Classifying Adenomyosis: Progress and Challenges
Abstract
:1. Introduction
2. Epidemiology
3. Defining Adenomyosis
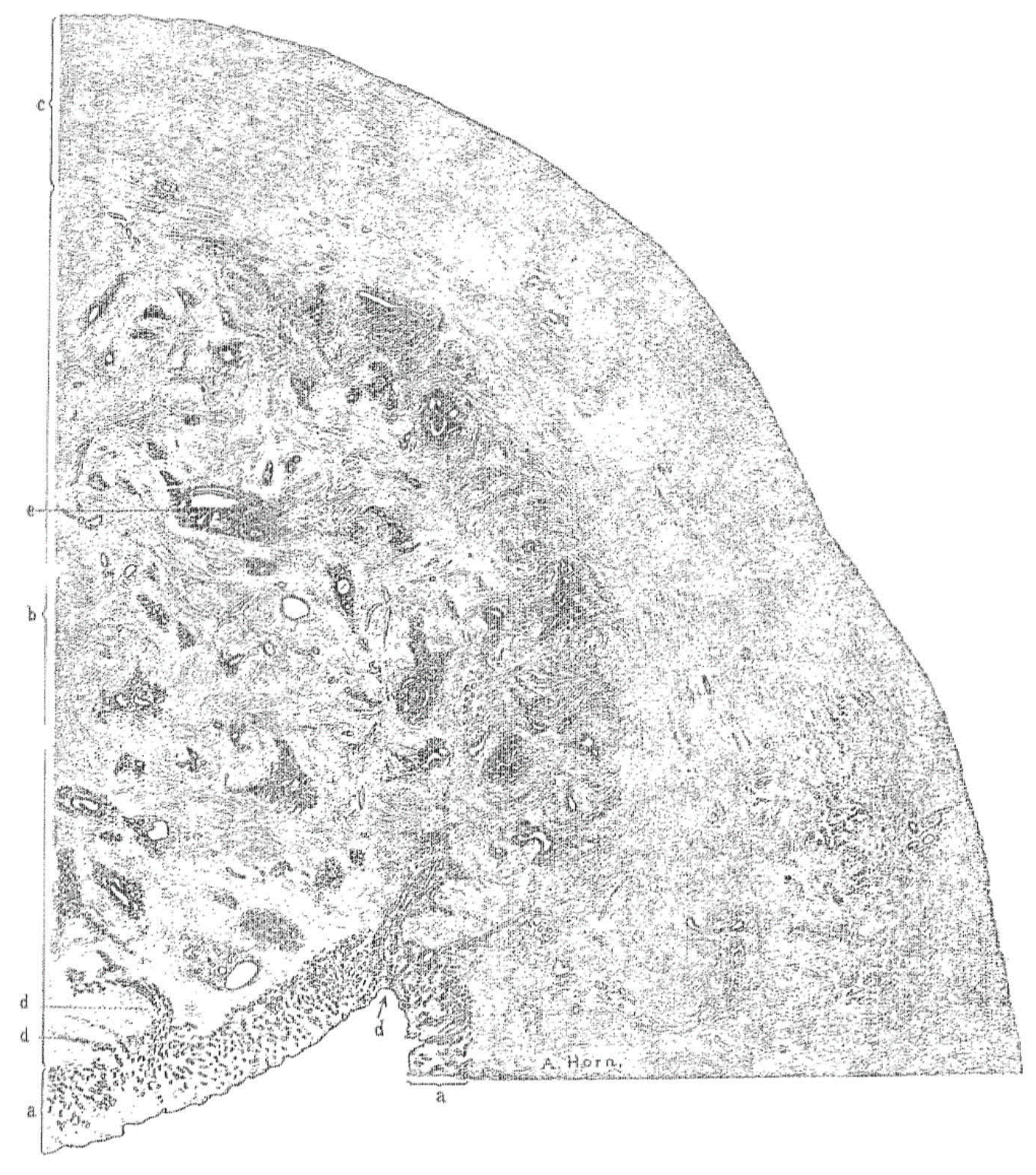
3.1. Histopathology
3.2. Imaging
- (a)
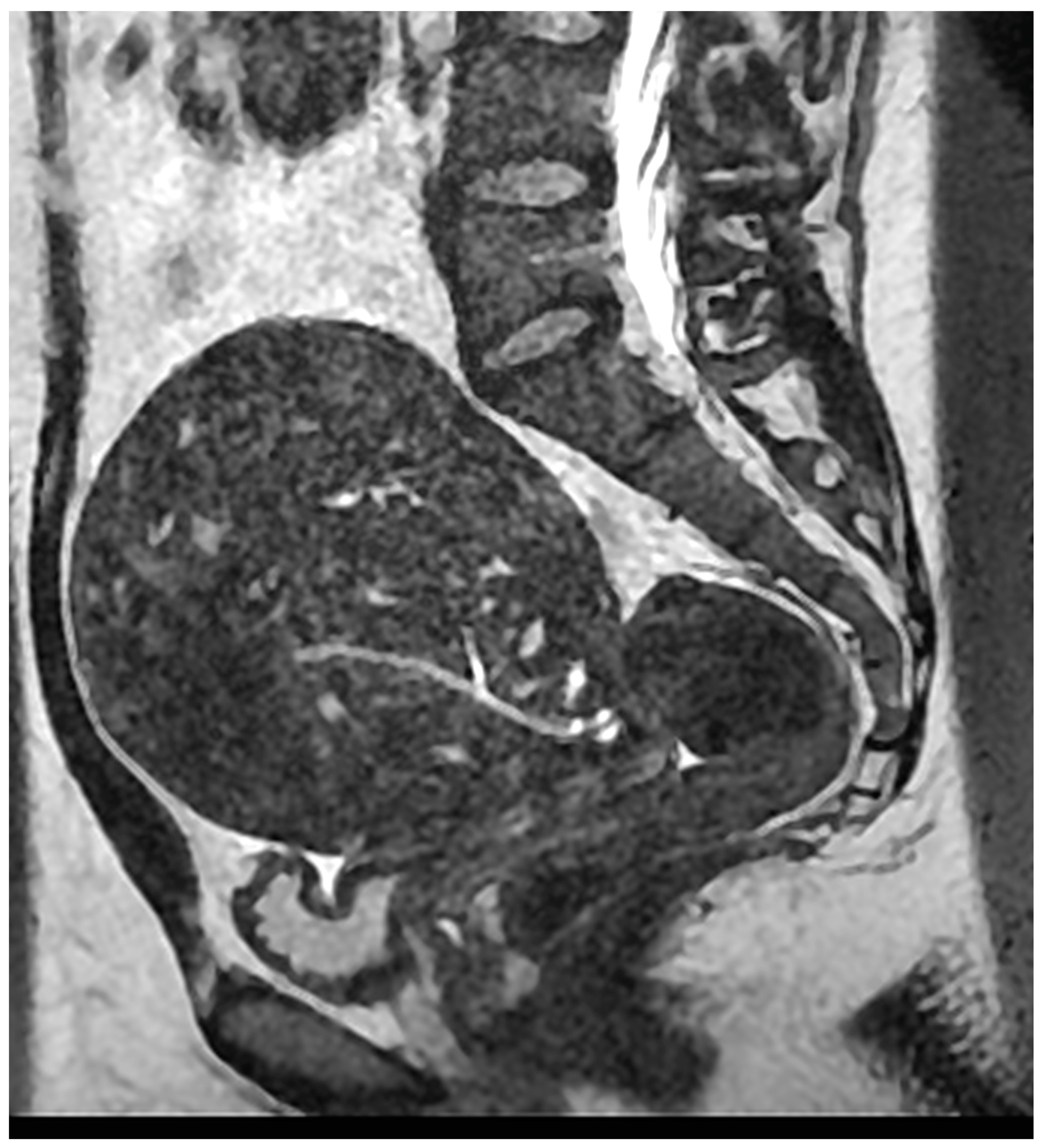
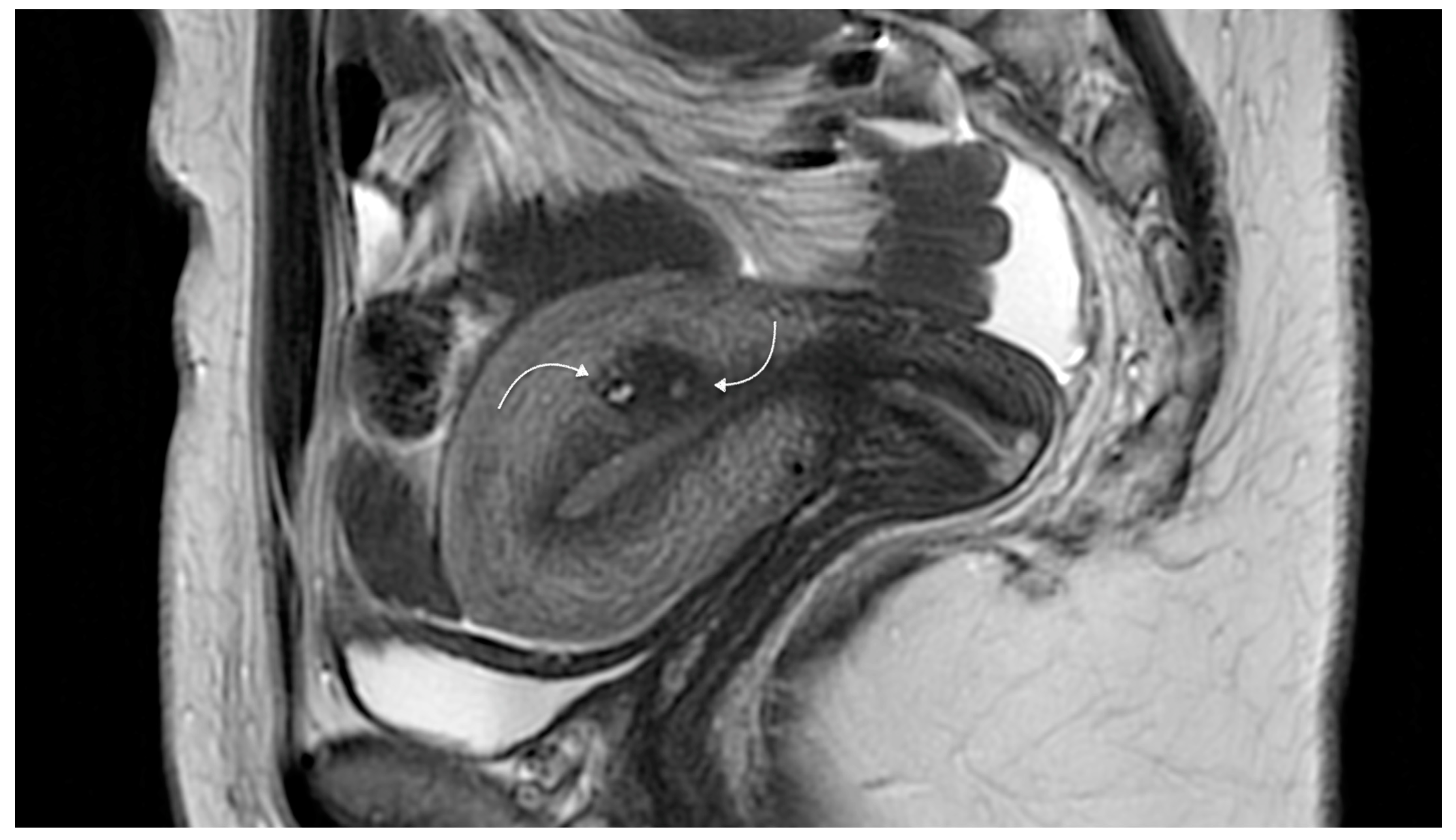
- Magnetic resonance imaging (MRI)
- (b)
- Ultrasound imaging
4. Variant Forms Related to Adenomyosis
- (a)
- Uterine cystic adenomyosis
- (b)
- Other rare variants
5. Co-Existing Pathology
Relation between Adenomyosis and Endometriosis
6. Pathophysiology of Adenomyosis
7. Classifying Adenomyosis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habiba, M.; Benagiano, G.; Brosens, I. The pathophysiology of adenomyosis. In Uterine Adenomyosis; Habiba, M., Benagiano, G., Eds.; Springer Internat. Publishing AG: Heidelberg, Germany, 2016; pp. 45–70. [Google Scholar]
- Brosens, I.; Puttemans, P.; Benagiano, G. Endometriosis: A life cycle approach? Am. J. Obstet. Gynecol. 2013, 209, 307–316. [Google Scholar] [CrossRef]
- Benagiano, G.; Brosens, I.; Habiba, M. Adenomyosis: A life-cycle approach. Reprod. Biomed. Online 2015, 30, 220–232. [Google Scholar] [CrossRef] [Green Version]
- Mark, A.S.; Hricak, H.; Heinrichs, L.W.; Hendrickson, M.R.; Winkler, M.L.; Bachica, J.A.; Stickler, J.E. Adenomyosis and leiomyoma: Differential diagnosis with MR imaging. Radiology 1987, 163, 527–529. [Google Scholar] [CrossRef]
- Fedele, L.; Bianchi, S.; Dorta, M.; Arcaini, L.; Zanotti, F.; Carinelli, S. Transvaginal ultrasonography in the diagnosis of diffuse ade-nomyosis. Fertil. Steril. 1992, 58, 94–97. [Google Scholar] [CrossRef]
- Habiba, M.; Gordts, S.; Bazot, M.; Brosens, I.; Benagiano, G. Exploring the challenges for a new classification of adenomyosis. Reprod. Biomed. Online 2020, 40, 569–581. [Google Scholar] [CrossRef]
