Adenomyosis as a Risk Factor for Myometrial or Endometrial Neoplasms—Review
Abstract
1. Introduction
2. Adenomyosis and Risk of Malignant Transformation
2.1. Established Pathological Pathways in Adenomyosis
2.2. Adenomyosis as an Oligoclonal Disorder Strongly Associated with KRAS Mutation
2.3. Possible Interactions between Adenomyosis, Endometriosis and Gynecological Cancers
2.4. Adenomyosis Originating from the Invasion and Migration of the Endometrium
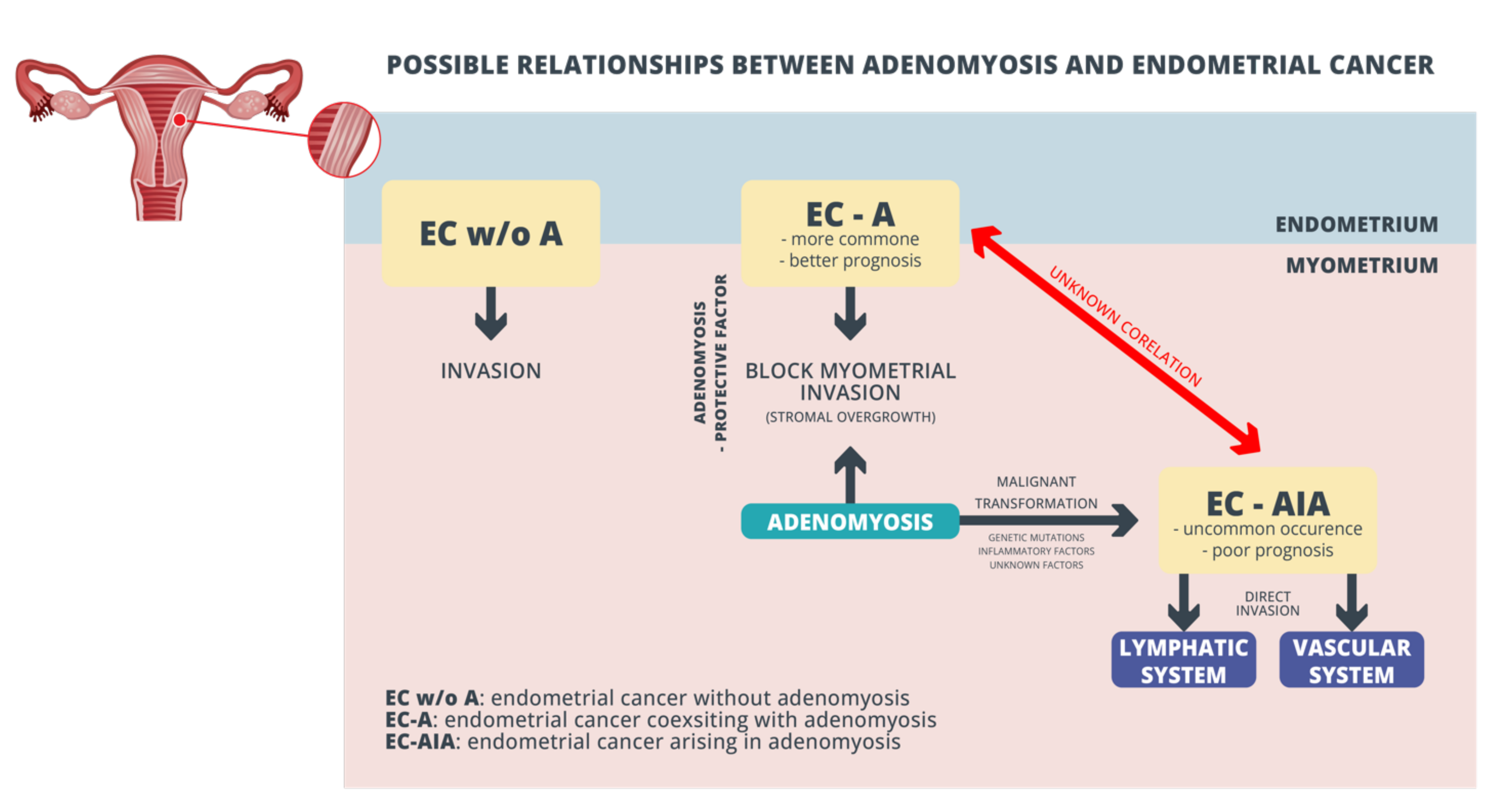
3. Clinical Studies
3.1. Adenomyosis as an Oncological Prognostic Marker in Endometrial Cancer—FIGO Stage and Grade
3.2. Prevalence of Adenomyosis in Gynecological Cancers Other Than Endometroid Endometrial Cancer
3.3. Adenomyosis and Endometrial Cancer as Two Different, Independent Entities—Influence on Survival
3.4. Direct Malignant Transformation of Adenomyosis Foci into Endometrial Cancer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graziano, A.; Lo Monte, G.; Piva, I.; Caserta, D.; Karner, M.; Engl, B.; Marci, R. Diagnostic findings in adenomyosis: A pictorial review on the major concerns. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1146–1154. [Google Scholar]
- Szubert, M.; Koziróg, E.; Olszak, O.; Krygier-Kurz, K.; Kazmierczak, J.; Wilczynski, J. Adenomyosis and Infertility-Review of Medical and Surgical Approaches. Int. J. Environ. Res. Public Health 2021, 18, 1235. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, V.H.; Arbib, N.; Schiff, E.; Goldenberg, M.; Seidman, D.S.; Soriano, D. Sonographic Signs of Adenomyosis Are Prevalent in Women Undergoing Surgery for Endometriosis and May Suggest a Higher Risk of Infertility. Biomed. Res. Int. 2017, 2017, 8967803. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, T. Uterine Evaluation Using a Diagnostic Protocol Based on MUSA. In How to Perform Ultrasonography in Endometriosis; Guerriero, S., Condous, G., Alcázar, J.L., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Habiba, M.; Pluchino, N.; Petignat, P.; Bianchi, P.; Brosens, I.A.; Benagiano, G. Adenomyosis and Endometrial Cancer: Literature Review. Gynecol. Obstet. Invest. 2018, 83, 313–328. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: R Package and ShinyApp for Producing PRISMA 2020 Compliant Flow Diagrams; Zenodo: Geneva, Switzerland, 2021. [Google Scholar] [CrossRef]
- Verit, F.F.; Yucel, O. Endometriosis, leiomyoma and adenomyosis: The risk of gynecologic malignancy. Asian Pac. J. Cancer Prev. 2013, 14, 5589–5597. [Google Scholar] [CrossRef]
- Kim, J.J.; Kurita, T.; Bulun, S.E. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr. Rev. 2013, 34, 130–162. [Google Scholar] [CrossRef]
