Abstract
(1) Background: The aim of this systematic review was to compare the cost-effectiveness of two follow-up methods (face-to-face and telemedicine) used in dermatology in the last ten years. (2) Methods: A search for articles that included economic analyses was conducted in August 2021 in the databases PubMed, Medline, Scielo and Scopus using the following keywords: “Cost–Benefit Analysis”, “Dermatology”, “Telemedicine”, “Primary Health Care”, as well as other search terms and following the PICOS eligibility criteria. (3) Results: Three clinical trials and five observational studies were analyzed, providing information for approximately 16,539 patients (including four cost-minimization or saving analyses, three cost-effectiveness analyses, and one cost–utility analysis) in Europe and the United States. They describe the follow-up procedures in each of the cases and measure and analyze the direct and indirect costs and effectiveness. All the articles indicate that teledermatology lowers costs and proves satisfactory to both patients and professionals. (4) Conclusions: Although it has been found that follow-up via teledermatology can be more efficient than traditional hospital follow-up, more work is needed to establish evaluation protocols and procedures that measure key variables more equally and demonstrate the quality of the evidence of said studies.
1. Introduction
Telemedicine is proving to be an efficient tool for improving remote medical assistance []. Dermatology is the clinical speciality best suited for telemedicine, owing to the visibility of dermatological conditions [,,].
The concept of teledermatology (TD) was first introduced in 1995 to offer remote dermatology services []. More recently, the arrival of the COVID-19 pandemic changed the scheme of in-person consultation within the healthcare system, except for those cases deemed emergencies [,]. Although TD was already in use worldwide, the arrival of COVID-19 and the subsequent confinement period consolidated the application of the tool [,]. Prior to this time, it had primarily been a resource aimed at rural populations with less convenient accessibility [].
Dermatological conditions are a frequent reason for primary care (PC) visits []. In general terms, TD is an easy-access tool for professionals in this field worldwide [,]. In some countries, TD service begins with PC physicians/nurses taking a photograph of the lesion in question and attaching it to the patient’s electronic medical records, along with a brief clinical description. Subsequently, at the referral hospital, the consultant dermatologists view the electronic medical records, observe the images and recommend a treatment or plan of action. Later, PC physicians evaluate these recommendations and contact the patient to convey the results [,].
There are several fields in TD care: synchronous, asynchronous or the combination of both [,]. Synchronous TD offers immediate diagnosis, as the dermatologist views the photograph of the patient in real time (live). In the asynchronous method, or storage TD, the images of the patients are loaded to a platform using a PC and sent to a dermatologist who later establishes a diagnosis. In clinical practice, the most widely utilized is the asynchronous model [,]. Those patients with an inconclusive diagnosis or who require an in-person appointment are contacted more quickly, thereby reducing any waiting lists.
TD offers certain advantages over traditional dermatology consultations. It reduces waiting times and costs, improves access in rural areas and/or for patients who experience difficulties in attending in-person appointments and decreases appointment cancellations and absenteeism. Moreover, TD proves to be a useful diagnostic and follow-up tool, favoring early referral in emergency cases [,,]. There are, however, other aspects that adversely affect TD. These include technological barriers (poor internet connection, limited access to platforms, poor image quality) or lack of technological skills among professionals and patients, which could hinder attention to geriatric patients or those with a different language, ultimately resulting in the making of possibly erroneous diagnoses [,].
The use of TD is greater in countries in North America and Europe []. Spain is one of the leading countries with active TD programs []. However, in other countries, such as Argentina, TD is currently in its early stages of development []. Approximately 38% of countries worldwide have integrated TD programs. In Australia, Holland and some parts of the United States, TD represents a key part of the healthcare system [,], as many rural areas lack dermatologists, thereby favoring its utilization [].
It seems that the development and expansion of TD is having a positive influence in terms of cost-effectiveness, a fact supported by the few studies and reviews found of this recent practice [,,]. However, there are even fewer investigations that focus on the analysis of the cost-effectiveness of TD compared to traditional consultations. It would be rather interesting to establish a comparative between both treatment methods and extract results that could be extrapolated and applied to improve patient care. Bearing this in mind, the aim of this systematic review is to compare the cost-effectiveness of two follow-up methods (face-to-face and telemedicine) used in dermatology over the last ten years.
