Cognitive Impairment in End Stage Renal Disease Patients Undergoing Hemodialysis: Markers and Risk Factors
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Markers Related to the Inflammatory Process and Cell Damage
3.2. Markers Related to Uremic Toxins
3.3. Disorders Associated with ESRD
3.4. Cognitive Function and Systemic Cardiovascular Risk Factors
3.5. Fluctuations in Cerebral Blood Flow and Cognitive Function
| Correlates/Surrogates of Cognitive Impairment | Studied Population | Cognitive Impairment Assessment | Ref. |
|---|---|---|---|
| Markers Related to the Inflammatory Process and Cell Damage | |||
| Neurobiomarker S100 calcium binding protein B (S100B); S100B level was independent predictor of CI (cut-off values for predicting CI was 36.1 pg/mL). | 30 HD * | MMSE | [6] |
| Bone turnover marker RANKL (Receptor activator of nuclear factor-kappa B ligand) level linked with better cognitive function. MoCA (β = 1.14, 95% CI 0.17 to 2.11) and CASI (β = 3.06, 95% CI 0.24 to 5.88). | 251 HD 37 HC ** | MoCA scale Cognitive Abilities Screening Instrument (CASI) | [10] |
| Endothelium-related biomarkers: syndecan-1, intercellular adhesion molecule-1 (ICAM-1), and angiopoietin-2 (AGPT2) correlated with better CI. | 216 HD | Cambridge Cognitive Examination MMSE | [12] |
| Brain-derived neurotrophic factor (BDNF) and platelets count correlated with cognitive test scores. | 58 HD 20 HC | MMSE MoCA scale | [13] |
| Insulin-like growth factor-1 (IGF-1) low levels is risk factor for severe CI and dementia. | 93 HD | MMSE | [19] |
| FGF-23 linked with worse performance on a composite memory score; FGF-23 was independently associated with a lower memory score. | 263 HD | Wechsler Memory Scale-III, Word List Learning Subtest, Wechsler Adult Intelligence Scale-III, Block Design and Digit Symbol-Coding Subtests, Trail Making Tests A and B | [25] |
| Uremic Toxins | |||
| Uric acid level showed negative correlation with MMSE score (r = −0.307, p = 0.014). | 180 HD | MMSE | [31] |
| Protein-bound uremic solute—indole-3-acetic acid (IAA) serum level was associated with a poor MMSE (β = −0.90) and a poor CASI (β = −3.29). | 230 HD | MMSE MoCA CASI | [34] |
| Circulating free indoxyl sulfate levels were negatively associated with the MMSE scores (β = −0.62) and the CASI scores (β = −1.97). | 260 HD | MMSE CASI | [35] |
| 4-hydroxyphenylacetate (RR = 1.16), hippurane (RR = 1.24), phenylacetylglutamine (RR = 1.39), prolyl-hydroxyproline (RR = 1.20) showed association with CI scores. | 141 HD 180 HC | Trail Making Test Part B Digit Symbol Substitution Test | [36] |
| Hyponatremia correlated with symptoms of depression. | 200 HD | Patient Health Questionnaire Perceived Deficit Questionnaire-5 | [37] |
| Plasma phosphorus level (>6 mg/dL, p = 0.034), inadequate dialysis dose (Kt/V < 1, 2, p = 0.023) and hyponatremia (Na < 135 mEq/L, p = 0.001) infuenced poor executive and functional status. | 56 HD | Modified Mini-Mental State (3MS) Trail Making Test A and B | [38] |
| Disorders Associated with ESRD | |||
| Vitamin D—25(OH)D levels correlated with executive functions (β = 0.16; p < 0.05) but no with memory assessment tests. | 255 HD | MMSE, Wechsler Memory Scale-III (WMS-III), Word List Learning Subtest Wechsler Adult Intelligence Scale-III (WAIS-III) Block Design and Digit Symbol-Coding subtests Trail Making Test A and B | [44] |