- Bukulmez, O.; Hardy, D.B.; Carr, B.R.; Word, R.A.; Mendelson, C.R. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology 2008, 149, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.L.; Cover, P.O.; Buckingham, J.C.; Gavins, F.N. Role and interactions of annexin A1 and oestrogens in the manifestation of sexual dimorphisms in cerebral and systemic inflammation. Br. J. Pharmacol. 2013, 169, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yi, T.; Liu, R.; Bian, C.; Qi, X.; He, X.; Wang, K.; Li, J.; Zhao, X.; Huang, C.; et al. Proteomics identification of annexin A2 as a key mediator in the metastasis and proangiogenesis of endometrial cells in human adenomyosis. Mol. Cell Proteomics 2012, 11, M112.017988. [Google Scholar] [CrossRef]
- Deng, L.; Gao, Y.; Li, X.; Cai, M.; Wang, H.; Zhuang, H.; Tan, M.; Liu, S.; Hao, Y.; Lin, B. Expression and clinical significance of annexin A2 and human epididymis protein 4 in endometrial carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 96. [Google Scholar] [CrossRef]
- Sakuragi, N.; Watari, H.; Ebina, Y.; Yamamoto, R.; Steiner, E.; Koelbl, H.; Yano, M.; Tada, M.; Moriuchi, T. Functional analysis of p53 gene and the prognostic impact of dominant-negative p53 mutation in endometrial cancer. Int. J. Cancer 2005, 116, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Abushahin, N.; Zhang, T.; Chiang, S.; Zhang, X.; Hatch, K.; Zheng, W. Serous endometrial intraepithelial carcinoma arising in adenomyosis: A report of 5 cases. Int. J. Gynecol. Pathol. 2011, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yildiz, S.; Adli, M.; Wei, J.J. Adenomyosis pathogenesis: Insights from next-generation sequencing. Hum. Reprod. Update 2021, 27, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Hirota, Y.; Ueno, T.; Fukui, Y.; Yoshida, E.; Hayashi, T.; Kojima, S.; Takeyama, R.; Hashimoto, T.; Kiyono, T.; et al. Uterine adenomyosis is an oligoclonal disorder associated with KRAS mutations. Nat. Commun. 2019, 10, 5785. [Google Scholar] [CrossRef]
- Lièvre, A.; Bachet, J.B.; Le Corre, D.; Boige, V.; Landi, B.; Emile, J.F.; Côté, J.F.; Tomasic, G.; Penna, C.; Ducreux, M.; et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006, 66, 3992–3995. [Google Scholar] [CrossRef]
- Gounaris, I.; Charnock-Jones, D.S.; Brenton, J.D. Ovarian clear cell carcinoma—Bad endometriosis or bad endometrium? J. Pathol. 2011, 225, 157–160. [Google Scholar] [CrossRef]
- Santoro, A.; Angelico, G.; Inzani, F.; Spadola, S.; Arciuolo, D.; Valente, M.; Fiorentino, V.; Mulè, A.; Scambia, G.; Zannoni, G.F. The Many Faces of Endometriosis-Related Neoplasms in the Same Patient: A Brief Report. Gynecol. Obstet. Invest. 2020, 85, 371–376. [Google Scholar] [CrossRef]
- Ohta, Y.; Hamatani, S.; Suzuki, T.; Ikeda, K.; Kiyokawa, K.; Shiokawa, A.; Kushima, M.; Ota, H. Clear cell adenocarcinoma arising from a giant cystic adenomyosis: A case report with immunohistochemical analysis of laminin-5 gamma2 chain and p53 overexpression. Pathol. Res. Pract. 2008, 204, 677–682. [Google Scholar] [CrossRef]
- De, P.; Aske, J.C.; Dale, A.; Rojas Espaillat, L.; Starks, D.; Dey, N. Addressing activation of WNT beta-catenin pathway in diverse landscape of endometrial carcinogenesis. Am. J. Transl. Res. 2021, 13, 12168–12180. [Google Scholar]