- Munro, M.G. Classification and Reporting Systems for Adenomyosis. J. Minim. Invasive Gynecol. 2020, 27, 296–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.; Yong, P.; Bedaiwy, M. A critical review of recent advances in the diagnosis, classification, and management of uterine adenomyosis. Curr. Opin. Obstet. Gynecol. 2019, 31, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Gordts, S.; Grimbizis, G.; Campo, R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil. Steril. 2018, 109, 380–388.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, C.C.; McElin, T.W.; Manalo-Estrella, P. The elusive adenomyosis of the uterus—Revisited. Am. J. Obstet. Gynecol. 1972, 112, 583–593. [Google Scholar] [CrossRef]
- Bergholt, T.; Eriksen, L.; Berendt, N.; Jacobsen, M.; Hertz, J. Prevalence and risk factors of adenomyosis at hysterectomy. Hum. Reprod. 2001, 16, 2418–2421. [Google Scholar] [CrossRef] [Green Version]
- Vercellini, P.; Parazzini, F.; Oldani, S.; Panazza, S.; Bramante, T.; Crosignani, P.G. Adenomyosis at hysterectomy: A study on fre-quency distribution and patient characteristics. Hum. Reprod 1995, 10, 1160–1162. [Google Scholar] [CrossRef]
- Seidman, J.D.; Kjerulff, K.H. Pathologic findings from the Maryland Women’s Health Study: Practice patterns in the diagnosis of adenomyosis. Int. J. Gynecol. Pathol. 1996, 15, 217–221. [Google Scholar] [CrossRef]
- Naftalin, J.; Hoo, W.; Pateman, K.; Mavrelos, D.; Holland, T.; Jurkovic, D. How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum. Reprod. 2012, 27, 3432–3439. [Google Scholar] [CrossRef] [Green Version]
- Morassutto, C.; Monasta, L.; Ricci, G.; Barbone, F.; Ronfani, L. Incidence and Estimated Prevalence of Endometriosis and Adeno-myosis in Northeast Italy: A Data Linkage Study. PLoS ONE 2016, 11, e0154227. [Google Scholar] [CrossRef] [Green Version]