| Anemia correlated with CI (increase in Hb values improved cognitive functions); improvement in Hb (p < 0.05) correlated with cerebral artery blood flow. | 120 HD | MMSE | [46] |
| Patients receiving EPO had a 39% lower risk of general dementia than those in the non-EPO group. The risk of dementia was further reduced in HD patients with EPO treatment in combination with iron. | 43, 906 HD | Clinical data | [47] |
| Systemic cardiovascular risk factors | |||
| Ankle-brachial index ABI < 0.9 showed association with the MoCA score (β = 0.62, p = 0.011) and the CASI score (β = 1.43, p = 0.026). Arterial stiffness surrogate—baPWV showed negative correlation with CASI (β = −0.70, p = 0.009). | 136 HD | MoCA CASI | [50] |
| Pulse wave velocity (PWV) values were associated with worse MMSE scores (β = −0.36, p = 0.001), and MiniCog scores (β = −0.26, p = 0.02). PWV value was significantly associated with TMTA but not with TMTB. | 72 HD | MMSE, Part A (TMTA) and Part B (TMTB) Mini-Cog Test | [51] |
| Maximum orthostatic systolic blood pressure reduction was independently and negatively associated with short (β = −0.05, p = 0.029) and delayed (β = −0.05, p = 0.035) recall memory in dialysis patients but not in controls. | 80 HD 80 HC | MoCA Auditory Verbal Learning Test (AVLT) | [56] |
| Common carotid artery pulsation index (CCAPI) had an independent effect on attention retention in HD patients (β = −0.36, p = 0.01). | 37 HD 18 HC | MoCA | [57] |
| Left ventricle function—LVEF showed inverse association with cognitive impairment (β = 0.87, p = 0.022). | 72 HD | MMSE | [61] |
| Fluctuations in cerebral blood flow | |||
| Marker of ischaemic cerebral small-vessel disease: prevalence of white matter hyperintensities (WMH) on magnetic resonance imaging was significantly higher in HD patients than in the healthy subjects (p < 0.01). | 179 HD 58 HC | WMH (white matter hyperintensities) on MRI is known CI risk factor in the general population | [64] |
| Cerebral arterial mean flow velocity (MFV) decline was correlated with the intradialytic decline in cognitive functions, including global functions, executive functions, and verbal fluency (p < 0.01). | 97 HD | National Institute of Neurologic Disorders and Stroke-Canadian Stroke Network Neuropsychological Battery | [65] |
| Reduced regional cerebral venous oxygen saturation (SvO) of two bilateral cortical, thalamic, septal, internal cerebral and basal regions in HD patients was significantly lower than in HC. | 54 HD 54 HC | MMSE MoCA | [66] |
| Cerebral exigenation (rSO2) values in HD patients was lower compared to cognitively healthy people. The relation between rSO2 and MoCA score was significant after adjustment for age and gender (p = 0.007). | 39 HD | MoCA | [67] |
3.6. Other Unclassified Factors
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Johansen, K.L.; Chertow, G.M.; Foley, R.N.; Gilbertson, D.T.; Herzog, C.A.; Ishani, A.; Israni, A.K.; Ku, E.; Tamura, M.K.; Li, S.; et al. US Renal Data System 2020 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2021, 77, A7–A8. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.M.; Malecki, A.K.; Müller, K.; Schönfeld, R.; Girndt, M.; Mohr, P.; Hiss, M.; Kielstein, H.; Jäger, K.; Kielstein, J.T. Effect of a single dialysis session on cognitive function in CKD5D patients: A prospective clinical study. Nephrol. Dial. Transplant. 2015, 30, 1551–1559. [Google Scholar] [CrossRef]
