Protective Effect of Leisure-Time Physical Activity and Resistance Training on Nonalcoholic Fatty Liver Disease: A Nationwide Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
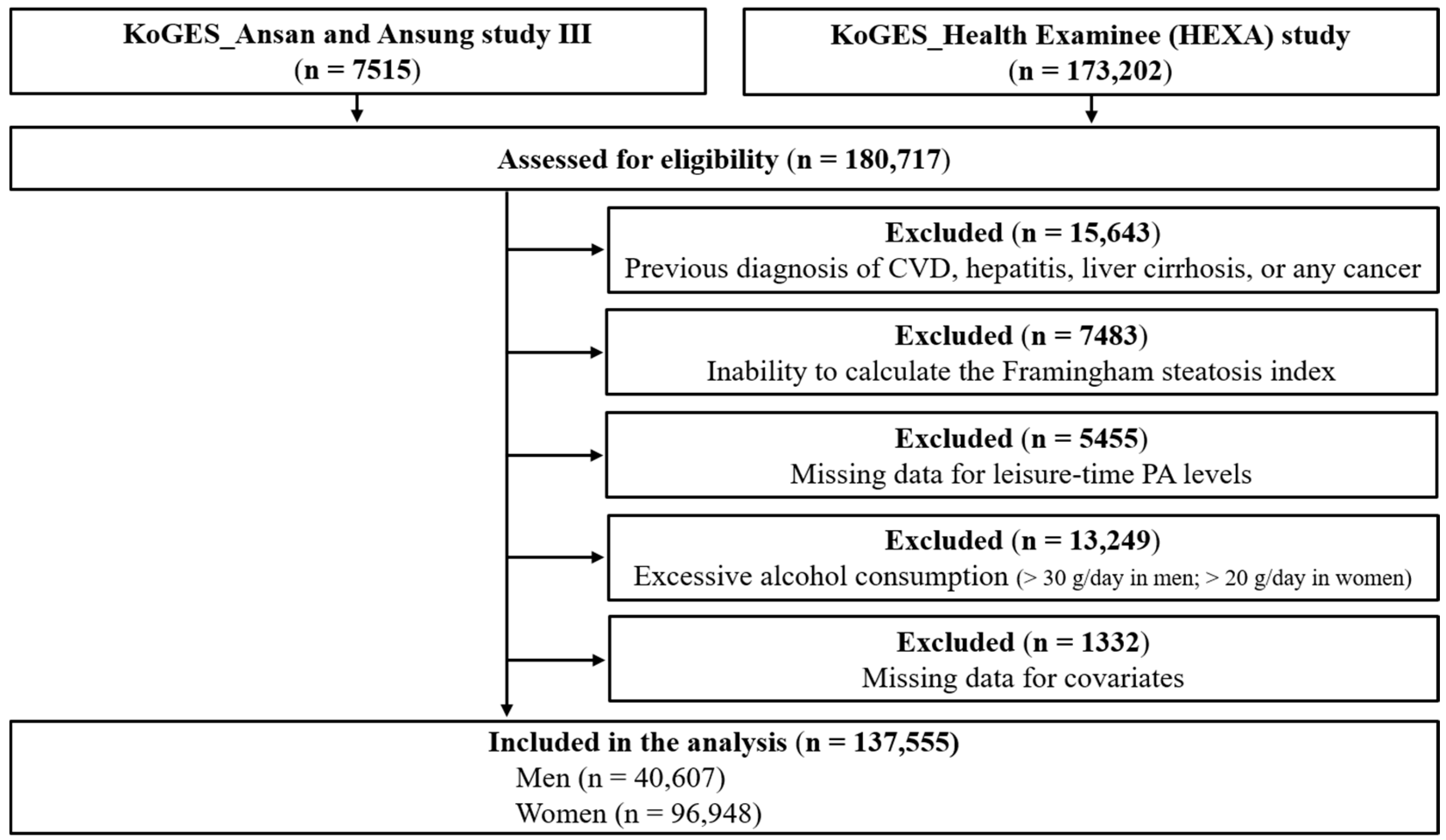
2.1. Study Participants
2.2. Definition of NAFLD
2.3. Measurement of PA
2.4. Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grefhorst, A.; van de Peppel, I.P.; Larsen, L.E.; Jonker, J.W.; Holleboom, A.G. The role of lipophagy in the development and treatment of non-alcoholic fatty liver disease. Front Endocrinol. 2020, 11, 601627. [Google Scholar] [CrossRef] [PubMed]
- Kasper, P.; Martin, A.; Lang, S.; Kütting, F.; Goeser, T.; Demir, M.; Steffen, H.M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2021, 110, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.E.; Zaccardi, F.; Khunti, K.; Davies, M.J. Causality between non-alcoholic fatty liver disease and risk of cardiovascular disease and type 2 diabetes: A meta-analysis with bias analysis. Liver Int. 2019, 39, 557–567. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Kim, Y.J.; Park, H.S. Trends in the prevalence of non-alcoholic fatty liver disease and its future predictions in Korean men, 1998-2035. J. Clin. Med. 2020, 9, 2626. [Google Scholar] [CrossRef] [PubMed]
- Im, H.J.; Ahn, Y.C.; Wang, J.H.; Lee, M.M.; Son, C.G. Systematic review on the prevalence of nonalcoholic fatty liver disease in South Korea. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101526. [Google Scholar] [CrossRef]
- Abdelbasset, W.K.; Tantawy, S.A.; Kamel, D.M.; Alqahtani, B.A.; Elnegamy, T.E.; Soliman, G.S.; Ibrahim, A.A. Effects of high-intensity interval and moderate-intensity continuous aerobic exercise on diabetic obese patients with nonalcoholic fatty liver disease: A comparative randomized controlled trial. Medicine 2020, 99, e19471. [Google Scholar] [CrossRef] [PubMed]
- Shamsoddini, A.; Sobhani, V.; Ghamar Chehreh, M.E.; Alavian, S.M.; Zaree, A. Effect of aerobic and resistance exercise training on liver enzymes and hepatic fat in Iranian men with nonalcoholic fatty liver disease. Hepat. Mon. 2015, 15, e31434. [Google Scholar] [CrossRef]
- Zhang, H.J.; He, J.; Pan, L.L.; Ma, Z.M.; Han, C.K.; Chen, C.S.; Chen, Z.; Han, H.W.; Chen, S.; Sun, Q.; et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: A randomized clinical trial. JAMA Intern. Med. 2016, 176, 1074–1082. [Google Scholar] [CrossRef]
- Byambasukh, O.; Zelle, D.; Corpeleijn, E. Physical activity, fatty liver, and glucose metabolism over the life course: The lifelines cohort. Am. J. Gastroenterol. 2019, 114, 907–915. [Google Scholar] [CrossRef]