- Yu, O.; Schulze-Rath, R.; Grafton, M.J.; Hansen, M.K.; Scholes, D.; Reed, S.D. Adenomyosis incidence, prevalence and treatment: United States population-based study 2006–2015. Am. J. Obstet. Gynecol. 2020, 223, 94.e1–94.e10. [Google Scholar] [CrossRef]
- Frankl, O. Adenomyosis uteri. Am. J. Obstet. Gynecol. 1925, 10, 680–684. [Google Scholar] [CrossRef]
- Habiba, M.; Lippi, D.; Benagiano, G. The History of the Discovery of Ectopic Epithelial Cells in Lower Peritoneal Organs: The So-Called Mucosal Invasion. Reprod. Med. 2021, 2, 68–84. [Google Scholar] [CrossRef]
- Lockyer, C. Fibroids and Allied Tumours (Myoma and Adenomyoma); MacMillan: London, UK, 1918. [Google Scholar]
- Babeș, G. Über epitheliale Geschwulste in Uterusmyomem [About epithelial tumors in uterine fibroids]. Allgem Wiener Med. Ztschr. 1882, 27, 36–48. [Google Scholar]
- Hendrickson, M.R.; Kempson, R.L. Endometrial epithelial metaplasias: Proliferations frequently misdiagnosed as adeno-carcinoma. Report of 89 cases and proposed classification. Am. J. Surg. Pathol. 1980, 4, 525–542. [Google Scholar] [CrossRef]
- Habiba, M.; Benagiano, G. The incidence and clinical significance of adenomyosis. In Uterine Adenomyosis; Habiba, M., Benagiano, G., Eds.; Springer Internat. Publishing AG: Heidelberg, Germany, 2016; pp. 9–44. [Google Scholar]
- Sammour, A.; Pirwany, I.; Usubutun, A.; Arseneau, J.; Tulandi, T. Correlations between extent and spread of adenomyosis and clinical symptoms. Gynecol. Obstet. Investig. 2002, 54, 213–216. [Google Scholar] [CrossRef]
- Cullen, T.S. Adenomyoma of the Uterus; WB. Saunders: Philadelphia, PA, USA; London, UK, 1908. [Google Scholar]
- Walsh, J.; Taylor, K.; Rosenfield, A. Gray scale ultrasonography in the diagnosis of endometriosis and adenomyosis. Am. J. Roentgenol. 1979, 132, 87–90. [Google Scholar] [CrossRef]