- Pereira, A.A.; Weiner, D.E.; Scott, T.; Chandra, P.; Bluestein, R.; Griffith, J.; Sarnak, M.J. Subcortical cognitive impairment in dialysis patients. Hemodial. Int. 2007, 11, 309–314. [Google Scholar] [CrossRef]
- Murray, A.M.; Knopman, D.S. Cognitive Impairment in CKD: No Longer an Occult Burden. Am. J. Kidney Dis. 2010, 56, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, C.W. Recurrent circulatory stress: The dark side of dialysis. Semin. Dial. 2010, 23, 449–451. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, H.W.; Lee, Y.J.; Park, S.; Kim, Y.W.; Kim, S.E.; Kim, I.H.; Park, J.H.; Park, K.M. Serum S100B represents a biomarker for cognitive impairment in patients with end-stage renal disease. Clin. Neurol. Neurosurg. 2020, 195, 105902. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007, 9 (Suppl. S1), S1. [Google Scholar] [CrossRef]
- Walsh, M.C.; Choi, Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front. Immunol. 2014, 5, 511. [Google Scholar] [CrossRef] [PubMed]
- Van Campenhout, A.; Golledge, J. Osteoprotegerin, vascular calcification and atherosclerosis. Atherosclerosis 2009, 204, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-H.; Lin, Y.-T.; Chen, C.-S.; Chiu, Y.-W.; Tsai, J.-C.; Kuo, P.-L.; Hsu, Y.-L.; Ljunggren, Ö.; Fellström, B.; Kuo, M.-C. Associations of Bone Turnover Markers with Cognitive Function in Patients Undergoing Hemodialysis. Dis. Markers 2020, 2020, 8641749. [Google Scholar] [CrossRef]
- Martins-Filho, R.K.; Zotin, M.C.; Rodrigues, G.; Pontes-Neto, O. Biomarkers Related to Endothelial Dysfunction and Vascular Cognitive Impairment: A Systematic Review. Dement. Geriatr. Cogn. Disord. 2020, 49, 365–374. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, C.M.M.F.; Da Silva, B.R.D.; Costa, B.G.; Sartori, V.F.; Meneses, G.C.; Bezerra, G.F.; Martins, A.M.C.; Libório, A.B. Cognitive impairment, endothelial biomarkers and mortality in maintenance haemodialysis patients: A prospective cohort study. Nephrol. Dial. Transplant. 2020, 35, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Jin, L.-N.; Shen, J.-Q.; Liu, J.-F.; Jiang, R.-Y.; Yang, L.; Zhang, J.; Luo, A.-L.; Miao, L.-Y.; Yang, C. Differential expression of serum biomarkers in hemodialysis patients with mild cognitive decline: A prospective single-center cohort study. Sci. Rep. 2018, 8, 12250. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and Synaptic Plasticity, Cognitive Function, and Dysfunction. Inflammation 2014, 220, 223–250. [Google Scholar] [CrossRef]
- Leal, G.; Comprido, D.; Duarte, C.B. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 2014, 76, 639–656. [Google Scholar] [CrossRef] [PubMed]
- Viana, J.L.; Kosmadakis, G.C.; Watson, E.L.; Bevington, A.; Feehally, J.; Bishop, N.C.; Smith, A.C. Evidence for Anti-Inflammatory Effects of Exercise in CKD. J. Am. Soc. Nephrol. 2014, 25, 2121–2130. [Google Scholar] [CrossRef]
- Carlsson, A.C.; Carrero, J.-J.; Stenvinkel, P.; Bottai, M.; Barany, P.; Larsson, A.; Ärnlöv, J. Endostatin, Cathepsin S, and Cathepsin L, and Their Association with Inflammatory Markers and Mortality in Patients Undergoing Hemodialysis. Blood Purif. 2015, 39, 259–265. [Google Scholar] [CrossRef]
- Norden, D.M.; Godbout, J.P. Review: Microglia of the aged brain: Primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 2013, 39, 19–34. [Google Scholar] [CrossRef]