- Kim, D.; Vazquez-Montesino, L.M.; Li, A.A.; Cholankeril, G.; Ahmed, A. Inadequate physical activity and sedentary behavior are independent predictors of nonalcoholic fatty liver disease. Hepatology 2020, 72, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.V.; Zandvakili, I.; Thaiss, C.A.; Schneider, K.M. Physical activity is associated with reduced risk of liver disease in the prospective UK Biobank cohort. JHEP Rep. 2021, 3, 100263. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Hashida, R.; Kawaguchi, T.; Bekki, M.; Omoto, M.; Matsuse, H.; Nago, T.; Takano, Y.; Ueno, T.; Koga, H.; George, J.; et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J. Hepatol. 2017, 66, 142–152. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Nitzan-Kaluski, D.; Goldsmith, R.; Webb, M.; Zvibel, I.; Goldiner, I.; Blendis, L.; Halpern, Z.; Oren, R. Role of leisure-time physical activity in nonalcoholic fatty liver disease: A population-based study. Hepatology 2008, 48, 1791–1798. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.G.; The KoGES Group. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef]
- Long, M.T.; Pedley, A.; Colantonio, L.D.; Massaro, J.M.; Hoffmann, U.; Muntner, P.; Fox, C.S. Development and validation of the Framingham steatosis index to identify persons with depatic steatosis. Clin. Gastroenterol. Hepatol. 2016, 14, 1172–1180.e2. [Google Scholar] [CrossRef]
- Motamed, N.; Nikkhah, M.; Karbalaie Niya, M.H.; Khoonsari, M.; Perumal, D.; Ashrafi, G.H.; Faraji, A.H.; Maadi, M.; Ajdarkosh, H.; Safarnezhad Tameshkel, F.; et al. The ability of the Framingham steatosis index (FSI) to predict non-alcoholic fatty liver disease (NAFLD): A cohort study. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101567. [Google Scholar] [CrossRef]
- Jung, T.Y.; Kim, M.S.; Hong, H.P.; Kang, K.A.; Jun, D.W. Comparative assessment and external validation of hepatic steatosis formulae in a community-based setting. J. Clin. Med. 2020, 9, 2851. [Google Scholar] [CrossRef]
- Kechagias, S.; Nasr, P.; Blomdahl, J.; Ekstedt, M. Established and emerging factors affecting the progression of nonalcoholic fatty liver disease. Metabolism 2020, 111, 154183. [Google Scholar] [CrossRef]
- de Sousa, E.C.; Abrahin, O.; Ferreira, A.L.L.; Rodrigues, R.P.; Alves, E.A.C.; Vieira, R.P. Resistance training alone reduces systolic and diastolic blood pressure in prehypertensive and hypertensive individuals: Meta-analysis. Hypertens. Res. 2017, 40, 927–931. [Google Scholar] [CrossRef] [PubMed]
- McLeod, J.C.; Stokes, T.; Phillips, S.M. Resistance exercise training as a primary countermeasure to age-related chronic disease. Front Physiol. 2019, 10, 645. [Google Scholar] [CrossRef]
- Westcott, W.L. Resistance training is medicine: Effects of strength training on health. Curr. Sports Med. Rep. 2012, 11, 209–216. [Google Scholar] [CrossRef]
