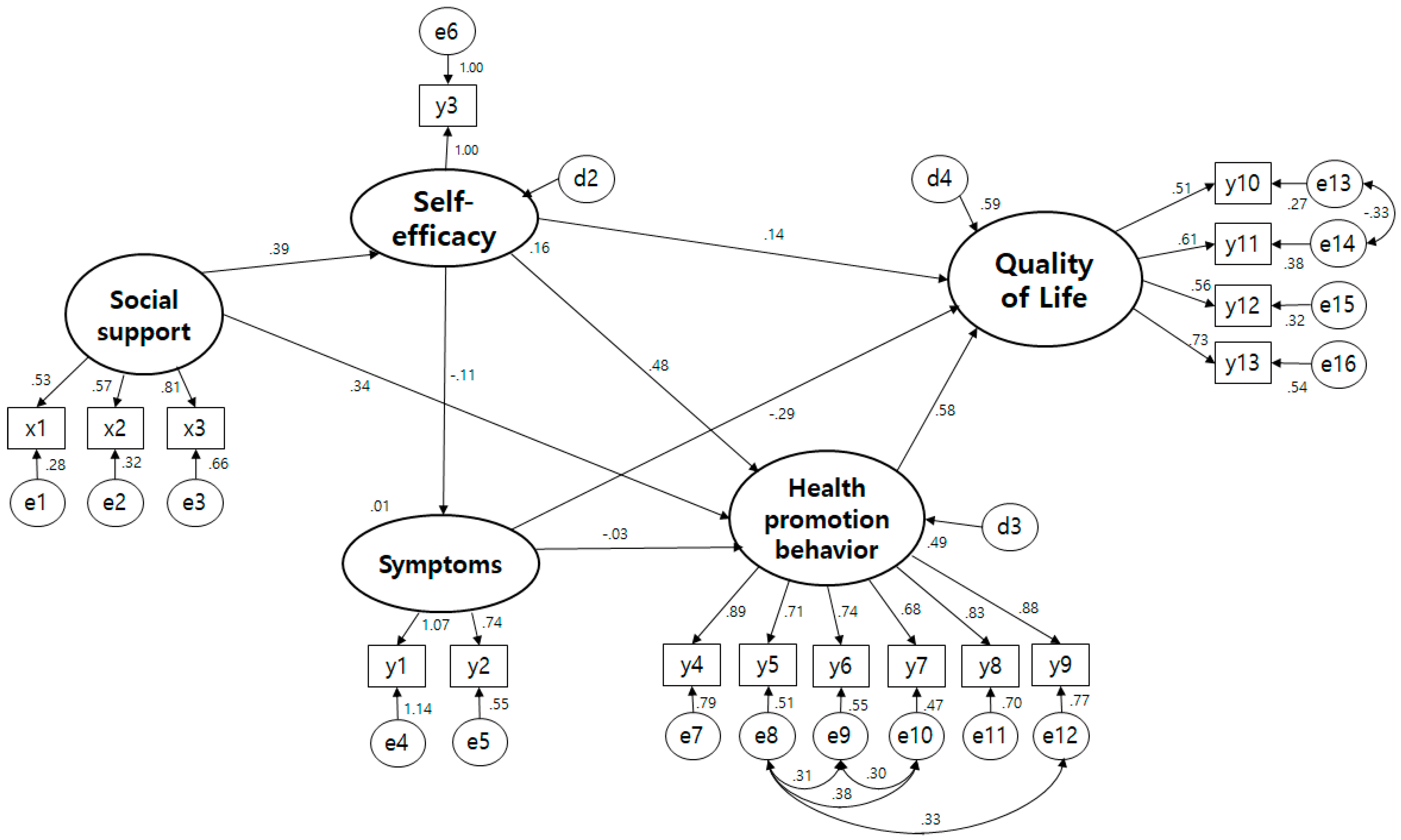
3.5. The Structural Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ministry of Health and Welfare; Korea Central Cancer Registry; National Cancer Center. Annual Report of Cancer Statistics in Korea in 2019; Annual Report; Ministry of Health and Welfare, Korea Central Cancer Registry, National Cancer Center: Seoul, Korea, 2021; Available online: https://www.ncc.re.kr/cancerStatsView.ncc?bbsnum=478&searchKey=total&searchValue=&pageNum=1 (accessed on 18 February 2022).
- Eom, J.W. Practice Guideline for Colorectal Cancer v.1.0. Korean Surg. Soc. 2012, 54–70. Available online: http://www.dbpia.co.kr/journal/articleDetail?nodeId=NODE01957171 (accessed on 19 February 2022).