- Prelevic, V.; Radunovic, D.; Antunovic, T.; Ratkovic, M.; Gligorovic-Bahranovic, N.; Gledovic, B.; Vujosevic, S.; Nedovic-Vukovic, M.; Basic-Jukic, N. Increased Serum Level of IGF-1 Correlates with Better Cognitive Status in End-Stage Renal Disease Patients Undergoing Hemodialysis. Ther. Apher. Dial. 2018, 22, 118–123. [Google Scholar] [CrossRef]
- Kurella Tamura, M.; Larive, B.; Unruh, M.L.; Stokes, J.B.; Nissenson, A.; Mehta, R.L.; Chertow, G.M.; The Frequent Hemodialysis Network Trial Group. Prevalence and correlates of cognitive impairment in hemodialysis patients: The Frequent Hemodialysis Network trials. Clin. J. Am. Soc. Nephrol. 2010, 5, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, O.M.; Januzzi, J.L.; Isakova, T.; Laliberte, K.; Smith, K.; Collerone, G.; Sarwar, A.; Hoffmann, U.; Coglianese, E.; Christenson, R.; et al. Fibroblast Growth Factor 23 and Left Ventricular Hypertrophy in Chronic Kidney Disease. Circulation 2009, 119, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Kirkpantur, A.; Balci, M.; Gurbuz, O.A.; Afsar, B.; Canbakan, B.; Akdemir, R.; Ayli, M.D. Serum fibroblast growth factor-23 (FGF-23) levels are independently associated with left ventricular mass and myocardial performance index in maintenance haemodialysis patients. Nephrol. Dial. Transplant. 2010, 26, 1346–1354. [Google Scholar] [CrossRef]
- Yamashita, T.; Yoshioka, M.; Itoh, N. Identification of a Novel Fibroblast Growth Factor, FGF-23, Preferentially Expressed in the Ventrolateral Thalamic Nucleus of the Brain. Biochem. Biophys. Res. Commun. 2000, 277, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, R.; Simpson, L.G.; Xiao, Z.-S.; Burnham, C.E.; Quarles, L.D. Regulation of Fibroblastic Growth Factor 23 Expression but Not Degradation by PHEX. J. Biol. Chem. 2003, 278, 37419–37426. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Tighiouart, H.; Scott, T.M.; Lou, K.V.; Fan, L.; Shaffi, K.; Weiner, D.E.; Sarnak, M.J. FGF-23 and cognitive performance in hemodialysis patients. Hemodial. Int. 2014, 18, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Romani, A.; Seripa, D.; Cremonini, E.; Bosi, C.; Magon, S.; Passaro, A.; Bergamini, C.M.; Pilotto, A.; Zuliani, G. Oxidative balance, homocysteine, and uric acid levels in older patients with Late Onset Alzheimer’s Disease or Vascular Dementia. J. Neurol. Sci. 2014, 337, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; The Brisighella Heart Study Group; Desideri, G.; Grossi, G.; Urso, R.; Rosticci, M.; D’Addato, S.; Borghi, C. Serum uric acid and impaired cognitive function in a cohort of healthy young elderly: Data from the Brisighella Study. Intern. Emerg. Med. 2014, 10, 25–31. [Google Scholar] [CrossRef]
- Al-Khateeb, E.; Althaher, A.; Al-Khateeb, M.; Al-Musawi, H.; Azzouqah, O.; Al-Shweiki, S.; Shafagoj, Y. Relation between Uric Acid and Alzheimer’s Disease in Elderly Jordanians. J. Alzheimer’s Dis. 2015, 44, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Canas, J.-A.; Dore, G.A.; Beydoun, H.A.; Rostant, O.S.; Fanelli-Kuczmarski, M.T.; Evans, M.K.; Zonderman, A.B. Serum Uric Acid and Its Association with Longitudinal Cognitive Change Among Urban Adults. J. Alzheimer’s Dis. 2016, 52, 1415–1430. [Google Scholar] [CrossRef]
- Sautin, Y.Y.; Johnson, R.J. Uric Acid: The Oxidant-Antioxidant Paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, L.; Hu, J.; Wang, Y.; Xu, Y. Uric acid is associated with cognitive impairment in the elderly patients receiving maintenance hemodialysis—A two-center study. Brain Behav. 2020, 10, e01542. [Google Scholar] [CrossRef] [PubMed]