- Hricak, H.; Alpers, C.E.; Crooks, L.E.; Sheldon, P.E. Magnetic resonance imaging of the female pelvis: Initial experience. Am. J. Roentgenol. 1983, 141, 1119–1128. [Google Scholar] [CrossRef]
- Lee, J.K.; Gersell, D.J.; Balfe, D.M.; Worthington, J.L.; Picus, D.; Gapp, G. The uterus: In vitro MR-anatomic correlation of normal and abnormal specimens. Radiology 1985, 157, 175–179. [Google Scholar] [CrossRef]
- Novellas, S.; Chassang, M.; Delotte, J.; Toullalan, O.; Chevallier, A.; Bouaziz, J.; Chevallier, P. MRI Characteristics of the Uterine Junctional Zone: From Normal to the Diagnosis of Adenomyosis. Am. J. Roentgenol. 2011, 196, 1206–1213. [Google Scholar] [CrossRef]
- Togashi, K.; Nishimura, K.; Itoh, K.; Fujisawa, I.; Noma, S.; Kanaoka, M.; Nakano, Y.; Itoh, H.; Ozasa, H.; Fujii, S. Adenomyosis: Diagnosis with MR imaging. Radiology 1988, 166, 111–114. [Google Scholar] [CrossRef]
- Mehasseb, M.K.; Bell, S.C.; Brown, L.; Pringle, J.H.; Habiba, M. Phenotypic Characterisation of the Inner and Outer Myometrium in Normal and Adenomyotic Uteri. Gynecol. Obstet. Investig. 2011, 71, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Dartmouth, K. A systematic review with meta-analysis: The common sonographic characteristics of adenomyosis. Ultrasound 2014, 22, 148–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canis, M.; Gremeau, A.S.; Bourdel, N. Elusive adenomyosis: A plea for an international classification system to allow artificial intelligence approaches to reset our clinical management. Fertil. Steril. 2018, 110, 1039–1040. [Google Scholar] [CrossRef] [Green Version]
- Bulić, M.; Kasnar, V.; Duković, I. Primjenaultrazvuka, u dijagnostici genitalne endometrioze [Use of ultrasound in the diagnosis of genital endometriosis]. Jugosl. Ginekol. Perinatol. 1986, 26, 33–34. [Google Scholar]
- Bohlman, M.E.; Ensor, R.E.; Sanders, R.C. Sonographic findings in adenomyosis of the uterus. Am. J. Roentgenol. 1987, 148, 765–766. [Google Scholar] [CrossRef] [PubMed]