- Maeda, D.; Shih, I.M. Pathogenesis and the role of ARID1A mutation in endometriosis-related ovarian neoplasms. Adv. Anat. Pathol. 2013, 20, 45–52. [Google Scholar] [CrossRef]
- Guo, Y.; Lang, X.; Lu, Z.; Wang, J.; Li, T.; Liao, Y.; Jia, C.; Zhao, W.; Fang, H. MiR-10b Directly Targets ZEB1 and PIK3CA to Curb Adenomyotic Epithelial Cell Invasiveness via Upregulation of E-Cadherin and Inhibition of Akt Phosphorylation. Cell Physiol. Biochem. 2015, 35, 2169–2180. [Google Scholar] [CrossRef]
- Gadducci, A.; Guerrieri, M.E.; Genazzani, A.R. New insights on the pathogenesis of ovarian carcinoma: Molecular basis and clinical implications. Gynecol. Endocrinol. 2012, 28, 582–586. [Google Scholar] [CrossRef]
- Mandai, M.; Yamaguchi, K.; Matsumura, N.; Baba, T.; Konishi, I. Ovarian cancer in endometriosis: Molecular biology, pathology, and clinical management. Int. J. Clin. Oncol. 2009, 14, 383–391. [Google Scholar] [CrossRef]
- Attar, E.; Tokunaga, H.; Imir, G.; Yilmaz, M.B.; Redwine, D.; Putman, M.; Gurates, B.; Attar, R.; Yaegashi, N.; Hales, D.B.; et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J. Clin. Endocrinol. Metab. 2009, 94, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Brennan, B.A.; Chan, T.; Netreba, J. WT1 expression in endometrioid ovarian carcinoma with and without associated endometriosis. Pathology 2008, 40, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, J.; Okuda, T.; Sekizawa, A.; Amemiya, S.; Saito, H.; Okai, T.; Kushima, M.; Tachikawa, T. K-ras mutation may promote carcinogenesis of endometriosis leading to ovarian clear cell carcinoma. Med. Electron. Microsc. 2004, 37, 188–192. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kajihara, H.; Yamada, Y.; Tanase, Y.; Kanayama, S.; Furukawa, N.; Noguchi, T.; Haruta, S.; Yoshida, S.; Naruse, K.; et al. Risk of carcinoma in women with ovarian endometrioma. Front. Biosci. 2011, 3, 529–539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murphy, M.A.; Wentzensen, N. Frequency of mismatch repair deficiency in ovarian cancer: A systematic review This article is a US Government work and, as such, is in the public domain of the United States of America. Int. J. Cancer. 2011, 129, 1914–1922. [Google Scholar] [CrossRef]
- Ali-Fehmi, R.; Khalifeh, I.; Bandyopadhyay, S.; Lawrence, W.D.; Silva, E.; Liao, D.; Sarkar, F.H.; Munkarah, A.R. Patterns of loss of heterozygosity at 10q23.3 and microsatellite instability in endometriosis, atypical endometriosis, and ovarian carcinoma arising in association with endometriosis. Int. J. Gynecol. Pathol. 2006, 25, 223–229. [Google Scholar] [CrossRef]
- Finn, O.J.; Gantt, K.R.; Lepisto, A.J.; Pejawar-Gaddy, S.; Xue, J.; Beatty, P.L. Importance of MUC1 and spontaneous mouse tumor models for understanding the immunobiology of human adenocarcinomas. Immunol. Res. 2011, 50, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.W.; Shah, K.; Brosens, J.J. The diversity of sex steroid action: The role of micro-RNAs and FOXO transcription factors in cycling endometrium and cancer. J. Endocrinol. 