- Kim, J.H. Update on distress management for cancer patients. J. Korean Med. Assoc. 2019, 62, 167–173. [Google Scholar] [CrossRef]
- Wilson, I.B.; Cleary, P.D. Linking clinical variables with health-related quality of life: A conceptual model of patient outcomes. JAMA 1995, 273, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Tae, Y.S. Quality of Life in Patients with Cancer. J. Nurs. Query 2007, 16, 52–78. [Google Scholar]
- Anwar, S.; Tan, W.; Yu, J.; Hutson, A.; Javle, M.; Iyer, R. Quality-of-life (QoL) as a predictive biomarker in patients with advanced pancreatic cancer (APC) receiving chemotherapy: Results from a prospective multicenter phase 2 trial. J. Gastrointest. Oncol. 2014, 5, 433. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Sung, S.W.; Lee, J.K. Management of cancer survivors in Korea. J. Korean Med. Assoc. 2015, 58, 216–226. [Google Scholar] [CrossRef]
- Mao, L.; Mondal, K.; Manna, M. A comparative study on quality of life of older adults. Indian J. Contin. Nurs. Educ. 2019, 20, 73. [Google Scholar] [CrossRef]
- Saengsiri, A.O.; Thanasilp, S.; Preechawong, S. Factors predicting quality of life for coronary artery disease patients after percutaneous coronary intervention. Asian Biomed. 2014, 8, 31–42. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.K. Structural Equation Modeling on Health-related Quality of Life among Patients with Thyroid Cancer. Korea Soc. Adult Nurs. 2018, 30, 171–182. [Google Scholar] [CrossRef]
- Haviland, J.; Sodergren, S.; Calman, L.; Corner, J.; Din, A.; Fenlon, D.; Grimmett, C.; Richardson, A.; Smith, P.W.; Winter, J.; et al. Social support following diagnosis and treatment for colorectal cancer and associations with health-related quality of life: Results from the UK ColoREctal Wellbeing (CREW) cohort study. Psycho-Oncology 2017, 26, 2276–2284. [Google Scholar] [CrossRef]
- Jeong, G.H.; Kim, K.H.; Kwak, Y.H. Quality of Life in Colorectal Cancer Patients according to the Severity of Symptom Clusters Classification. Asian Oncol. Nurs. 2014, 14, 74–83. [Google Scholar] [CrossRef][Green Version]
- Qian, H.; Yuan, C. Factors associated with self-care self-efficacy among gastric and colorectal cancer patients. Cancer Nurs. 2012, 35, E22–E31. [Google Scholar] [CrossRef] [PubMed]
- Iyer, N.S.; Osann, K.; Hsieh, S.; Tucker, J.A.; Monk, B.J.; Nelson, E.L.; Wenzel, L. Health behaviors in cervical cancer survivors and associations with quality of life. Clin. Ther. 2016, 38, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Han, C.J.; Gigic, B.; Schneider, M.; Kulu, Y.; Peoples, A.R.; Ose, J.; Koelsch, T.; Jacobsen, P.B.; Colditz, G.; Figueiredo, J.C.; et al. Prospective, longitudinal study of risk factors for cancer-related distress in colorectal cancer survivors from prior to surgery until one year after surgery: Results from the ColoCare study. J. Clin. Oncol. 2019, 37, 146. [Google Scholar] [CrossRef]
- Eom, C.-S.; Shin, D.W.; Kim, S.Y.; Yang, H.K.; Jo, H.S.; Kweon, S.S.; Kang, Y.S.; Kim, J.-H.; Cho, B.-L.; Park, J.-H. Impact of perceived social support on the mental health and health-related quality of life in cancer patients: Results from a nationwide, multicenter survey in South Korea. Psycho-Oncology 2013, 22, 1283–1290. [Google Scholar] [CrossRef]
- Korean Society of Coloproctology. Colon Anal Disease Information. 2002. Available online: http://www.colon.or.kr/colonlife_new/bbs/index.kin?category_id=&page_id=&bbs_mode=view&f_wbcode=board_story_1&f_master_idx=31&f_page=1&f_template_type=list&f_query=&f_target=1 (accessed on 18 February 2022).