- Coleman, B.G.; Arger, P.H.; Grumbach, K.; Menard, M.K.; Mintz, M.C.; Allen, K.S.; Arenson, R.L.; Lamon, K.A. Transvaginal and transabdominal sonogra-phy: Prospective comparison. Radiology 1988, 168, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Timor-Tritsch, I.E.; Bar-Yam, Y.; Elgali, S.; Rottem, S. The technique of transvaginal sonography with the use of a 6.5 MHz probe. Am. J. Obstet. Gynecol. 1988, 158, 1019–1024. [Google Scholar] [CrossRef]
- Brosens, J.J.; de Souza, N.M.; Barker, F.G.; Paraschos, T.; Winston, R.M. Endovaginal ultrasonography in the diagnosis of adenomyo-sis uteri: Identifying the predictive characteristics. BJOG Int. J. Obstet. Gynecol. 1995, 102, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Hanafi, M. Ultrasound diagnosis of adenomyosis, leiomyoma, or combined with histopathological correlation. J. Hum. Reprod. Sci. 2013, 6, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, C.; Tafazoli, F.; Wang, L. Imaging features of adenomyosis. Hum. Reprod. Updat. 1998, 4, 337–349. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, R.K.; Horrow, M.M.; Smith, R.J.; Springer, J. Adenomyosis: A Sonographic Diagnosis. Radiographics 2018, 38, 1576–1589. [Google Scholar] [CrossRef]
- Meredith, S.M.; Sanchez-Ramos, L.; Kaunitz, A.M. Diagnostic accuracy of transvaginal sonography for the diagnosis of adeno-myosis: Systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2009, 201, 107.e1–107.e6. [Google Scholar] [CrossRef]
- Exacoustos, C.; Brienza, L.; Di Giovanni, A.; Szabolcs, B.; Romanini, M.E.; Zupi, E.; Arduini, D. Adenomyosis: Three-dimensional so-nographic findings of the junctional zone and correlation with histology. Ultrasound Obs. Gynecol. 2011, 37, 471–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andres, M.P.; Borrelli, G.M.; Ribeiro, J.; Baracat, E.C.; Abrão, M.S.; Kho, R.M. Transvaginal Ultrasound for the Diagnosis of Adeno-myosis: Systematic Review and Meta-Analysis. J. Minim. Invasive. Gynecol. 2018, 25, 257–264. [Google Scholar] [CrossRef]