- Bacchi, E.; Negri, C.; Targher, G.; Faccioli, N.; Lanza, M.; Zoppini, G.; Zanolin, E.; Schena, F.; Bonora, E.; Moghetti, P. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology 2013, 58, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- de Piano, A.; de Mello, M.T.; Sanches Pde, L.; da Silva, P.L.; Campos, R.M.; Carnier, J.; Corgosinho, F.; Foschini, D.; Masquio, D.L.; Tock, L.; et al. Long-term effects of aerobic plus resistance training on the adipokines and neuropeptides in nonalcoholic fatty liver disease obese adolescents. Eur. J. Gastroenterol. Hepatol. 2012, 24, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Saeidifard, F.; Medina-Inojosa, J.R.; West, C.P.; Olson, T.P.; Somers, V.K.; Bonikowske, A.R.; Prokop, L.J.; Vinciguerra, M.; Lopez-Jimenez, F. The association of resistance training with mortality: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2019, 26, 1647–1665. [Google Scholar] [CrossRef] [PubMed]
- Hallsworth, K.; Fattakhova, G.; Hollingsworth, K.G.; Thoma, C.; Moore, S.; Taylor, R.; Day, C.P.; Trenell, M.I. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011, 60, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Buch, A.; Yeshua, H.; Vaisman, N.; Webb, M.; Harari, G.; Kis, O.; Fliss-Isakov, N.; Izkhakov, E.; Halpern, Z.; et al. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J. Gastroenterol. 2014, 20, 4382–4392. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Chen, L.L.; Yang, X.P. The efficacy of resistance training for non-alcoholic fatty-liver disease: A meta-analysis of randomized controlled trials. Int. J. Clin. Exp. Med. 2019, 12, 13188–13195. [Google Scholar]
- Xiong, Y.; Peng, Q.; Cao, C.; Xu, Z.; Zhang, B. Effect of different exercise methods on non-alcoholic fatty liver disease: A meta-analysis and meta-regression. Int. J. Environ. Res. Public Health 2021, 18, 3242. [Google Scholar] [CrossRef]
- Ralston, G.W.; Kilgore, L.; Wyatt, F.B.; Baker, J.S. The effect of weekly set volume on strength gain: A meta-analysis. Sports Med. 2017, 47, 2585–2601. [Google Scholar] [CrossRef] [PubMed]
- Borde, R.; Hortobágyi, T.; Granacher, U. Dose-response relationships of resistance training in healthy old adults: A systematic review and meta-analysis. Sports Med. 2015, 45, 1693–1720. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.M.; Nuckols, G.; Krieger, J.W. Sex differences in resistance training: A systematic review and meta-analysis. J. Strength Cond. Res. 2020, 34, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Schoenfeld, B.J.; Davies, T.B.; Lazinica, B.; Krieger, J.W.; Pedisic, Z. Effect of resistance training frequency on gains in muscular strength: A systematic review and meta-analysis. Sports Med. 2018, 48, 1207–1220. [Google Scholar] [CrossRef]
| Variables | Men (n = 40,607) | p-Value | Women (n = 96,948) | p-Value | ||
|---|---|---|---|---|---|---|
| Low-PA (n = 24,239) | High-PA (n = 16,368) | Low-PA (n = 62,436) | High-PA (n = 34,512) | |||
