Cumulative Incidence of SARS-CoV-2 in Healthcare Workers at a General Hospital in Germany during the Pandemic—A Longitudinal Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Management and Statistical Methods
3. Results
4. Discussion
4.1. PCR
4.2. Seroprevalence
4.3. Long COVID or Post-COVID Syndrome (PCS)
4.4. Vaccination
4.5. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koh, D. Occupational risks for COVID-19 infection. Occup. Med. 2020, 70, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Airoldi, C.; Patrucco, F.; Milano, F.; Alessi, D.; Sarro, A.; Rossi, M.; Cena, T.; Borrè, S.; Faggiano, F. High Seroprevalence of SARS-CoV-2 among Healthcare Workers in a North Italy Hospital. Int. J. Environ. Res. Public Health 2021, 18, 3343. [Google Scholar] [CrossRef]
- Galán, M.I.; Velasco, M.; Casas, M.L.; Goyanes, M.J.; Rodríguez-Caravaca, G.; Losa-García, J.E.; Noguera, C.; Castilla, V.; Weber, A.A.; Punter, J.C.A.; et al. Hospital-Wide SARS-CoV-2 seroprevalence in health care workers in a Spanish teaching hospital. Enferm. Infecc. Y Microbiol. Clín. 2020. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Krastinova, E.; Garrait, V.; Lecam, M.-T.; Coste, A.; Varon, E.; Delacroix, I.; Ali, A.S.; Jung, C.; Smati, M.; Cherbit, M.; et al. Household transmission and incidence of positive SARS-CoV-2 RT-PCR in symptomatic healthcare workers, clinical course and outcome: A French hospital experience. Occup. Environ. Med. 2020, 78, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Mutambudzi, M.; Niedzwiedz, C.; Macdonald, E.B.; Leyland, A.; Mair, F.; Anderson, J.; Celis-Morales, C.; Cleland, J.; Forbes, J.; Gill, J.; et al. Occupation and risk of severe COVID-19: Prospective cohort study of 120 075 UK Biobank participants. Occup. Environ. Med. 2020, 78, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.V.; Wood, R.; Gribben, C.; Caldwell, D.; Bishop, J.; Weir, A.; Kennedy, S.; Reid, M.; Smith-Palmer, A.; Goldberg, D.; et al. Risk of hospital admission with coronavirus disease 2019 in healthcare workers and their households: Nationwide linkage cohort study. BMJ 2020, 371, m3582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M. Estimation of differential occupational risk of COVID-19 by comparing risk factors with case data by occupational group. Am. J. Ind. Med. 2020, 64, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Möhner, M.; Wolik, A. Differences in COVID-19 Risk Between Occupational Groups and Employment Sectors in Germany. Dtsch. Ärztebl. Int. 2020, 117, 641–642. [Google Scholar] [CrossRef]
- Ferland, L.; Carvalho, C.; Dias, J.G.; Lamb, F.; Adlhoch, C.; Suetens, C.; Beauté, J.; Kinross, P.; Plachouras, D.; Hannila-Handelberg, T.; et al. Risk of hospitalization and death for healthcare workers with COVID-19 in nine European countries, January 2020–January 2021. J. Hosp. Infect. 2021, 119, 170–174. [Google Scholar] [CrossRef]
- Gholamia, M.; Fawada, I.; Shadana, S.; Rowaieea, R.; Ghanema, H.A.; Khamisb, A.H.; Hoa, S.B. COVID-19 and healthcare workers: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 104, 335–346. [Google Scholar] [CrossRef]