- Champaneria, R.; Abedin, P.; Daniels, J.; Balogun, M.; Khan, K.S. Ultrasound scan and magnetic resonance imaging for the diag-nosis of adenomyosis: Systematic review comparing test accuracy. Acta Obstet. Gynecol. Scand. 2010, 89, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Cortez, A.; Darai, E.; Rouger, J.E.; Chopier, J.; Antoine, J.-M.; Uzan, S. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: Correlation with histopathology. Hum. Reprod. 2001, 16, 2427–2433. [Google Scholar] [CrossRef] [Green Version]
- Van den Bosch, T.; Dueholm, M.; Leone, F.P.G.; Valentin, L.; Rasmussen, C.K.; Votino, A.; Van Schoubroeck, D.; Landolfo, C.; Installé, A.J.F.; Guerriero, S.; et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: A consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet. Gynecol. 2015, 46, 284–298. [Google Scholar] [CrossRef]
- Van den Bosch, T.; Van Schoubroeck, D. Ultrasound diagnosis of endometriosis and adenomyosis: State of the art. Best Pract. Res. Clin. Obstet. Gynecol. 2018, 51, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Gordts, S.; Habiba, M.; Benagiano, G. Uterine Cystic Adenomyosis: A Disease of Younger Women. J. Pediatr. Adolesc. Gynecol. 2015, 28, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Habiba, M.; Brosens, I.; Zannoni, G.F.; Benagiano, G. Uncommon Mixed Endometrial-Myometrial Benign Tumors of the Repro-ductive Tract: Literature Review. Gynecol. Obstet. Investig. 2019, 84, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra Babu, Y.P.; Karki, R.K.; Menezes, R.G.; Jagadish Rao, P.P.; Shetty, S.K.; Sahu, K.K. Adenomyomatous polyp of the uterus: Report of an autopsy case and review of the literature. J. Forensic Leg. Med. 2012, 19, 236–238. [Google Scholar] [CrossRef]