| Age (years) | 53.25 ± 8.87 | 55.11 ± 8.61 | <0.0001 | 52.43 ± 8.33 | 52.95 ± 7.67 | <0.0001 |
| Education level, n (%) | <0.0001 | <0.0001 | ||||
| ≤Elementary school | 3407 (14.06) | 1275 (7.79) | 15,843 (25.38) | 6392 (18.52) | ||
| Middle/high school | 12,801 (52.81) | 8337 (50.93) | 35,223 (56.41) | 21,885 (63.41) | ||
| ≥College | 8031 (33.13) | 6756 (41.28) | 11,370 (18.21) | 6235 (18.07) | ||
| Drinking habit, n (%) | <0.0001 | <0.0001 | ||||
| Never drinker | 6336 (26.14) | 3793 (23.17) | 42,828 (68.59) | 22,851 (66.21) | ||
| Ex-drinker | 1881 (7.76) | 1269 (7.75) | 1252 (2.01) | 652 (1.89) | ||
| Current drinker | 16,022 (66.10) | 11,306 (69.08) | 18,356 (29.40) | 11,009 (31.90) | ||
| Smoking habit, n (%) | <0.0001 | <0.0001 | ||||
| Never smoker | 7267 (29.98) | 5229 (31.95) | 60,210 (96.44) | 33,665 (97.54) | ||
| Ex-smoker | 8486 (35.01) | 7407 (45.25) | 739 (1.18) | 382 (1.11) | ||
| Current smoker | 8486 (35.01) | 3732 (22.80) | 1487 (2.38) | 465 (1.35) | ||
| PA-time (min/week) | 20.64 ± 40.59 | 397.69 ± 265.87 | <0.0001 | 17.70 ± 38.79 | 364.37 ± 218.41 | <0.0001 |
| RT (%) | 2000 (8.25) | 4176 (25.51) | <0.0001 | 2087 (3.34) | 6342 (18.38) | <0.0001 |
| BMI (kg/m2) | 24.22 ± 2.81 | 24.44 ± 2.59 | <0.0001 | 23.74 ± 3.07 | 23.66 ± 2.78 | <0.0001 |
| WC (cm) | 85.50 ± 7.68 | 85.49 ± 7.23 | 0.93 | 78.98 ± 8.47 | 78.25 ± 7.86 | <0.0001 |
| SBP (mmHg) | 124.35 ± 14.94 | 125.34 ± 14.64 | <0.0001 | 120.52 ± 15.74 | 121.01 ± 15.58 | <0.0001 |
| DBP (mmHg) | 78.26 ± 10.02 | 78.47 ± 9.62 | <0.05 | 74.75 ± 9.92 | 74.93 ± 9.77 | <0.01 |
| T-Chol (mg/dL) | 194.31 ± 34.26 | 193.75 ± 33.56 | 0.11 | 199.55 ± 35.76 | 200.67 ± 35.43 | <0.0001 |
| HDL-C (mg/dL) | 48.00 ± 11.35 | 49.53 ± 11.65 | <0.0001 | 55.17 ± 12.62 | 57.22 ± 13.10 | <0.0001 |
| TG (mg/dL) | 152.01 ± 106.54 | 140.56 ± 96.60 | <0.0001 | 116.43 ± 76.71 | 110.07 ± 70.46 | <0.0001 |
| FBG (mg/dL) | 97.54 ± 24.25 | 98.64 ± 22.77 | <0.0001 | 92.69 ± 19.26 | 92.98 ± 18.61 | <0.05 |
| AST (IU/L) | 25.11 ± 13.25 | 24.90 ± 13.24 | 0.12 | 22.53 ± 22.20 | 22.54 ± 9.43 | 0.93 |
| ALT (IU/L) | 27.24 ± 21.18 | 25.49 ± 17.53 | <0.0001 | 19.97 ± 23.21 | 19.45 ± 14.69 | <0.0001 |
| Hypertension, n (%) | 7667 (31.63) | 5836 (35.65) | <0.0001 | 15,769 (25.26) | 9114 (26.41) | <0.0001 |
| DM, n (%) | 2621 (10.81) | 2250 (13.75) | <0.0001 | 4300 (6.89) | 2746 (7.96) | <0.0001 |
| FSI | 21.21 ± 20.10 | 20.22 ± 18.57 | <0.0001 | 12.46 ± 14.10 | 11.60 ± 12.87 | <0.0001 |
| NAFLD, n (%) | 7593 (31.33) | 4807 (29.37) | <0.0001 | 8815 (14.12) | 4109 (11.91) | <0.0001 |
| N | NAFLD (%) | Model 1 OR (95% CI) | Model 2 OR (95% CI) | Model 3 OR (95% CI) | |
|---|---|---|---|---|---|
| Total | 137,555 | 18.41 | |||
| Low-PA | 86,675 | 18.93 | 1 (reference) | 1 (reference) | 1 (reference) |
| High-PA | 50,880 | 17.52 | 0.82 (0.80–0.85) ** | 0.88 (0.85–0.91) ** | 0.80 (0.77–0.84) ** |