- Elfeki, H.; Larsen, H.M.; Emmertsen, K.J.; Christensen, P.; Youssef, M.; Khafagy, W.; Omar, W.; Laurberg, S. Bowel dysfunction after sigmoid resection for cancer and its impact on quality of life. Br. J. Surg. 2019, 106, 142–151. [Google Scholar] [CrossRef]
- Grimmett, C.; Haviland, J.; Winter, J.; Calman, L.; Din, A.; Richardson, A.; Foster, C. Colorectal cancer patient’s self-efficacy for managing illness-related problems in the first 2 years after diagnosis, results from the ColoREctal Well-being (CREW) study. J. Cancer Surviv. 2017, 11, 634–642. [Google Scholar] [CrossRef]
- Johansson, A.C.; Axelsson, M.; Grankvist, G.; Berndtsson, I.; Brink, E. Symptoms, illness perceptions, self-efficacy and health-related quality of life following colorectal cancer treatment. Open J. Nurs. 2018, 8, 591–604. [Google Scholar] [CrossRef][Green Version]
- Moug, S.J.; Bryce, A.; Mutrie, N.; Anderson, A.S. Lifestyle interventions are feasible in patients with colorectal cancer with potential short-term health benefits: A systematic review. Int. J. Colorectal Dis. 2017, 32, 765–775. [Google Scholar] [CrossRef]
- Je, Y.; Jeon, J.Y.; Giovannucci, E.L.; Meyerhardt, J.A. Association between physical activity and mortality in colorectal cancer: A meta-analysis of prospective cohort studies. Int. J. Cancer 2013, 133, 1905–1913. [Google Scholar] [CrossRef]
- Yun, Y.H.; Sim, J.A.; Jung, J.Y.; Noh, D.-Y.; Lee, E.S.; Kim, Y.W.; Oh, J.H.; Ro, J.S.; Park, S.Y.; Cho, K.H.; et al. The association of self-leadership, health behaviors, and posttraumatic growth with health-related quality of life in patients with cancer. Psycho-Oncology 2014, 23, 1423–1430. [Google Scholar] [CrossRef]
- Lee, B.G.; Lee, T.S.; Lim, S.H. Mediation effect of Self-Efficacy on the relationship between perceived self-management support and health-related quality of life among cancer survivors. J. Korean Acad. Nurs. 2019, 49, 298–306. [Google Scholar] [CrossRef]
- Kim, S.K.; Ruy, S.Y. Influence of Social Support for a Cancer Patient undergoing Radiation Treatment on Quality of Life. J. Korean Soc. Radiol. 2016, 10, 145–152. [Google Scholar] [CrossRef][Green Version]
- Husson, O.; Denollet, J.; Ezendam, N.P.; Mols, F. Personality, health behaviors, and quality of life among colorectal cancer survivors: Results from the PROFILES registry. J. Psychosoc. Oncol. 2017, 35, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Mrabti, H.; Amziren, M.; Elghissassi, I.; Bensouda, Y.; Berrada, N.; Abahssain, H.; Boutayeb, S.; El Fakir, S.; Nejjari, C.; Benider, A.; et al. Quality of life of early stage colorectal cancer patients in Morocco. BMC Gastroenterol. 2016, 16, 131. [Google Scholar] [CrossRef]
- Kim, H.J.; Chu, S.H.; Ruy, G.S.; Kim, N.G. Nutritional Risk and Physical Activity on Quality of Life in Patients with Colorectal Cancer. Asian Oncol. Nurs. 2014, 14, 66–73. [Google Scholar] [CrossRef]
- Yeom, J.W.; Suh, Y.O. The Effect of Symptom Experience, Nutritional Status, and Self Care on Quality of Life in Elderly Patients with Colorectal Cancer. Korean Acad. Soc. Rehabil. Nurs. 2019, 22, 48–57. [Google Scholar] [CrossRef]
- Kline, R.B. Principles and Practice of Structural Equation Modeling, 2nd ed.; Guilford Press: New York, NY, USA, 2004. [Google Scholar]
- Zimet, G.D.; Dahlem, N.W.; Zimet, S.G.; Farley, G.K. The multidimensional scale of perceived social support. J. Personal. Assess. 1988, 52, 30–41. [Google Scholar] [CrossRef]
- Oh, P.J.; Lee, E.O.; Tae, Y.S.; Um, D.C. Effects of a Program to Promoto Self-Efficacy and Hope on the Self-Care Behaviors and the Quality of Life in Patients with Leukemia. J. Korean Acad. Nurs. 1997, 27, 627–638. [Google Scholar]