2. Materials and Methods
In August 2021, a systematic review was conducted following the standards set out in the PRISMA statement for systematic reviews [,]. Said review was previously registered with the International Prospective Register of systematic reviews (PROSPERO) and was assigned number CRD42021267213. A meta-analysis could not be carried out due to the range of study designs used and inconsistent outcome reporting.
A search was conducted for articles published in the last 10 years in PubMed, Medline, Scielo and Scopus. The target population was users of dermatology services and the material was gathered from the studies present in the databases mentioned, existing gray literature and Web of Science. Additionally, manual searches were undertaken to locate bibliographic references deemed to be of interest—those included in systematic reviews and prior meta-analyses. The inclusion criteria were:
- Articles containing the MeSH terms: “Cost–Benefit Analysis”, “Dermatology”, “Telemedicine”, “Primary Health Care”. Other search terms included: “Cost–Utility Analysis”, “Economic Evaluation”, and “Cost-Effectiveness Analysis”, “Teledermatology”, “Skin Disease”, “Telehealth”, “Remote Consult” (conventional or “face-to-face” or standard or in-person).
- Clinical trials or observational studies comparing the two follow-up methods (face-to-face versus TD).
The exclusion criteria were:
- Description of a single method for intervention or follow-up, without comparison.
- Clinical guidelines, systematic reviews and meta-analyses.
The search strategy in the different databases is described in Table 1.

Table 1.
Databases and search terms.
The PICOS eligibility criteria (participants, intervention, comparison intervention, results and study design) were used for article selection: participants would be users of dermatology services. Intervention would involve the application of face-to-face dermatological monitoring follow-up (in-person at a hospital) or remote monitoring follow-up using telemedicine (teledermatology). The results measured were the costs of these follow-up methods for dermatological conditions, as well as secondary measures, such as the effectiveness or satisfaction of users and professionals. As for the type of study required, investigations had to be observational studies or clinical trials.
Articles should evaluate (main variables): number of visits, economic impact or identification of costs, such as those directly attributable to TD and/or face-to-face dermatology service (including cameras, hardware and staff).
Among the other variables considered were: costs not directly attributable to dermatology service (such as building maintenance, information technology (IT) services, gas, electricity, telephone–internet connections and medical insurance). Also taken into account were costs incurred by patients and society, for example, lost productive time, lost salaries, leisure time lost, time spent traveling to visits and petrol.
Following the application of the search strategy, a total of 71 articles were selected (between 13 and 31 August 2021), which were analyzed by title and abstract.
The extraction and reading of all titles and abstracts of the studies initially selected was carried out independently by two researchers (R.L.-L. and M.Á.V.-M.), who consulted a third individual (A.L.-V.) if there was any disagreement about the inclusion/exclusion of an article.
After an initial screening of the studies considered to be potentially relevant, a full-text critical reading of 27 articles was performed, paying particular attention to the intervention (type of treatment) and cost–benefit evaluation. Finally, it was determined that a total of eight articles met the objective and fulfilled the criteria proposed for this review (Figure 1). A descriptive analysis of the selected results was carried out. Disagreements were resolved through discussion among the reviewers until a consensus was reached.
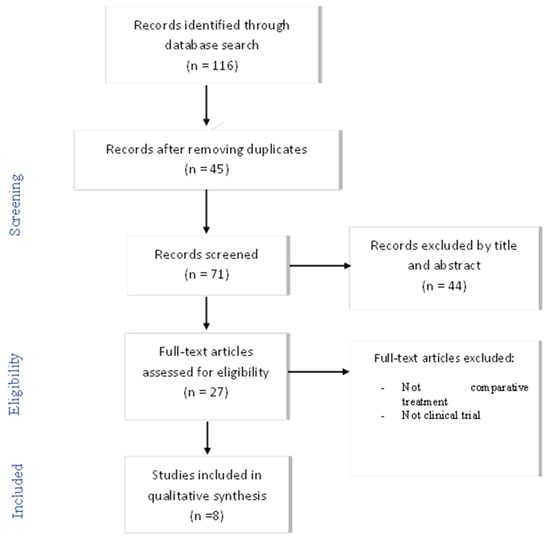
Figure 1.
Flowchart of articles selection process. n = number of articles.