- Gondouin, B.; Cerini, C.; Dou, L.; Sallée, M.; Duval-Sabatier, A.; Pletinck, A.; Calaf, R.; Lacroix, R.; Jourde-Chiche, N.; Poitevin, S.; et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int. 2013, 84, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-L.; Yang, C.-M. Role of Redox Signaling in Neuroinflammation and Neurodegenerative Diseases. BioMed Res. Int. 2013, 2013, 484613. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Wu, P.-H.; Lee, H.-H.; Mubanga, M.; Chen, C.-S.; Kuo, M.-C.; Chiu, Y.-W.; Kuo, P.-L.; Hwang, S.-J. Indole-3 acetic acid increased risk of impaired cognitive function in patients receiving hemodialysis. NeuroToxicology 2019, 73, 85–91. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Wu, P.-H.; Liang, S.-S.; Mubanga, M.; Yang, Y.-H.; Hsu, Y.-L.; Kuo, M.-C.; Hwang, S.-J.; Kuo, P.-L. Protein-bound uremic toxins are associated with cognitive function among patients undergoing maintenance hemodialysis. Sci. Rep. 2019, 9, 20388. [Google Scholar] [CrossRef]
- Tamura, M.K.; Chertow, G.M.; Depner, T.A.; Nissenson, A.R.; Schiller, B.; Mehta, R.L.; Liu, S.; Sirich, T.L. Metabolic Profiling of Impaired Cognitive Function in Patients Receiving Dialysis. J. Am. Soc. Nephrol. 2016, 27, 3780–3787. [Google Scholar] [CrossRef]
- Fan, S.; Lin, L.; Chen, V.C.; Hsieh, C.; Hsiao, H.; McIntyre, R.S.; Iacobucci, M.; Coles, A.S.; Tsai, D.; Weng, J.; et al. Effects of Lower Past-Year Serum Sodium and Hyponatremia on Depression Symptoms and Cognitive Impairments in Patients with Hemodialysis. Ther. Apher. Dial. 2020, 24, 169–177. [Google Scholar] [CrossRef]
- Shavit, L.; Mikeladze, I.; Torem, C.; Slotki, I. Mild hyponatremia is associated with functional and cognitive decline in chronic hemodialysis patients. Clin. Nephrol. 2014, 82, 313–319. [Google Scholar] [CrossRef]
- Zyada, F.; Makar, S.H.; Abdelrahman, S.M.; Labana, A.H. Assessment of cognitive functions in children on regular hemodialysis and after renal transplantation. Middle East Curr. Psychiatry 2017, 24, 128–133. [Google Scholar] [CrossRef]
- Massieu, L.; Montiel, T.; Robles, G.; Quesada, O. Brain amino acids during hyponatremia in vivo: Clinical observations and ex-perimental studies. Neurochem. Res. 2004, 29, 73–81. [Google Scholar] [CrossRef]
- van de Rest, O.; van der Zwaluw, N.L.; de Groot, L.C.P.G.M. Literature review on the role of dietary protein and amino acids in cognitive functioning and cognitive decline. Amino Acids 2013, 45, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.K.; Wadley, V.; Yaffe, K.; McClure, L.A.; Howard, G.; Go, R.; Allman, R.M.; Warnock, D.G.; McClellan, W. Kidney function and cognitive impairment in US adults: The Reasons for Geo-graphic and Racial Differences in Stroke (REGARDS) Study. Am. J. Kidney Dis. 2008, 52, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.M.; Tupper, D.E.; Knopman, D.S.; Gilbertson, D.T.; Pederson, S.L.; Li, S.; Smith, G.; Hochhalter, A.K.; Collins, A.J.; Kane, R.L. Cognitive impairment in hemodialysis patients is common. Neurology 2006, 67, 216–223. [Google Scholar] [CrossRef]
- Shaffi, K.; Tighiouart, H.; Scott, T.; Lou, K.; Drew, D.; Weiner, D.; Sarnak, M. Low 25-Hydroxyvitamin D Levels and Cognitive Impairment in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2013, 8, 979–986. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.-X. Intravenous Iron Sucrose in Chinese Hemodialysis Patients with Renal Anemia. Blood Purif. 2008, 26, 151–156. [Google Scholar] [CrossRef]