2012, 212, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Munksgaard, P.S.; Blaakaer, J. The association between endometriosis and ovarian cancer: A review of histological, genetic and molecular alterations. Gynecol Oncol. 2012, 124, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Tokyol, C.; Aktepe, F.; Dilek, F.H.; Sahin, O.; Arioz, D.T. Expression of cyclooxygenase-2 and matrix metalloproteinase-2 in adenomyosis and endometrial polyps and its correlation with angiogenesis. Int. J. Gynecol. Pathol. 2009, 28, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, X.; Jeschke, U.; von Schönfeldt, V. COX-2-PGE2-EPs in gynecological cancers. Arch. Gynecol. Obstet. 2020, 301, 1365–1375. [Google Scholar] [CrossRef]
- Kim, K.H.; Jo, M.S.; Suh, D.S.; Yoon, M.S.; Shin, D.H.; Lee, J.H.; Choi, K.U. Expression and significance of the TLR4/MyD88 signaling pathway in ovarian epithelial cancers. World J. Surg. Oncol. 2012, 10, 193. [Google Scholar] [CrossRef]
- Newman, A.C.; Hughes, C.C. Macrophages and angiogenesis: A role for Wnt signaling. Vasc. Cell 2012, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- González-Ramos, R.; Van Langendonckt, A.; Defrère, S.; Lousse, J.C.; Colette, S.; Devoto, L.; Donnez, J. Involvement of the nuclear factor-κB pathway in the pathogenesis of endometriosis. Fertil. Steril. 2010, 94, 1985–1994. [Google Scholar] [CrossRef]
- Herrmann Lavoie, C.; Fraser, D.; Therriault, M.J.; Akoum, A. Interleukin-1 stimulates macrophage migration inhibitory factor secretion in ectopic endometrial cells of women with endometriosis. Am. J. Reprod. Immunol. 2007, 58, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Carli, C.; Metz, C.N.; Al-Abed, Y.; Naccache, P.H.; Akoum, A. Up-regulation of cyclooxygenase-2 expression and prostaglandin E2 production in human endometriotic cells by macrophage migration inhibitory factor: Involvement of novel kinase signaling pathways. Endocrinology 2009, 150, 3128–3137. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Z.; Liu, H.; Niu, W.; Wang, X.; Liang, N.; Wang, X.; Wang, Y.; Shi, Y.; Xu, L.; et al. Single-cell transcriptomic analysis of eutopic endometrium and ectopic lesions of adenomyosis. Cell. Biosci. 2021, 11, 51. [Google Scholar] [CrossRef]
- Ismiil, N.; Rasty, G.; Ghorab, Z.; Nofech-Mozes, S.; Bernardini, M.; Ackerman, I.; Thomas, G.; Covens, A.; Khalifa, M.A. Adenomyosis involved by endometrial adenocarcinoma is a significant risk factor for deep myometrial invasion. Ann. Diagn. Pathol. 2007, 11, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Gizzo, S.; Patrelli, T.S.; Dall’asta, A.; Gangi, S.; Giordano, G.; Migliavacca, C.; Monica, M.; Merisio, C.; Nardelli, G.B.; Quaranta, M.; et al. Coexistence of adenomyosis and endometrioid endometrial cancer: Role in surgical guidance and prognosis estimation. Oncol. Lett. 2016, 11, 1213–1219. [Google Scholar] [CrossRef]
- Gisser, S.D.; Toker, C. Endometrial stromal sarcoma and leiomyosarcoma arising in adenomyosis: A possible presentation of occult extra-genital malignancy. Mt. Sinai. J. Med. 1978, 45, 218–224. [Google Scholar]