The quality of the studies was evaluated using the Downs and Black quality assessment method []. This method contains 27 items divided into five sections: study quality, external validity, study bias, confounding and selections bias and study power. Scores range from 0 to 28; higher scores indicate a better methodological quality of the study: excellent (26–28), good (20–25), fair (15–19), and poor (<14) This scale has high validity and reliability, ranked as one of the six best quality assessment scales suitable for systematic reviews [,].
3. Results
Eight articles were included which provided information on 16,539 patients (including four cost-minimization or saving analyses, three cost-effectiveness analyses and one cost–utility analysis]. They described how the follow-ups were conducted for each of the methods and contained at least a cost analysis.
The following Table 2 presents a summary of the studies selected, indicating: type of study, country, number of participants, follow-up method in each group, the variables that measured direct and indirect costs, effectiveness and the main results obtained.

Table 2.
Description of the main results in the selected articles.
The following section presents the content analysis of the information based on the most important variables (Table 3):

Table 3.
Description of “Cost and Effectiveness” Variables.
3.1. Description of TD Procedure versus Face-to-face Follow-Up
Among the various procedures used in dermatology, all the articles use an asynchonous model, in which the images of the patient are uploaded to a platform along with a brief clinical history, after which the dermatologist establishes a diagnosis and treatment plan [,,,,,,].
Patients attend an initial consultation with a PC dermatology doctor [,,,,,] or nurse [] who takes the photos of the affected area and uploads them to an online platform which a dermatologist can access remotely. In the study by Parsi [], the patients themselves take photos of their own skin and upload them to the platform.
For example, in the study by Zakaria et al. [], the referring clinicians are required to upload patient photographs and a brief history through a web-based telemedicine platform. An attending dermatologist reviews TD cases weekly and determines which patients require an in-person appointment at the dermatology clinic. When a dermatology visit is not recommended, the referring clinician is expected to coordinate the dermatologist’s recommended workup and treatment plan.
Os-Medendorp [] describes an e-health portal for patients consisting of e-consultation, a patient-tailored website, monitoring and self-management training.
Some studies consider the preliminary training of the doctor or patient in taking photographs correctly [,,]. Only in the studies by Os-Medendorp et al. [] and Parsi et al. [] do patients receive advice on self-care in addition to general information and individualized guidance on their dermatological condition.
In the article by López-Villegas et al. [], photographs are taken with a dermascope. All others use images taken with a mobile phone or similar devices [,,,,,,].
The face-to-face or conventional dermatological practice used in all the studies consists of an in-person consultation at a hospital [,,,,,,,].
Follow-up periods vary between three [], six [] and nine [] months and one [,,] and five years [].
3.2. Variables That Measure Direct and Indirect Costs
The types of economic analysis and economic perspectives vary greatly among these studies.
In general, nearly all the articles include the labour costs of the heathcare professionals and the patients [,,,,,]. However, in the case of Zarca [], the salary of patients is not included, as the patients being studied were inmates. The article by Yang [] includes the costs of PC, dermatology and TD consultations by establishing estimations (minimum, mean and maximum).
Zakaria [], López-Villegas [] and Vidal-Alaball [], assess personal costs (salary and time), technological costs, as well as the number of PC, dermatological and TD consultations. The three studies exclude maintenance costs and those incurred by patients’ companions.
In order to determine the number of face-to-face consultations saved in Spain, López- Villegas [] and Vidal-Alaball [] utilize the following equation:
Nº TD consultations − Nº consultations requested following
Zakaria [] utilizes a decision tree model to analyze the difference in costs for patient management and real costs instead of estimations.
López-Villegas [] and Vidal-Alaball [] assess, from the patients’ perspective, travel time (according to distance obtained using Google Maps) and the cost of gasoline (km/h, established by the Spanish Ministry of Finance). Both studies assume that the patients use their personal means of transport. They also consider waiting time, consultation time and legal average salary per hour.
3.3. Variables That Measure the Effectiveness of TD versus Face-to-Face Follow-Up
A medical treatment is generally considered to be cost-effective based on the following conditions: it provides an added health benefit at an equal or lower cost than the opposing treatment; it provides an added health benefit that is worth an additional cost; or it provides a lesser health benefit but comes with cost savings that are more valuable than the health benefit lost [].