- Spilchuk, V.; Arrandale, V.H.; Armstrong, J. Potential risk factors associated with COVID-19 in health care workers. Occup. Med. 2021, 72, 35–42. [Google Scholar] [CrossRef]
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Di Cristanziano, V.; Osebold, L.; et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. Lancet Reg. Health Eur. 2021, 6, 100122. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Cranen, R.; Peters, C.; Wisplinghoff, H.; Nienhaus, A.; Bach, A.D.; Michels, G. Prävalenz von SARS-CoV-2 bei Mitarbeitern eines Krankenhauses der Regel-/Schwerpunktversorgung in Nordrhein-Westfalen. DMW—Dtsch. Med. Wochenschr. 2021, 146, e30–e38. [Google Scholar] [CrossRef] [PubMed]
- Menting, T.; Krause, K.; Benz-Tettey, F.; Boehringer, R.; Laufer, D.; Gruber, B.; Crump, M.; Schieferdecker, R.; Reuhl, S.; Kaeferstein, A.; et al. Low-threshold SARS-CoV-2 testing facility for hospital staff: Prevention of COVID-19 outbreaks. Int. J. Hyg. Environ. Health 2021, 231, 113653. [Google Scholar] [CrossRef]
- Brehm, T.T.; Schwinge, D.; Lampalzer, S.; Schlicker, V.; Küchen, J.; Thompson, M.; Ullrich, F.; Huber, S.; Schmiedel, S.; Addo, M.M.; et al. Seroprevalence of SARS-CoV-2 antibodies among hospital workers in a German tertiary care center: A sequential follow-up study. Int. J. Hyg. Environ. Health 2020, 232, 113671. [Google Scholar] [CrossRef] [PubMed]
- Korth, J.; Wilde, B.; Dolff, S.; Anastasiou, O.E.; Krawczyk, A.; Jahn, M.; Cordes, S.; Ross, B.; Esser, S.; Lindemann, M.; et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J. Clin. Virol. 2020, 128, 104437. [Google Scholar] [CrossRef]
- Ständige Impfkommission (STIKO). Beschluss der STIKO zur 4. Aktualisierung der COVID-19-Impfempfehlung und die Dazugehörende Wissenschaftliche Begründung. 2021. Available online: https://www.rki.de/DE/Content/Kommissionen/STIKO/Empfehlungen/Vierte_Empfehlung_01042021_Download.pdf?__blob=publicationFile (accessed on 8 October 2021).
- Kozak, A.; Nienhaus, A. COVID-19 Vaccination: Status and Willingness to Be Vaccinated among Employees in Health and Welfare Care in Germany. Int. J. Environ. Res. Public Health 2021, 18, 6688. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, L.T.; Brunink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [Green Version]
- Euroimmun. Characteristics of EUROIMMUN ELISA for COVID-19 Diagnostics. 2020. Available online: https://www.coronavirus-diagnostics.com/documents/Indications/Infections/Coronavirus/YI_2606_I_U.K._C.pdf (accessed on 16 January 2022).
- Euroimmun. Anti-SARS-CoV-2-QuantiVac-ELISA (IgG). 2021. Available online: https://www.coronavirus-diagnostik.de/documents/Indications/Infections/Coronavirus/EI_2606_D_DE_E.pdf (accessed on 16 January 2022).
- Roche. Elecsys Anti-SARS-CoV-2. 2021. Available online: https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html. (accessed on 16 January 2022).
- Van Elslande, J.; Houben, E.; Depypere, M.; Brackenier, A.; Desmet, S.; André, E.; Van Ranst, M.; Lagrou, K.; Vermeersch, P. Diagnostic performance of seven rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients. Clin. Microbiol. Infect. 2020, 26, 1082–1087. [Google Scholar] [CrossRef]
- Team R. R—A Language and Environment for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 16 January 2022).