- Talia, K.L.; Naaman, Y.; McCluggage, W.G. Uterine Adenosarcoma Originating in Adenomyosis: Report of an Extremely Rare Phenomenon and Review of Published Literature. Int. J. Gynecol. Pathol. 2021, 40, 342–348. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, J.Y. A Rare Case of Intramural Müllerian Adenosarcoma Arising from Adenomyosis of the Uterus. J. Pathol. Transl. Med. 2017, 51, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Puppa, G.; Shozu, M.; Perin, T.; Nomura, K.; Gloghini, A.; Campagnutta, E.; Canzonieri, V. Small primary adenocarcinoma in adenomyosis with nodal metastasis: A case report. BMC Cancer 2007, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Chen, Q.; Zhang, X.; Cheng, L. Serous carcinoma arising from uterine adenomyosis/adenomyotic cyst of the cervical stump: A report of 3 cases. Diagn. Pathol. 2016, 11, 46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Izadi-Mood, N.; Samadi, N.; Sarmadi, S.; Eftekhar, Z. Papillary serous carcinoma arising from adenomyosis presenting as intramural leiomyoma. Arch. Iran Med. 2007, 10, 258–260. [Google Scholar]
- Choi, J.I.; Lee, H.J.; Shin, Y.J.; Lim, H.W.; Lee, H.N.; Kim, M.J. Rapid enlargement of endometrial stromal sarcoma after uterine fibroid embolization for presumed adenomyosis: A case report and literature review. Eur. J. Gynaecol. Oncol. 2016, 37, 876–881. [Google Scholar]
- Stefanko, D.P.; Eskander, R.; Aisagbonhi, O. Disseminated Endometriosis and Low-Grade Endometrioid Stromal Sarcoma in a Patient with a History of Uterine Morcellation for Adenomyosis. Case Rep. Obstet. Gynecol. 2020, 2020, 7201930. [Google Scholar] [CrossRef]
- Machida, H.; Maeda, M.; Cahoon, S.S.; Scannell, C.A.; Garcia-Sayre, J.; Roman, L.D.; Matsuo, K. Endometrial cancer arising in adenomyosis versus endometrial cancer coexisting with adenomyosis: Are these two different entities? Arch. Gynecol. Obstet. 2017, 295, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Hermens, M.; van Altena, A.M.; Velthuis, I.; van de Laar, D.C.M.; Bulten, J.; van Vliet, H.A.A.M.; Siebers, A.G.; Bekkers, R.L.M. Endometrial Cancer Incidence in Endometriosis and Adenomyosis. Cancers 2021, 13, 4592. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Seracchioli, R.; Raimondo, D.; Maletta, M.; Travaglino, A.; Raimondo, I.; Giaquinto, I.; Orsini, B.; Insabato, L.; Pellicano, M.; et al. Prevalence of adenomyosis in endometrial cancer patients: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2021, 303, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, D.; Raffone, A.; Travaglino, A.; Maletta, M.; Casadio, P.; Ambrosio, M.; Chiara Aru, A.; Santoro, A.; Franco Zannoni, G.; Insabato, L.; et al. Impact of adenomyosis on the prognosis of patients with endometrial cancer. Int. J. Gynecol. Obstet. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Duan, H.; Zhang, Y. Prognostic significance of co-existent adenomyosis on outcomes and tumor characteristics of endometrial cancer: A meta-analysis. J. Obstet. Gynaecol. Res. 2020, 46, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Casadio, P.; Raffone, A.; Maletta, M.; Travaglino, A.; Raimondo, D.; Raimondo, I.; Santoro, A.; Paradisi, R.; Zannoni, G.F.; Mollo, A.; et al. Clinical Characteristics of Patients with Endometrial Cancer and Adenomyosis. Cancers 2021, 13, 4918. [Google Scholar] [CrossRef]