A variety of questionnaires were used to measure effectiveness. On the one hand, in the study by Yang et al. [], the PC doctor has to respond to the question: “Without the TD service, how would this patient have otherwise received care for this condition?” with these options: (1)“I would take care of the issue myself”, (2) “I would refer the patient for an in-person dermatologist visit” or (3) “I would refer the patient to urgent care or an emergency room (ER)”. Based on their answers, 60% of cases would be handled by the PC doctor, 35.6% would be referred for a dermatologist visit and 4.4.% to an emergency room. On the other hand, Zarca [], uses the MAST Model (Model for Assessment of Telemedicine) to conduct a multidimensional evaluation in which PC doctors and dermatologists had to respond to a 6-item survey.
To measure quality of life, Os-Medendorp [] and Parsi [] utilize the Dermatology Life Quality Index (DLQI). Os-Medendorp [] also uses the Infants’ Dermatititis Quality of Life Index (IDQIL), while Parsi [], applies the “European Quality of Life Survey—5 Dimensions”, along with Quality-Adjusted Life Expectancy (QALE) and Quality-Adjusted Life Year (QALY).
As for the study by Datta et al. [], quality of life is determined according to the patient’s health condition: living longer with a dermatological condition versus living for a shorter time with perfect health.
For the purpose of gathering information on the status of skin, the “Impact of Chronic Skin Disease on Daily Life” questionnaire was used, along with the VAS scale to measure itch intensity and the “Health and Labour Questionnaire” to determine the loss of productivity [].
3.4. Comparison of Cost-Effectiveness in Each Method of Follow-Up
In the study by Parsi [], TD proves to have the greatest savings in relation to in-person consultation, which is in keeping with that of Zakaria [], in which TD obtains a mean savings of $110.12 per patient. The cost reduction, albeit lower, is also found to be evident in the studies by Vidal-Alaball [] and Datta [].
The study by López-Villegas [] indicates that TD effectively saves more than 50% of visits with respect to face-to-face follow-up within the public healthcare system. In the article by Vidal-Alaball [], only a small percentage of participants required an in-person consultation.
Yang [] finds that in-person consultations were reduced to 14%, representing significant cost savings. Zarca [] also identifies an evident cost reduction, yet one that depends on the number of patients treated.
Once again referencing the study by Datta [], it does not show statistically significant differences between both methods in terms of effectiveness.
Similary, Os-Medendorp [] fails to indentify any significant differences in terms of the quality of life of patients with atopic dermatitis, finding TD to be as effective as in-person consultation. However, the article by Parsi [] cites the existence of a slightly higher improvement in the quality of life of patients with the in-person method, yet, in terms of preference, the patients support online treatment.
Regarding satisfaction among doctors, Zarca [] finds that doctors display greater satisfaction with TD.
3.5. Level of Evidence and Quality of Articles Included
Table 4 shows the score of each item of the Downs and Black scale []. The mean score of the studies included was 22.12 (range: 17–26), considering that the highest possible score was 28 points. Based on the cut-off points suggested for categorizing studies according to their quality, one article was evaluated as “fair” (15–19 points) and seven were classified as “good” (20–25 points).

Table 4.
Methodological Quality of Studies.
4. Discussion
This systematic review has described studies that compare an online model (telemedicine) with in-office care in dermatology services. Although both models can be considered highly cost-effective, herein, it has been found that teledermatology reduces costs and is satisfactory to both patients and professionals. Reviews, such as those by Barbieri et al. [], agree that TD has the potential to provide access to suitable, quality care.
Nonetheless, the implementation of TD requires either investments or improvements in healthcare infrastructures and training for professionals, as well as for patients as relates to prevention [,]. From the legislative and ethical point of view, TD is considered to be a care method that should respect patient autonomy. According to European legislation, the informed consent of the patient is only necessary in cases where the images taken reveal the identity of the subject and/or are used for teaching or scientific purposes [].
Zakaria [] demonstrated that TD favours the remote classification and treatment of patients with dermatological conditions by means of implementing a triage system, which in turn contributes to a reduction of costs. The study by Barbieri et al. [] also suggests that TD is effective for classification in dermatological consultations and could increase their efficiency.
Several economic analyses have been performed on traditional TD models; however, most are cost-minimization studies and do not regularly use validated clinical outcomes or standardized cost-effectiveness measures [,].
It would be interesting to promote the use of dermascopes to take photographs of patients, as used in the study by López-Villegas []. Ferrandiz [] considers that images taken with a dermascope significantly improve diagnoses when compared to other clinical images. This study highlights the importance of a strong interconnection between PC and specialised care (hospital).