- Walker, S.N.; Sechrist, K.R.; Pender, N.J. The Health-Promoting Lifestyle Profile: Development and psychometric characteristics. Nurs. Res. 1987, 36, 76–81. [Google Scholar] [CrossRef]
- Ward, W.L.; Hahn, E.A.; Mo, F.; Hernandez, L.; Tulsky, D.S.; Cella, D. Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual. Life Res. 1999, 8, 81–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Tae, Y.S. A Predictive Model of Quality of Life for Stomach Cancer Patients with Gastrectomy. Korean J. Adult Nurs. 2015, 27, 613–623. [Google Scholar] [CrossRef]
- Vonk-Klaassen, S.M.; de Vocht, H.M.; den Ouden, M.E.; Eddes, E.H.; Schuurmans, M.J. Ostomy-related problems and their impact on quality of life of colorectal cancer ostomates: A systematic review. Qual. Life Res. 2016, 25, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J. Structural Equation Model of Health-related Quality of Life after Percutaneous Coronary Intervention. Ph.D. Thesis, State National University, Seoul, Korea, 2014; pp. 1–104. [Google Scholar]
- Hwang, S.H.; Chun, N.M. Health Promotion Behavior in Colorectal Cancer Patients and General Adults. Asian Oncol. Nurs. 2016, 16, 94–102. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Fuchs, C.S.; Niedzwiecki, D.; Zhang, S.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Association of survival with adherence to the American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors after colon cancer diagnosis: The CALGB 89803/Alliance trial. JAMA Oncol. 2018, 4, 783–790. [Google Scholar] [CrossRef]
- Jo, H.S.; Kim, B.G.; Lee, H.J.; Lee, B.Y. Perceived Social Support as Influencing Factors on Quality of Life among Cancer Patients. Korea J. Health Educ. Promot. 2010, 27, 51–59. [Google Scholar]
- Jeon, Y.H.; Park, G.J. Relationships between Specific self-efficacy, Family support, and Self-care performance for Patients with Stomach Cancer after Gastrectomy. J. Korea Acad.-Ind. Coop. Soc. 2018, 19, 456–465. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, S.K.; An, J.Y.; Kim, G.S. Development and Evaluation of Standardized Telephone Counseling Guidelines on Symptom Management for Patients Discharged after Colorectal Cancer Surgery. Asian Oncol. Nurs. 2014, 14, 191–201. [Google Scholar] [CrossRef]
- Baek, Y.; Yi, M. Factors influencing quality of life during chemotherapy for colorectal cancer patients in South Korea. J. Korean Acad. Nurs. 2015, 45, 604–612. [Google Scholar] [CrossRef]
| Characteristics | Categories | n (%) | M ± SD |
|---|---|---|---|
| Age (year) | ≥49 | 23 (11.0) | 61.33 ± 11.21 |
| 50~59 | 70 (33.5) | ||
| 60~69 | 70 (33.5) | ||
| ≤70 | 46 (22.0) | ||
| Gender | Male | 121 (57.9) | |
| Female | 88 (42.1) | ||
| Marriage status | Unmarried | 11 (5.3) | |
| Married | 172 (82.3) | ||
| Diverse/Separation | 26 (12.4) | ||
| Occupation | Yes | 54 (25.8) | |
| No | 155 (74.2) | ||
| Period of received Surgery | ≥5 | 34 (16.3) | 31.3 ± 27.4 |
| (month) | 6–12 | 31 (14.8) | |
| 13–59 | 119 (56.9) | ||
| ≤60 | 25 (12.0) | ||
| Stage | I | 36 (17.2) | |
| II | 53 (25.4) | ||
| III | 93 (44.5) | ||
| IV | 27 (12.9) | ||
| Cancer location | Ascending colon | 56 (26.8) | |
| Descending colon | 12 (5.7) | ||
| Sigmoid colon | 79 (37.8) | ||
| Rectum | 62 (29.7) | ||
| Stoma | Yes | 31 (14.8) | |
| No | 178 (85.2) | ||
| Chemotherapy | Yes | 140 (67.0) | |
| No | 69 (33.0) | ||
| Radiation therapy | Yes | 40 (19.1) | |
| No | 169 (80.9) |