- Kuwashima, Y.; Uehara, T.; Kishi, K.; Tajima, H.; Shiromizu, K.; Matsuzawa, M.; Takayama, S. Intramural adenocarcinoma of the uterus, arisen from adenomyosis uteri, showing unique histologic appearances. Report of two cases. Eur. J. Gynaecol. Oncol. 1994, 15, 418–423. [Google Scholar]
- Khalifa, M.A.; Atri, M.; Klein, M.E.; Ghatak, S.; Murugan, P. Adenomyosis As a Confounder to Accurate Endometrial Cancer Staging. Semin. Ultrasound CT MR 2019, 40, 358–363. [Google Scholar] [CrossRef]

| ADENOMYOSIS IN ULTRASOUND |
|---|
| globally enlarged uterus |
| asymmetric thickness anterior and posterior wall = pseudo-widening sign |
| cystic myometrium (cystic anechoic spaces) |
| junctional zone (JZ) not clearly visible, JZ interrupted, irregular, thickened; with anechoic cysts, hyperechoic dots |
| heterogeneous echogenicity of the myometrium |
| ill-defined lesion (difficult to delineate) |
| focal disturbances in myometrium layer |
| sometimes focal form diagnosed as intramural myoma |
| anechoic cysts or cysts of ground-glass appearance |
| absence of blood flow in lesions |
| Genetic Factors | Function | Association |
|---|---|---|
| Laminin-5 gamma2 chain | membrane glycoprotein, ligand of various transmembrane receptors | overexpressed in clear cell adenocarcinoma arising from adenomyosis [20] |
| β-Catenin (CTNNB1) | plasma membrane, responsibility for cell differentiation | β-Catenin pathways are involved in endometriosis and endometrial cancer, play important role in the pathogenesis of adenomyosis through epithelial–mesenchymal transition [21] |
| AT-Rich Interaction Domain 1A (ARID1A) | suppressor gene | occurrence in endometriosis tissues, mutation and loss of function in EAOC [22] |
| PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase) | suppressor gene | upregulated expression in adenomyosis, frequently observed in EC [23], detected in precursor endometriosis tissues, strongly associated with Ovarian Clear Cell Carcinoma (CCC) [22] |
| Phosphatase and Tensin Homolog (PTEN gene) | suppressor gene | occurrence in endometrial cyst, inactivation in up to 40% of clear carcinoma cells [24] |
| Protein 53 (p53) | suppressor gene | detected in hyperplastic and atypical epithelium of carcinoma arising from adenomyosis foci [12], mutation and loss of function in ovarian cancer [24], not observed in endometriosis, observed in endometriotic cells located near ovarian cancer cells [25] |
| Wilms tumor suppressor gene (WT1) | regulates the expression of insulin growth factor IGF-1, and transforming growth factor associated with DNA mismatch repair system | significant downregulated in endometriosis (downregulation of WT1 increased level of P450 aromatase expression and estrogen formation in endometriosis) [26], correlated with high-grade serous ovarian carcinomas [27] |