The pandemic and the situation caused by COVID-19 have consolidated the use of TD, with no need to interrupt treatments. What is more, these events favored access to specialized care for individuals who, due to specific circumstances, are unable to attend in person [] or who live in areas that are distant or difficult to reach []. Several studies promote the use of TD in rural or isolated areas, such as Cutler et al. [], for Haiti; Byamba et al. [], for Mongolia, one of the least densely populated countries worldwide; and Tran et al. [], for Cairo, Egypt. Similarly, the review by Kozera et al. [] supports the use of TD as a valuable service that allows patients in these rural areas to access care.
A systematic review of cost-effectiveness of store-and-forward TD in 2016 [] suggests that TD can be cost-effective when used as a triage mechanism to reduce face-to-face appointment requirements, although evidence was sparse. It suggests that further economic research is required for TD, which uses dermoscopes in combination with smartphone applications, as well as regarding the possibility and consequences of patients self-capturing and transmitting images.
In the present systematic review, all the studies utilized the method of saving and forwarding images [,,,,,,,]. Hadeler et al. [] states that this type of method is highly satisfactory for the patient, albeit some either declare feeling embarrassed by having photographs taken of their skin or express rejection due to social or religious issues.
All the studies analysed in our revision included pathologies that could be followed-up by TD. Some of them did not specify the dermatological condition [,,]. The rest of the articles included diverse diseases: benign growths [,], infection and eczematous dermatitis [], acnea [], atopic dermatitis [], psoriasis [] or non-benign pathologies (various types of cancer of the skin) []. Only one study [] refers to the cost-effectiveness TD in terms of skin cancer follow-up, although a face-to-face consultation was sometimes necessary.
It would also be necessary to discuss the cost-effectiveness of TD in terms of skin cancer follow-up comparing clinical trials, as during the COVID-19 pandemic this topic or the lack of this follow-up has been of great importance. We have found a study that analyzed the potential benefits gained from the addition of dermoscopic images to an internet-based skin cancer screening system []. In this trial, teledermoscopy has rendered high sensitivity and specificity to assist the dermatologist in making referral decisions, consequently improving the proportion of correct decisions where the relevant lesions were skin cancer (melanoma), premalignant lesions (actinic keratosis) or lesions suitable for follow-up (atypical nevus or others) [].
Finally, all the articles in this review state that both treatment methods are effective [,,,,,,,]. All the studies found improvements in quality of life with both online and in-office patients. Studies, such as those by Vyas et al. [], continue their commitment to TD as being a safe tool with comparable or superior effectiveness to traditional consultation, as well as favoring interprofessional collaboration in time and space. This method eliminates a large number of unnecessary referrals and could be utilized as an education tool for patients.
The limitations of this review resemble those found in the cost-effectiveness studies. They are related to the variability of the direct and indirect costs included or excluded and the sample of patients. It is necessary that the patients have similar characteristics and the sample number not be large so as to obtain more evident and reliable results that can be extrapolated to the rest of the population. In addition to the variety of methods used to evaluate costs, QALY and satisfaction have also been measured differently, which could lead to different results and interpretations. Finally, the protocols or method of treatment and the results obtained in each of the studies are quite heterogeneous when compared.
This present study carries implications for clinical practice, supporting the development and expansion of healthcare policies regarding telemedicine services in dermatology, and the results could be extrapolated to other healthcare services, such as online models focused on the patient [].
Current clinical practice shows that chronic dermatological conditions often require regular dermatological visits and are generally associated with high costs for medical care systems []. The TD model represents an innovative and profitable method that provides follow-up care to patients when applied properly with relatively low-risk conditions [].
This work is intended to be a tool to promote the creation of TD protocols that favor diagnoses and optimal treatments, ultimately reducing both societal and economic costs and providing dermatological care in our society that is more accessible and of higher quality [].
5. Conclusions
The studies included in this investigation consider that follow-up via teledermatology can be more cost-effective than face-to-face follow up.
Nevertheless, teledermatology can vary from one country to another, according to the healthcare system in the studies analyzed. Therefore, more work remains to be done for the purpose of establishing cost-effectiveness evaluation protocols that measure the main variables more heterogeneously and with larger samples of participants, making it possible to conduct analyses and thus demonstrate the quality of the evidence produced by rigorous systematic reviews.