- Treibel, T.A.; Manisty, C.; Burton, M.; McKnight, Á.; Lambourne, J.; Augusto, J.; Couto-Parada, X.; Cutino-Moguel, T.; Noursadeghi, M.; Moon, J.C. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet 2020, 395, 1608–1610. [Google Scholar] [CrossRef]
- Diel, R.; Hittel, N.; Nienhaus, A. Point-of-Care COVID-19 Antigen Testing in Exposed German Healthcare Workers—A Cost Model. Int. J. Environ. Res. Public Health 2021, 18, 10767. [Google Scholar] [CrossRef] [PubMed]
- Hunter, E.; Price, D.A.; Murphy, E.; van der Loeff, I.S.; Baker, K.F.; Lendrem, D.; Lendrem, C.; Schmid, M.L.; Pareja-Cebrian, L.; Welch, A.; et al. First experience of COVID-19 screening of health-care workers in England. Lancet 2020, 395, e77–e78. [Google Scholar] [CrossRef]
- Keehner, J.; Horton, L.E.; Pfeffer, M.A.; Longhurst, C.A.; Schooley, R.T.; Currier, J.S.; Abeles, S.R.; Torriani, F.J. SARS-CoV-2 Infection after Vaccination in Health Care Workers in California. N. Engl. J. Med. 2021, 384, 1774–1775. [Google Scholar] [CrossRef]
- Herzberg, J.; Vollmer, T.; Fischer, B.; Becher, H.; Becker, A.-K.; Sahly, H.; Honarpisheh, H.; Guraya, S.Y.; Strate, T.; Knabbe, C. Half-Year Longitudinal Seroprevalence of SARS-CoV-2-Antibodies and Rule Compliance in German Hospital Employees. Int. J. Environ. Res. Public Health 2021, 18, 10972. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Schiebel, J.; Hufert, F.; Gremmels, H.-D.; Spallek, J. COVID-19 among Healthcare Workers: A Prospective Serological-Epidemiological Cohort Study in a Standard Care Hospital in Rural Germany. Int. J. Environ. Res. Public Health 2021, 18, 10999. [Google Scholar] [CrossRef]
- Gaber, T.A.-Z.K.; Ashish, A.; Unsworth, A. Persistent post-covid symptoms in healthcare workers. Occup. Med. 2021, 71, 144–146. [Google Scholar] [CrossRef]
- Rao, S.; Amara, V.; Chaudhuri, S.; Rao, B.K.; Todur, P. “Post-COVID-19 syndrome:” The New Pandemic Affecting Healthcare Workers and How the Frontline Warriors Are Battling it. Indian J. Palliat. Care 2021, 27, 313–318. [Google Scholar] [CrossRef]
- Karagiannidis, C.; Spies, C.; Kluge, S.; Marx, G.; Janssens, U. Impfbereitschaft unter Intensivmedizinischem Personal: Ängsten Entgegenwirken. Med. Klin.-Intensiv. Notf. 2021, 116, 216–219. [Google Scholar] [CrossRef]