- Shaker, A.M.; Mohamed, O.M.; Mohamed, M.F.; El-Khashaba, S.O. Impact of correction of anemia in end-stage renal disease patients on cerebral circulation and cognitive functions. Saudi J. Kidney Dis. Transplant. 2018, 29, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.-H.; Yeh, C.-C.; Sung, F.-C.; Hsiao, C.-Y.; Muo, C.-H.; Hung, K.-Y.; Tsai, K.-J. Erythropoietin prevents dementia in hemodialysis patients: A nationwide population-based study. Aging 2019, 11, 6941–6950. [Google Scholar] [CrossRef]
- Blacher, J.; Safar, M.E.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; London, G.M. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003, 63, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Guerchet, M.; Aboyans, V.; Nubukpo, P.; Lacroix, P.; Clément, J.P.; Preux, P.M. Ankle-brachial index as a marker of cognitive im-pairment and dementia in general population. A systematic review. Atherosclerosis 2011, 216, 251–257. [Google Scholar] [CrossRef]
- Wu, P.-H.; Lin, Y.-T.; Wu, P.-Y.; Huang, J.-C.; Chen, S.-C.; Chang, J.-M.; Chen, H.-C. A Low Ankle-Brachial Index and High Brachial-Ankle Pulse Wave Velocity Are Associated with Poor Cognitive Function in Patients Undergoing Hemodialysis. Dis. Markers 2019, 2019, 9421352. [Google Scholar] [CrossRef]
- Tasmoc, A.; Donciu, M.; Veisa, G.; Nistor, I.; Covic, A. Increased arterial stiffness predicts cognitive impairment in hemodialysis patients. Hemodial. Int. 2016, 20, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Fedorowski, A.; Stavenow, L.; Hedblad, B.; Berglund, G.; Nilsson, P.M.; Melander, O. Consequences of orthostatic blood pressure variability in middle-aged men (The Malmö Preventive Project). J. Hypertens. 2010, 28, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Canney, M.; O’Connell, M.D.L.; Sexton, D.J.; O’Leary, N.; Kenny, R.A.; Little, M.A.; O’Seaghdha, C.M. Graded Association Between Kidney Function and Impaired Orthostatic Blood Pressure Stabilization in Older Adults. J. Am. Hear. Assoc. 2017, 6, e005661. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Gao, S.A.; Friberg, P.; Annerstedt, M.; Bergström, G.; Carlström, J.; Ivarsson, T.; Jensen, G.; Ljungman, S.; Mathillas, Ö.; et al. Reduced Baroreflex Effectiveness Index in Hypertensive Patients with Chronic Renal Failure. Am. J. Hypertens. 2005, 18, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Jassal, S.V.; Douglas, J.F.; Stout, R.W. Prevalence of central autonomic neuropathy in elderly dialysis patients. Nephrol. Dial. Transplant. 1998, 13, 1702–1708. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, W.; Wang, L.; Huang, X.; Yuan, C.; Li, H.; Yang, J. Orthostatic blood pressure reduction as a possible explanation for memory deficits in dialysis patients. Hypertens. Res. 2019, 42, 1049–1056. [Google Scholar] [CrossRef]
- Post, J.B.; Morin, K.G.; Handrakis, J.P.; Rivera, D.R.; Yen, C.; Sano, M.; Spungen, A.M. Cognition may be related to arterial pulsatility index in HD patients. Clin. Nephrol. 2014, 81, 313–319. [Google Scholar] [CrossRef]