Author Contributions
All authors contributed to the conception of the study and participated in critically appraising and revising the intellectual content of the manuscript. R.L.-L., M.Á.V.-M., A.L.-V. and F.A.V.-R. participated in the acquisition of data. R.J.B.-M.; S.P. and C.L.-C. were responsible for data analysis. M.Á.V.-M. prepared the manuscript draft. All authors have read and agreed to the published version of the manuscript.
Funding
This study has been funded by the Consejería de Salud y Familias of the Junta de Andalucía through the project No. AP-0011-2020C1-F2 AJ-1, belonging to the Call “Research and Innovation Projects in the field of Primary Care and Regional Hospitals and High Resolution Hospital Centers of the Andalusian Public Health System”, 2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Byamba, K.; Syed-Abdul, S.; Garcia-Romero, M.T.; Huang, C.-W.; Nergyi, S.; Nyamdorj, A.; Nguyen, P.-A.; Iqbal, U.; Paik, K.; Celi, L.; et al. Mobile teledermatology for a prompter and more efficient dermatological care in rural Mongolia. Br. J. Dermatol. 2014, 173, 265–267. [Google Scholar] [CrossRef]
- Andrees, V.; Klein, T.; Augustin, M.; Otten, M. Live interactive teledermatology compared to in-person care—A systematic review. J. Eur. Acad. Dermatol. Venereol. 2019, 34, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; English, J.C. Teledermatology: A Review and Update. Am. J. Clin. Dermatol. 2017, 19, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Salerni, G.; Fernández-Bussy, R. Teledermatología: Aplicaciones actuales y futuras. Dermatol. Argent. 2017, 23, 29–33. [Google Scholar]
- Kumar, S.; Bishnoi, A.; Vinay, K. Changing paradigms of dermatology practice in developing nations in the shadow of COVID -19: Lessons learnt from the pandemic. Dermatol. Ther. 2020, 33, e13472. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, P.; Romero-Aguilera, G.; Moreno-Ramírez, D. Teledermatología en tiempos de pandemia: El antes, el durante y el después. Dermatol. Práctica 2020, 112, 324–329. [Google Scholar] [CrossRef]
- Pearlman, R.L.; Le, P.B.; Brodell, R.T.; Nahar, V.K. Evaluation of patient attitudes towards the technical experience of synchronous teledermatology in the era of COVID-19. Arch. Dermatol. Res. 2021, 313, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Ayen-Rodríguez, A.; Llamas-Molina, J.M.; Cabrerizo-Carvajal, A.M.; Leon-López, F.J.; Ruiz-Villaverde, R. Teledermatología en el Área Sanitaria Centro Oeste de Granada desde atención primaria a especializada. Semergen 2021, 4, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Coustasse, A.; Sarkar, R.; Abodunde, B.; Metzger, B.J.; Slater, C.M. Use of Teledermatology to Improve Dermatological Access in Rural Areas. Telemed. E-Health 2019, 25, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Trettel, A.; Eissing, L.; Augustin, M. Telemedicine in dermatology: Findings and experiences worldwide—A systematic literature review. J. Eur. Acad. Dermatol. Venereol. 2017, 32, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Maddukuri, S.; Patel, J.; Lipoff, J.B. Teledermatology Addressing Disparities in Health Care Access: A Review. Curr. Dermatol. Rep. 2021, 10, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Stadler, P.; Senner, S.; Frey, S.; Clanner-Engelshofen, B.M.; Frommherz, L.H.; French, L.E.; Reinholz, M. Teledermatology in times of COVID-19. J. Dermatol. 2021, 48, 620–624. [Google Scholar] [CrossRef] [PubMed]