- Schug, C.; Erim, Y.; Geiser, F.; Hiebel, N.; Beschoner, P.; Jerg-Bretzke, L.; Albus, C.; Weidner, K.; Steudte-Schmiedgen, S.; Borho, A.; et al. Bereitschaft zur COVID-19-Impfung unter Beschäftigten im Gesundheitswesen in Deutschland. Bundesgesundh.-Gesundh.-Gesundh. 2021, 65, 74–85. [Google Scholar] [CrossRef]
- Schianchi, A.; Ughi, N.; Cassano, G.; Del Gaudio, F.; Dicuonzo, A.; Scaglione, F.; Alberti, P.M.; Rossetti, C.; Micheloni, G.; Zoppini, L.; et al. Sick leave request following anti-COVID-19 vaccine administration is low among healthcare workers: Results from a retrospective cross-sectional monocentric study. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7218–7222. [Google Scholar] [CrossRef] [PubMed]
- Prato’, S.; Paladino, M.E.; Riva, M.A.; Belingheri, M. COVID-19 Vaccination and Asymptomatic Infection. J. Occup. Environ. Med. 2021, 63, e868–e870. [Google Scholar] [CrossRef] [PubMed]
- Barriere, J.; Carles, M.; Audigier-Valette, C.; Re, D.; Zoubir, A.; Seitz-Polski, B.; Gounant, V.; Descamps, D.; Zalcman, G. Third dose of anti-SARS-CoV-2 vaccine for patients with cancer: Should humoral responses be monitored? A position paper. Eur. J. Cancer 2022, 162, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Berar-Yanay, N.; Freiman, S.; Shapira, M.; Saffoury, A.; Elemy, A.; Hamze, M.; Elhaj, M.; Zaher, M.; Matanis, L.; Armaly, Z.A. Waning Humoral Response 3 to 6 Months after Vaccination with the SARS-COV-2 BNT162b2 mRNA Vaccine in Dialysis Patients. J. Clin. Med. 2021, 11, 64. [Google Scholar] [CrossRef]
- Timmermann, L.; Globke, B.; Lurje, G.; Schmelzle, M.; Schöning, W.; Öllinger, R.; Pratschke, J.; Eberspächer, B.; Drosten, C.; Hofmann, J.; et al. Humoral Immune Response following SARS-CoV-2 Vaccination in Liver Transplant Recipients. Vaccines 2021, 9, 1422. [Google Scholar] [CrossRef]
- Maya, S.; Padda, G.; Close, V.; Wilson, T.; Ahmed, F.; Marseille, E.; Kahn, J.G. Optimal strategies to screen health care workers for COVID-19 in the US: A cost-effectiveness analysis. Cost Eff. Resour. Alloc. 2022, 20, 2. [Google Scholar] [CrossRef]
- Tadi, L.J.; Chunchu, S.R.; Srinivas, M.; Mallamgunta, S.; Ravula, U.; Ariyanachi, K.; Dara, C.; Sandepogu, T.S. Screening of Asymptomatic Healthcare Workers for SARS-COV-2 for Occult Infections: A Cross-Sectional Study. Cureus 2021, 13, e19341. [Google Scholar] [CrossRef]
- Johannesen, C.K.; Rezahosseini, O.; Gybel-Brask, M.; Kristensen, J.H.; Hasselbalch, R.B.; Pries-Heje, M.M.; Nielsen, P.B.; Knudsen, A.D.; Fogh, K.; Norsk, J.B.; et al. Risk Factors for Being Seronegative following SARS-CoV-2 Infection in a Large Cohort of Health Care Workers in Denmark. Microbiol. Spectr. 2021, 9, e0090421. [Google Scholar] [CrossRef]