| Men | 40,607 | 30.54 | |||
| Low-PA | 24,239 | 31.33 | 1 (reference) | 1 (reference) | 1 (reference) |
| High-PA | 16,368 | 29.37 | 0.91 (0.87–0.95) ** | 0.85 (0.80–0.89) ** | 0.80 (0.75–0.85) ** |
| Women | 96,948 | 13.33 | |||
| Low-PA | 62,436 | 14.12 | 1 (reference) | 1 (reference) | 1 (reference) |
| High-PA | 34,512 | 11.91 | 0.79 (0.76–0.82) ** | 0.92 (0.87–0.96) * | 0.83 (0.78–0.88) ** |
| N | NAFLD (%) | RT Levels | OR (95% CI) | |||
|---|---|---|---|---|---|---|
| Frequency | Training Period | |||||
| (Days/Week) | (Month) | ≥1 Year (%) | ||||
| Total | 137,555 | 18.41 | ||||
| Low-PA | 82,588 | 18.91 | - | - | - | 1 (reference) |
| Low-PA+RT | 4087 | 19.26 | 3.39 ± 1.71 a | 13.04 ± 24.12 a | 65.99 a | 0.94 (0.84–1.06) |
| High-PA | 40,362 | 17.91 | - | - | - | 0.83 (0.79–0.86) ** |
| High-PA+RT | 10,518 | 16.03 | 4.36 ± 1.57 a | 18.59 ± 34.22 a | 81.98 a | 0.70 (0.65–0.76) ** |
| Men | 40,607 | 30.54 | ||||
| Low-PA | 22,239 | 31.44 | - | - | - | 1 (reference) |
| Low-PA+RT | 2000 | 30.00 | 3.49 ± 1.81 a | 15.33 ± 31.06 a | 72.80 a | 0.94 (0.82–1.08) |
| High-PA | 12,192 | 30.24 | - | - | - | 0.84 (0.79–0.90) ** |
| High-PA+RT | 4176 | 26.82 | 4.44 ± 1.70 a | 21.95 ± 44.04 a | 85.56 a | 0.67 (0.61–0.75) ** |
| Women | 96,948 | 13.33 | ||||
| Low-PA | 60,349 | 14.30 | - | - | - | 1 (reference) |
| Low-PA+RT | 2087 | 8.96 | 3.29 ± 1.59 a | 10.85 ± 14.34 a | 59.46 a | 0.89 (0.73–1.10) |
| High-PA | 28,170 | 12.58 | - | - | - | 0.83 (0.78–0.88) ** |
| High-PA+RT | 6342 | 8.92 | 4.30 ± 1.48 a | 16.38 ± 25.56 a | 79.63 a | 0.80 (0.71–0.90) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Lim, N.-K.; Park, H.-Y. Protective Effect of Leisure-Time Physical Activity and Resistance Training on Nonalcoholic Fatty Liver Disease: A Nationwide Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 2350. https://doi.org/10.3390/ijerph19042350
Park JH, Lim N-K, Park H-Y. Protective Effect of Leisure-Time Physical Activity and Resistance Training on Nonalcoholic Fatty Liver Disease: A Nationwide Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2022; 19(4):2350. https://doi.org/10.3390/ijerph19042350
Chicago/Turabian StylePark, Jae Ho, Nam-Kyoo Lim, and Hyun-Young Park. 2022. "Protective Effect of Leisure-Time Physical Activity and Resistance Training on Nonalcoholic Fatty Liver Disease: A Nationwide Cross-Sectional Study" International Journal of Environmental Research and Public Health 19, no. 4: 2350. https://doi.org/10.3390/ijerph19042350
APA StylePark, J. H., Lim, N.-K., & Park, H.-Y. (2022). Protective Effect of Leisure-Time Physical Activity and Resistance Training on Nonalcoholic Fatty Liver Disease: A Nationwide Cross-Sectional Study. International Journal of Environmental Research and Public Health, 19(4), 2350. https://doi.org/10.3390/ijerph19042350