- McKoy, K.; Halpern, S.; Mutyambizi, K. International Teledermatology Review. Curr. Dermatol. Rep. 2021, 10, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Kaunitz, G.; Yin, L.; Nagler, A.R.; Sicco, K.L.; Kim, R.H. Assessing Patient Satisfaction with Live-Interactive Teledermatology Visits During the COVID-19 Pandemic: A Survey Study. Telemed. E-Health 2021. [Google Scholar] [CrossRef] [PubMed]
- Edwards, H.A.; Shen, X.; Soyer, H.P. Teledermatology Adaptations in the COVID-19 Era. Front. Med. 2021, 8, 675383. [Google Scholar] [CrossRef] [PubMed]
- Schuster, B.; Ziehfreund, S.; Tizek, L.; Krause, J.; Biedermann, T.; Zink, A. Wie offen ist die bayerische Bevölkerung für Teledermatologie? Eine Querschnittsstudie in ländlichen und städtischen Regionen Bayerns. Das Gesundh. 2019, 83, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Rustad, A.M.; Lio, P.A. Pandemic Pressure: Teledermatology and Health Care Disparities. J. Patient Exp. 2021, 8. [Google Scholar] [CrossRef]
- Arias, P.G.; Arenas, E.A.; Blanco, M.A.; Sánchez, J.R.; Gutiérrez, M.G.; García-Nieto, A.V. Aspectos medicolegales de la práctica de la teledermatología en España. Actas Dermosifiliogr. 2020, 112, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, G.; Bonfill, X. PRISMA declaration: A proposal to improve the publication of systematic reviews and meta-analyses. Med. Clín. 2010, 135, 507–511. [Google Scholar] [CrossRef]
- Hutton, B.; Catalá-López, F.; Moher, D. La extensión de la declaración PRISMA para revisiones sistemáticas que incorporan metaanálisis en red: PRISMA-NMA. Med. Clin. 2016, 147, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Deeks, J.J.; Dinnes, J.; D’Amico, R.; Sowden, A.J.; Sakarovitch, C.; Song, F.; Petticrew, M.; Altman, D.G.; International Stroke Trial Collaborative Group; European Carotid Surgery Trial Collaborative Group. Evaluating nonrandomized intervention studies. Health Technol. Assess 2003, 7, 1–173. [Google Scholar] [CrossRef]
- Saunders, L.D.; Soomro, G.M.; Buckingham, J.; Jamtvedt, G.; Raina, P. Assessing the Methodological Quality of Nonrandomized Intervention Studies. West. J. Nurs. Res. 2003, 25, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, A.; Miclau, T.A.; Maurer, T.; Leslie, K.S.; Amerson, E. Cost Minimization Analysis of a Teledermatology Triage System in a Managed Care Setting. JAMA Dermatol. 2021, 157, 52–58. [Google Scholar] [CrossRef]
- Lopez-Villegas, A.; Bautista-Mesa, R.J.; Baena-Lopez, M.A.; Alvarez-Moreno, M.L.; Montoro-Robles, J.E.; Vega-Ramirez, F.A.; Ordoñez-Naranjo, I.; Hernandez-Montoya, C.J.; Leal-Costa, C.; Peiró, S. Economic impact and cost savings of teledermatology units compared to conventional monitoring at hospitals in southern Spain. J. Telemed. Telecare 2020. [Google Scholar] [CrossRef]
- Vidal-Alaball, J.; Garcia-Domingo, J.L.; Cuyàs, F.G.; Peña, J.M.; Flores-Mateo, G.; Rosanas, J.D.; Valmaña, G.S. A cost savings analysis of asynchronous teledermatology compared to face-to-face dermatology in Catalonia. BMC Heal. Serv. Res. 2018, 18, 650. [Google Scholar] [CrossRef]
- Yang, X.; Barbieri, J.S.; Kovarik, C.L. Cost analysis of a store-and-forward teledermatology consult system in Philadelphia. J. Am. Acad. Dermatol. 2018, 81, 758–764. [Google Scholar] [CrossRef]
- Zarca, K.; Charrier, N.; Mahé, E.; Guibal, F.; Carton, B.; Moreau, F.; Durand-Zaleski, I. Tele-expertise for diagnosis of skin lesions is cost-effective in a prison setting: A retrospective cohort study of 450 patients. PLoS ONE 2018, 13, e0204545. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.K.; Warshaw, E.M.; Edison, K.; Kapur, K.; Thottapurathu, L.; Moritz, T.E.; Reda, D.J.; Whited, J.D. Cost and Utility Analysis of a Store-and-Forward Teledermatology Referral System. JAMA Dermatol. 2015, 151, 1323–1329. [Google Scholar] [CrossRef]