- Lopez, A.; Kosnik, R.; Blanc, P.D.; Taylor, B.R.; Guntur, S. Testing for SARS-CoV-2 in Symptomatic Vaccinated and Unvaccinated Health Care Workers During the Delta Variant Surge. J. Occup. Environ. Med. 2021, 64, 179–181. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
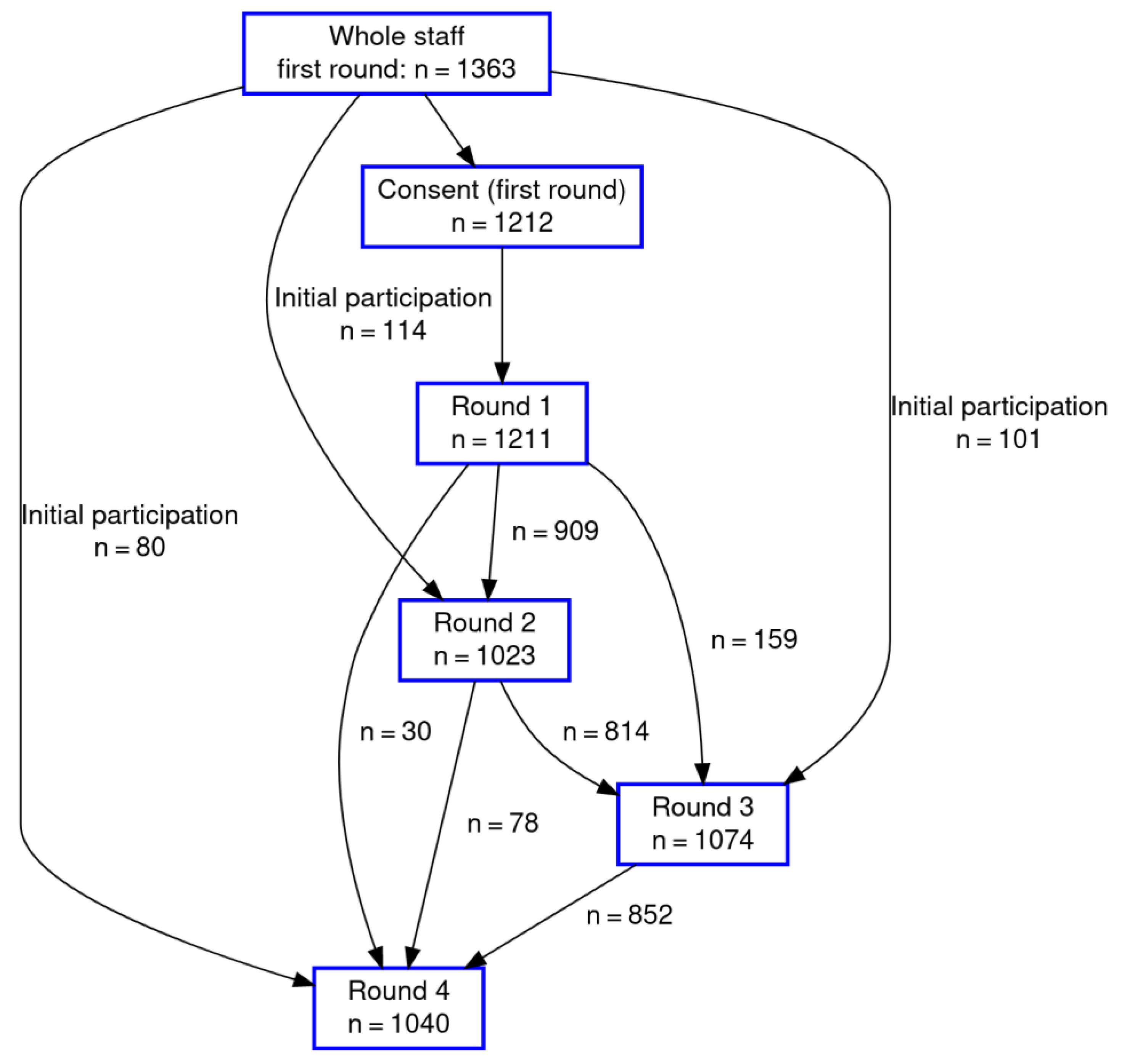

| Number of Rounds Participated | N/Mean | %/Range |
|---|---|---|
| 1 | 287 | 19.1 |
| 2 | 246 | 16.3 |
| 3 | 324 | 21.5 |
| 4 | 649 | 43.1 |
| Gender | ||
| Male | 206 | 13.7 |
| Female | 792 | 52.6 |
| Unknown | 508 | 33.6 |
| Age (mean ± SD) [min.–max.] | 43.7 ± 13.2 | 18–75 |
| Ward | ||
| Intensive care (with patients requiring ventilation) | 125 | 8.3 |
| Wards or tasks with patient contact | 657 | 43.6 |
| Tasks without patient contact | 113 | 7.5 |
| Unknown | 611 | 40.6 |
| Total | 1506 | 100.0 |
| PCR+ | IgG+ | IgG or PCR+ | Participants | ||||
|---|---|---|---|---|---|---|---|
| Time of Test | N | % | N | % | N | % | N (%) |
| Round 1 | 3 (16) * | 1.6 | 40 | 3.3 | 47 | 3.9 | 1211 |
| Round 2 | 8 | 0.8 | 31 | 3.0 | 38 | 3.7 | 1023 |
| Round 3 | 21 | 2.0 | 56 | 5.2 | 62 | 5.8 | 1074 |
| Round 4 (IgM+) * | |||||||
| All | 4 | 0.4 | 103 | 9.9 | 103 | 9.9 | 1040 |
| Not vaccinated (IgG) | 2 | 0.9 | 60 | 26.1 | 60 | 26.1 | 230 (22.1) |
| Vaccinated (IgG/IgM) ** | 2 | 0.2 | 43 | 5.3 | 43 | 5.3 | 810 (77.9) |
| Either PCR or Ig positive | 52 | 3.5 | 154 | 10.2 | 165 | 11.0 | 1506 |
| IgG and PCR− | IgG or PCR+ | Logistic Regression * | ||||
|---|---|---|---|---|---|---|
| Ward or Task | N | % | N | % | OR | 95% CI |
| Intensive care unit (ICU) | 101 | 80.8 | 24 | 19.2 | 4.42 | 1.73–13.6 |
| Normal care | 566 | 86.1 | 91 | 13.9 | 2.92 | 1.27–8.49 |
| No patient contact | 108 | 95.6 | 5 | 4.4 | 1 | -- |
| Symptom | N | % |
|---|---|---|
| Quality of life still diminished | 23 | 31.5 |
| General health still diminished | 21 | 28.8 |
| Physical fitness reduced | 26 | 35.6 |
| Weariness, tiredness increased | 35 | 47.9 |
| Memory problems increased | 31 | 42.5 |
| Shortness of breath increased | 22 | 30.1 |
| BioNTech/Pfizer | AstraZeneca | Other | All | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (% *) | |
| First vaccine dose | 397 (49.0) | 401 (49.5) | 12 (1.5) | 810 (77.8) |