- Bossola, M.; Tazza, L.; Vulpio, C.; Luciani, G. Reviews: Is Regression of Left Ventricular Hypertrophy in Maintenance Hemodialysis Patients Possible? Semin. Dial. 2008, 21, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Middleton, R.J.; Parfrey, P.S.; Foley, R.N. Left Ventricular Hypertrophy in the Renal Patient. J. Am. Soc. Nephrol. 2001, 12, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Prohovnik, I.; Post, J.; Uribarri, J.; Lee, H.; Sandu, O.; Langhoff, E. Cerebrovascular effects of hemodialysis in chronic kidney disease. J. Cereb. Blood Flow Metab. 2007, 27, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Bossola, M.; Laudisio, A.; Antocicco, M.; Tazza, L.; Colloca, G.; Tosato, M.; Zuccalà, G. Cognitive performance is associated with left ventricular function in older chronic hemodialysis patients: Result of a pilot study. Aging Clin. Exp. Res. 2014, 26, 445–451. [Google Scholar] [CrossRef]
- MacEwen, C.; Sutherland, S.; Daly, J.; Pugh, C.; Tarassenko, L. Relationship between hypotension and cerebral ischemia during hemodialysis. J. Am. Soc. Nephrol. 2017, 28, 2511–2520. [Google Scholar] [CrossRef] [PubMed]
- Polinder-Bos, H.A.; García, D.V.; Kuipers, J.; Elting, J.W.J.; Aries, M.J.; Krijnen, W.P.; Groen, H.; Willemsen, A.T.; Van Laar, P.J.; Strijkert, F.; et al. Hemodialysis Induces an Acute Decline in Cerebral Blood Flow in Elderly Patients. J. Am. Soc. Nephrol. 2018, 29, 1317–1325. [Google Scholar] [CrossRef]
- Naganuma, T.; Takemoto, Y.; Shoji, T.; Shima, H.; Ishimura, E.; Okamura, M.; Nakatani, T. Factors associated with cerebral white matter hyperintensities in haemodialysis patients. Neurology 2012, 17, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Findlay, M.D.; Dawson, J.; Dickie, D.A.; Forbes, K.P.; McGlynn, D.; Quinn, T.; Mark, P. Investigating the Relationship between Cerebral Blood Flow and Cognitive Function in Hemodialysis Patients. J. Am. Soc. Nephrol. 2019, 30, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.; Wang, H.; Chu, Z.; Li, J.; Qian, T.; Haacke, E.M.; Xia, S.; Shen, W. Reduced regional cerebral venous oxygen saturation is a risk factor for the cognitive impairment in hemodialysis patients: A quantitative susceptibility mapping study. Brain Imaging Behav. 2020, 14, 1339–1349. [Google Scholar] [CrossRef]
- Kovarova, L.; Valerianova, A.; Kmentova, T.; Lachmanova, J.; Hladinova, Z.; Malik, J. Low Cerebral Oxygenation Is Associated with Cognitive Impairment in Chronic Hemodialysis Patients. Nephron 2018, 139, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ookawara, S.; Ueda, Y.; Miyazawa, H.; Uchida, T.; Kofuji, M.; Hayasaka, H.; Minato, S.; Kaneko, S.; Mutsuyoshi, Y.; et al. Cerebral oxygenation improvement is associated with hemoglobin increase after hemodialysis initiation. Int. J. Artif. Organs 2020, 43, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Xu, C.; Li, Y.; Yu, L.; Shao, X.; Xie, K.; Gu, J.; Yu, Z.; Yan, Y.; Guan, Y.; et al. The Incidence Prognosis and Risk Factors of Cognitive Impairment in Maintenance Haemodialysis Patients. Blood Purif. 2018, 47, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.J.; Bhandari, S.; Dutta, S. Cognitive Impairment and its Correlates in Chronic Kidney Disease Patients Undergoing Haemodialysis. J. Evol. Med. Dent. Sci. 2019, 8, 2818–2822. [Google Scholar] [CrossRef] [PubMed]
- Gesualdo, G.D.; Duarte, J.G.; Zazzetta, M.S.; Kusumota, L.; Say, K.G.; Pavarini, S.C.I.; Orlandi, F.D.S. Cognitive impairment of patients with chronic renal disease on hemodialysis and its relationship with sociodemographic and clinical characteristics. Dement. Neuropsychol. 2017, 11, 221–226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fadili, W.; Al Adlouni, A.; Louhab, N.; Allah, M.H.; Kissani, N.; Laouad, I. Prevalence and risk factors of cognitive dysfunction in chronic hemodialysis patients. Aging Ment. Health 2013, 18, 207–211. [Google Scholar] [CrossRef]