| Variable | Categories | M ± SD | Skewmess | Kurtosis | Tolerance | VIF | Estimate | CR | AVE |
|---|---|---|---|---|---|---|---|---|---|
| Social support | Significant | 2.53 ± 1.19 | 1.40 | −0.93 | 0.529 | ||||
| Family | 4.04 ± 0.90 | −1.00 | 0.76 | 0.577 | |||||
| Friend | 3.30 ± 1.04 | −0.22 | −0.63 | 0.804 | |||||
| Total | 39.45 ± 0.67 | 0.12 | −0.17 | 0.78 | 1.28 | 0.677 | 0.420 | ||
| Symptoms | General | 2.61 ± 2.16 | 0.83 | −0.30 | 1.031 | ||||
| G-I | 2.57 ± 2.31 | 0.80 | −0.34 | 0.797 | |||||
| Total | 46.84 ± 7.81 | 0.80 | −0.34 | 0.59 | 1.68 | 0.865 | 0.686 | ||
| Self-efficacy | Total | 45.67 ± 0.64 | −0.70 | 0.45 | 0.63 | 1.59 | 0.312 | 0.661 | 0.661 |
| Health Promotion behavior | Self-realization | 2.60 ± 0.69 | −0.10 | −0.36 | 0.892 | ||||
| Heath responsibility | 2.38 ± 0.60 | 0.15 | −0.45 | 0.715 | |||||
| Physical activity | 2.42 ± 0.86 | 0.12 | −0.94 | 0.743 | |||||
| Nutrition | 2.67 ± 0.66 | −0.02 | −0.52 | 0.683 | |||||
| Interpersonal relations | 2.69 ± 0.68 | −0.23 | −0.23 | 0.833 | |||||
| Stress management | 2.92 ± 0.80 | 0.14 | −0.45 | 0.880 | |||||
| Total | 83.68 ± 1.33 | 0.03 | −0.33 | 0.55 | 1.81 | 0.919 | 0.657 | ||
| Quality of Life | Physical well being | 3.13 ± 0.79 | −1.19 | 1.17 | 0.525 | ||||
| Social/Family well being | 2.30 ± 0.85 | −0.50 | −0.31 | 0.639 | |||||
| Emotional well being | 2.93 ± 0.69 | −0.55 | −0.16 | 0.561 | |||||
| Functional well being | 2.55 ± 0.97 | −0.40 | −0.49 | 0.720 | |||||
| Total | 73.35 ± 1.30 | −0.12 | −0.63 | 0.52 | 1.92 | 0.785 | 0.480 |
| Pathway | SE | T(p) | SMC | Direct Effect (p) | Indirect Effect (p) | Total Effect (p) | ||
|---|---|---|---|---|---|---|---|---|
| Self-efficacy | ← | Social support | 0.448 | 4.323 (<0.001) | 0.155 | 0.394 (0.015) | 0.394 (0.015) | |
| Symptoms | ← | Social support | −0.045 (0.093) | −0.045 (0.093) | ||||
| ← | Self-efficacy | −0.363 | −1.773 (0.076) | 0.130 | −0.113 (0.104) | −0.113 (0.104) | ||
| Health promotion behavior | ← | Social support | 0.333 | 4.053 (<0.001) | 0.486 | 0.344 (0.004) | 0.191 (0.004) | 0.535 (0.004) |
| ← | Symptoms | −0.009 | −1.625 (0.532) | −0.032 (0.528) | −0.032 (0.528) | |||
| ← | Self-efficacy | 0.410 | 7.352 (<0.001) | 0.481 (0.011) | 0.481 (0.011) | |||
| Quality of Life | ← | Social support | 0.587 | 0.380 (0.011) | 0.380 (0.011) | |||
| ← | Health Promotion behavior | 0.386 | 5.169 (<0.001) | 0.579 (0.014) | 0.579 (0.014) | |||
| ← | Self-efficacy | 0.82 | 1.773 (0.076) | 0.144 (0.071) | 0.313 (0.009) | 0.458 (0.015) | ||
| ← | Symptoms | −0.051 | −3.322 (<0.001) | −0.290 (0.007) | −0.019 (0.546) | −0.308 (0.008) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeom, J.W.; Suh, Y.O. A Structural Model of Quality of Life in Patients after Colorectal Cancer Surgery. Int. J. Environ. Res. Public Health 2022, 19, 2564. https://doi.org/10.3390/ijerph19052564
Yeom JW, Suh YO. A Structural Model of Quality of Life in Patients after Colorectal Cancer Surgery. International Journal of Environmental Research and Public Health. 2022; 19(5):2564. https://doi.org/10.3390/ijerph19052564
Chicago/Turabian StyleYeom, Jeong Won, and Yeon Ok Suh. 2022. "A Structural Model of Quality of Life in Patients after Colorectal Cancer Surgery" International Journal of Environmental Research and Public Health 19, no. 5: 2564. https://doi.org/10.3390/ijerph19052564
APA StyleYeom, J. W., & Suh, Y. O. (2022). A Structural Model of Quality of Life in Patients after Colorectal Cancer Surgery. International Journal of Environmental Research and Public Health, 19(5), 2564. https://doi.org/10.3390/ijerph19052564