| Second vaccine dose | 382 (96.2) | 7 (1.8) | 8 (2.0) | 397 (38.2) |
| BioNTech/Pfizer | AstraZeneca | |||
|---|---|---|---|---|
| First Vaccination | N | % | N | % |
| No | 95 | 24.1 | 45 | 11.5 |
| Local | 213 | 53.9 | 41 | 10.5 |
| Systemic | 22 | 5.6 | 109 | 27.8 |
| Local and systemic | 65 | 16.5 | 197 | 50.3 |
| Total * | 395 | 100.0 | 392 | 100.0 |
| Sick leave in days | ||||
| 1 | 28 | 7.1 | 23 | 5.9 |
| 2 | 20 | 5.1 | 12 | 3.1 |
| 3–7 | 10 | 2.5 | 4 | 1.0 |
| Sick leave, all | 58 | 14.7 | 42 | 10.7 |
| Second vaccination | ||||
| No | 75 | 19.7 | 4 | 100.0 |
| Local | 100 | 26.3 | 0 | 0.0 |
| Systemic | 86 | 22.6 | 0 | 0.0 |
| Local and systemic | 119 | 31.3 | 0 | 0.0 |
| Total ** | 380 | 100.0 | 4 | 100.0 |
| Sick leave in days | ||||
| 1 | 23 | 6.1 | 0 | -- |
| 2 | 19 | 5.0 | 0 | -- |
| 3–5 | 3 | 0.8 | 0 | -- |
| Sick leave, all | 45 | 11.8 | 0 | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Platten, M.; Nienhaus, A.; Peters, C.; Cranen, R.; Wisplinghoff, H.; Kersten, J.F.; Bach, A.D.; Michels, G. Cumulative Incidence of SARS-CoV-2 in Healthcare Workers at a General Hospital in Germany during the Pandemic—A Longitudinal Analysis. Int. J. Environ. Res. Public Health 2022, 19, 2429. https://doi.org/10.3390/ijerph19042429
Platten M, Nienhaus A, Peters C, Cranen R, Wisplinghoff H, Kersten JF, Bach AD, Michels G. Cumulative Incidence of SARS-CoV-2 in Healthcare Workers at a General Hospital in Germany during the Pandemic—A Longitudinal Analysis. International Journal of Environmental Research and Public Health. 2022; 19(4):2429. https://doi.org/10.3390/ijerph19042429
Chicago/Turabian StylePlatten, Martin, Albert Nienhaus, Claudia Peters, Rita Cranen, Hilmar Wisplinghoff, Jan Felix Kersten, Alexander Daniel Bach, and Guido Michels. 2022. "Cumulative Incidence of SARS-CoV-2 in Healthcare Workers at a General Hospital in Germany during the Pandemic—A Longitudinal Analysis" International Journal of Environmental Research and Public Health 19, no. 4: 2429. https://doi.org/10.3390/ijerph19042429
APA StylePlatten, M., Nienhaus, A., Peters, C., Cranen, R., Wisplinghoff, H., Kersten, J. F., Bach, A. D., & Michels, G. (2022). Cumulative Incidence of SARS-CoV-2 in Healthcare Workers at a General Hospital in Germany during the Pandemic—A Longitudinal Analysis. International Journal of Environmental Research and Public Health, 19(4), 2429. https://doi.org/10.3390/ijerph19042429