- Zubair, U.B.; Butt, B. Association of Quality of Sleep with Cognitive Decline Among the Patients of Chronic Kidney Disease Undergoing Haemodialysis. J. Ayub. Med. Coll. Abbottabad. 2017, 29, 619–622. [Google Scholar] [PubMed]
- Stringuetta-Belik, F.; Shiraishi, F.G.; E Silva, V.R.O.; Barretti, P.; Caramori, J.D.S.C.T.; Bôas, P.J.F.V.; Martin, L.C.; Franco, R.J.D.S. Greater level of physical activity associated with better cognitive function in hemodialysis in end stage renal disease. J. Bras. Nefrol. 2012, 34, 378–386. [Google Scholar] [CrossRef] [PubMed]
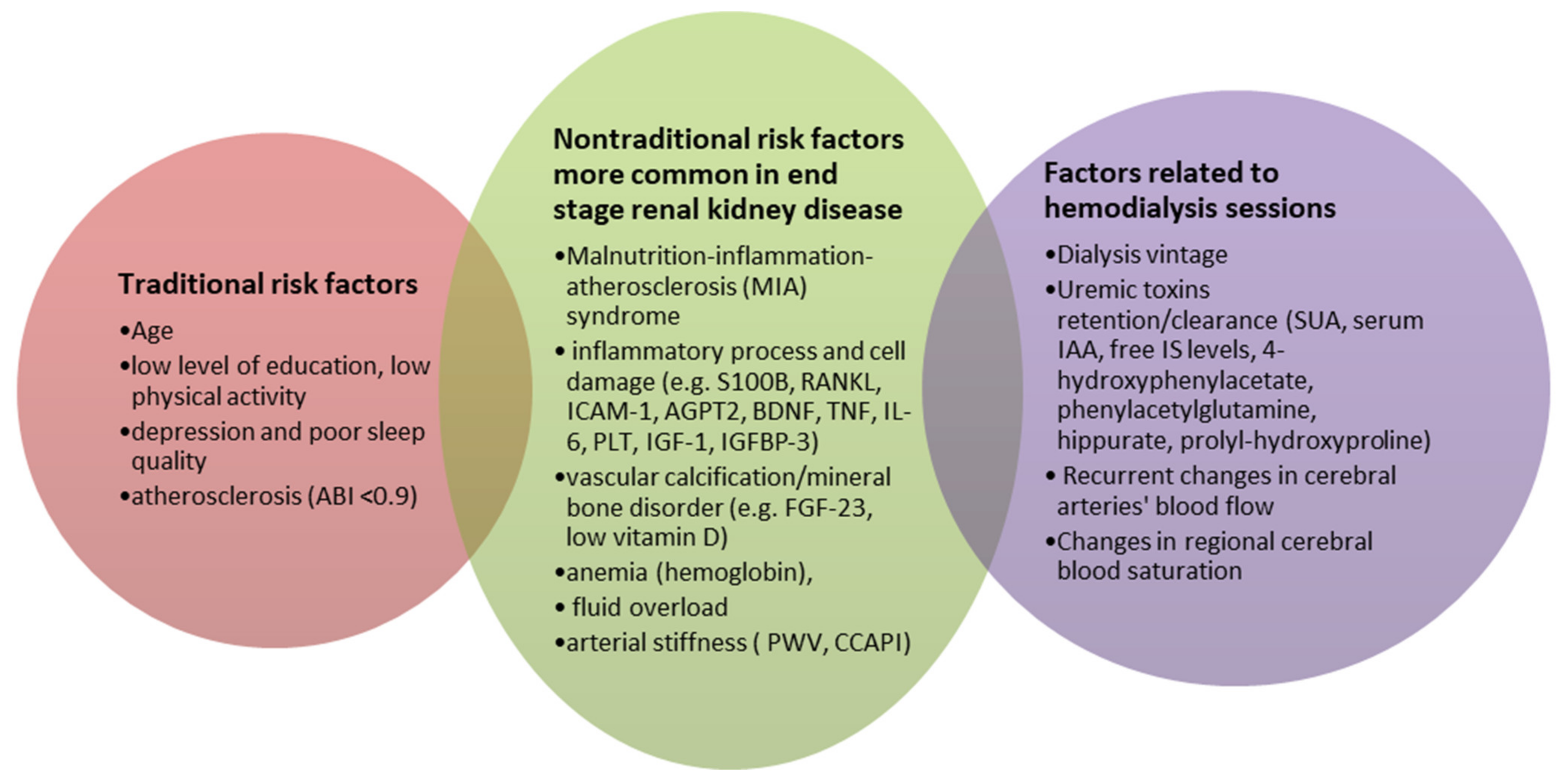
| Factors | Studied Population | CI Measures | Ref. |
|---|---|---|---|
| Factors correlating with CI were identified: Educational level (OR 2.234), spKt/V (OR 1.982), Post-dialysis diastolic blood (OR 1.982). | 219 HD * | MoCA | [69] |
| Identified correlates: socio-economic status and global cognition score (χ2 = 81.13, df = 48, p = 0.002), education level and orientation (χ2 = 29.78, df = 8, p = 0.000), recall (χ2 = 31.7, df = 12, p = 0.002). A negative correlation was found between dialysis vintage (r = −0.411, p = 0.003), depression (r = −0.721, p <0.01) and cognitive function. | 50 HD | MoCA Patient Health Questionnaire-9 (PHQ-9) | [70] |
| A positive correlations was found between cognitive function and years of education (r = 0.52, p ≤ 0.001), dialysis vintage (r = 0.26, p ≤ 0.001). | 99 HD | Addenbrooke’s Cognitive Examination-Revised (ACE-R) | [71] |
| Educational level (odd ratio = 0.564, p = 0.031), anemia (odd ratio = 0.743; p = 0.046) assiociated with cognitive functions. | 108 HD | MMSE | [72] |
| Sleep quality (OR 10.709 p = 0.002) independently associated with CI. | 106 HD | British Columbia Cognitive Complaints Inventory | [73] |
| Less physically active patients assiociated with CI. | 102 HD | MMSE | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olczyk, P.; Kusztal, M.; Gołębiowski, T.; Letachowicz, K.; Krajewska, M. Cognitive Impairment in End Stage Renal Disease Patients Undergoing Hemodialysis: Markers and Risk Factors. Int. J. Environ. Res. Public Health 2022, 19, 2389. https://doi.org/10.3390/ijerph19042389
Olczyk P, Kusztal M, Gołębiowski T, Letachowicz K, Krajewska M. Cognitive Impairment in End Stage Renal Disease Patients Undergoing Hemodialysis: Markers and Risk Factors. International Journal of Environmental Research and Public Health. 2022; 19(4):2389. https://doi.org/10.3390/ijerph19042389
Chicago/Turabian StyleOlczyk, Piotr, Mariusz Kusztal, Tomasz Gołębiowski, Krzysztof Letachowicz, and Magdalena Krajewska. 2022. "Cognitive Impairment in End Stage Renal Disease Patients Undergoing Hemodialysis: Markers and Risk Factors" International Journal of Environmental Research and Public Health 19, no. 4: 2389. https://doi.org/10.3390/ijerph19042389
APA StyleOlczyk, P., Kusztal, M., Gołębiowski, T., Letachowicz, K., & Krajewska, M. (2022). Cognitive Impairment in End Stage Renal Disease Patients Undergoing Hemodialysis: Markers and Risk Factors. International Journal of Environmental Research and Public Health, 19(4), 2389. https://doi.org/10.3390/ijerph19042389