| KRAS genes | oncogene | strongly associated with adenomyosis, detected in ovarian clear cell cancer, detected in atypical endometriosis [28] |
| Hepatocyte nuclear factor (HNF—1B) | oncogene, plays role in chemoresistance | detected in endometriosis and clear cell carcinoma [29] |
| Hypermetylation of MutL Homolog 1 (MLH1) | component of DNA mismatch repair, leading to PTEN dysfunctionMicro-satellite instability (MSI) | observed in epithelial ovarian cancer and endometriosis [30], observed in ovarian cancer at chromosome 10q23 region [31] |
| Mucin 1—transmembrane hetemrodimer molecules (MUC1) | member of the mucin family molecules | present in endometriotic lesions and overexpressed in epithelial ovarian tumors [32] |
| Inflammatory Factors | Function | Association |
|---|---|---|
| Cyclooxygenase-2 (COX-2) | promotion of angiogenesis in adenomyosis | upregulated expression in endometrial, ovarian, and cervical cancer [35,36] |
| Tumor necrosis- alfa (TNF alfa) | promote production of ROS | high level in endometriosis and ovarian cancer [37] |
| Toll-like receptors (TLRs)—intracellular signaling components | cell surface sensors initiate proliferation and modulate immune cells—connected with chemoresistance | well described in endometriosis and ovarian cancer [38] |
| Tumor associated macrophages (TAMS) | promote angiogenesis, tumorigenesis, matrix remodeling, inhibits adaptive immunity | infiltrated ovarian tumor [39] |
| Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-KB proteins) | active in tumor cells, mediate metastasis | involved in development of endometriosis and ovarian cancer [40] |
| Macrophage migration inhibitory factor (MIF protein) | regulator of immune and inflammatory response | found in active ectopic endometrial implants [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szubert, M.; Kozirog, E.; Wilczynski, J. Adenomyosis as a Risk Factor for Myometrial or Endometrial Neoplasms—Review. Int. J. Environ. Res. Public Health 2022, 19, 2294. https://doi.org/10.3390/ijerph19042294
Szubert M, Kozirog E, Wilczynski J. Adenomyosis as a Risk Factor for Myometrial or Endometrial Neoplasms—Review. International Journal of Environmental Research and Public Health. 2022; 19(4):2294. https://doi.org/10.3390/ijerph19042294
Chicago/Turabian StyleSzubert, Maria, Edward Kozirog, and Jacek Wilczynski. 2022. "Adenomyosis as a Risk Factor for Myometrial or Endometrial Neoplasms—Review" International Journal of Environmental Research and Public Health 19, no. 4: 2294. https://doi.org/10.3390/ijerph19042294
APA StyleSzubert, M., Kozirog, E., & Wilczynski, J. (2022). Adenomyosis as a Risk Factor for Myometrial or Endometrial Neoplasms—Review. International Journal of Environmental Research and Public Health, 19(4), 2294. https://doi.org/10.3390/ijerph19042294