- Os-Medendorp, H.; Koffijberg, H.; Kok, P.C.M.E.; Zalm, A.; Bruin-Weller, M.S.; Pasmans, S.G.M.A.; Ros, W.J.G.; Thio, H.B.; Knol, M.J.; Bruijnzeel-Koomen, C.A.F.M. E-health in caring for patients with atopic dermatitis: A randomized controlled cost-effectiveness study of internet-guided monitoring and online self-management training. Br. J. Dermatol. 2012, 166, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Parsi, K.; Chambers, C.J.; Armstrong, A.W. Cost-effectiveness analysis of a patient-centered care model for management of psoriasis. J. Am. Acad. Dermatol. 2012, 66, 563–570. [Google Scholar] [CrossRef]
- Barbieri, J.S.; Kovarik, C.L. Inpatient and Tertiary Consultations in Teledermatology. Curr. Dermatol. Rep. 2016, 5, 83–89. [Google Scholar] [CrossRef]
- Tognetti, L.; Fiorani, D.; Russo, F.; Lazzeri, L.; Trovato, E.; Flori, M.L.; Moscarella, E.; Cinotti, E.; Rubegni, P. Teledermatology in 2020: Past, present and future per-spectives. Ital. J. Dermatol. Venerol. 2021, 156, 198–212. [Google Scholar] [CrossRef]
- Barbieri, J.S.; Nelson, C.A.; James, W.D.; Margolis, D.J.; Littman-Quinn, R.; Kovarik, C.L.; Rosenbach, M. The Reliability of Teledermatology to Triage Inpatient Dermatology Consultations. JAMA Dermatol. 2014, 150, 419. [Google Scholar] [CrossRef]
- Ferrándiz, L.; Ojeda-Vila, T.; Corrales, A.; Martín-Gutiérrez, F.J.; Ruíz-De-Casas, A.; Galdeano, R.; Álvarez-Torralba, I.; Sánchez-Ibáñez, F.; Domínguez-Toro, J.M.; Encina, F.; et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: A randomized teledermoscopy trial. J. Am. Acad. Dermatol. 2017, 76, 676–682. [Google Scholar] [CrossRef]
- Nelson, C.A.; Takeshita, J.; Wanat, K.A.; Bream, K.; Holmes, J.H.; Koenig, H.C.; Roth, R.R.; Vuppalapati, A.; James, W.D.; Kovarik, C.L. Impact of store-and-forward (SAF) teledermatology on outpatient dermatologic care: A prospective study in an underserved urban primary care setting. J. Am. Acad. Dermatol. 2016, 74, 484–490.e1. [Google Scholar] [CrossRef]
- Cutler, L.; Ross, K.; Withers, M.; Chiu, M.; Cutler, D. Teledermatology: Meeting the Need for Specialized Care in Rural Haiti. J. Health Care Poor Underserved 2019, 30, 1394–1406. [Google Scholar] [CrossRef]
- Tran, K.; Ayad, M.; Weinberg, J.; Cherng, A.; Chowdhury, M.; Monir, S.; El Hariri, M.; Kovarik, C. Mobile teledermatology in the developing world: Implications of a feasibility study on 30 Egyptian patients with common skin diseases. J. Am. Acad. Dermatol. 2011, 64, 302–309. [Google Scholar] [CrossRef]
- Kozera, E.K.; Yang, A.; Murrell, D.F. Patient and practitioner satisfaction with tele-dermatology including Australia’s indigenous population: A systematic review of the literature. Int. J. Women’s Dermatol. 2016, 2, 70–73. [Google Scholar] [CrossRef][Green Version]
- Snoswell, C.; Finnane, A.; Janda, M.; Soyer, H.P.; Whitty, J.A. Cost-effectiveness of Store-and-Forward Teledermatology. JAMA Dermatol. 2016, 152, 702–708. [Google Scholar] [CrossRef]
- Hadeler, E.; Gitlow, H.; Nouri, K. Definitions, survey methods, and findings of patient satisfaction studies in teledermatology: A systematic review. Arch. Dermatol. Res. 2020, 313, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Vyas, K.S.; Hambrick, H.R.; Shakir, A.; Morrison, S.; Tran, D.C.; Pearson, K.; Vasconez, H.C.; Mardini, S.; Gosman, A.A.; Dobke, M.; et al. A Systematic Review of the Use of Telemedicine in Plastic and Reconstructive Surgery and Dermatology. Ann. Plast. Surg. 2017, 78, 736–768. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).