- Grimbizis, G.F.; Mikos, T.; Miliaras, D.; Kioussis, G.; Theodoridis, T.D.; Tsolakidis, D.; Tarlatzis, B.C. Management of atypical polypoid adenomyomas. A case series. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 215, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Maseelall, P.; Schott, L.L.; Brockwell, S.E.; Schocken, M.; Johnston, J.M. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women’s Health Across the Nation (SWAN). Fertil. Steril. 2009, 91, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, H.; Khan, K.S. Adenomyosis in Pakistani women: Four year experience at the Aga Khan University Medical Centre, Karachi. J. Clin. Pathol. 1990, 43, 817–819. [Google Scholar] [CrossRef] [Green Version]
- Kunz, G.; Beil, D.; Huppert, P.; Noe, M.; Kissler, S.; Leyendecker, G. Adenomyosis in endometriosis--prevalence and impact on fer-tility. Evidence from magnetic resonance imaging. Hum. Reprod. 2005, 20, 2309–2316. [Google Scholar] [CrossRef] [Green Version]
- Leyendecker, G.; Bilgicyildirim, A.; Inacker, M.; Stalf, T.; Huppert, P.; Mall, G.; Böttcher, B.; Wildt, L. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch. Gynecol. Obstet. 2015, 291, 917–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapron, C.; Tosti, C.; Marcellin, L.; Bourdon, M.; Lafay-Pillet, M.-C.; Millischer, A.-E.; Streuli, I.; Borghese, B.; Petraglia, F.; Santulli, P. Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum. Reprod. 2017, 32, 1393–1401. [Google Scholar] [CrossRef] [Green Version]
- Marcellin, L.; Santulli, P.; Bourdon, M.; Maignien, C.; Campin, L.; Lafay-Pillet, M.-C.; Millischer, A.-E.; Bordonne, C.; Borghese, B.; Dousset, B.; et al. Focal adenomyosis of the outer myometrium and deep infiltrating endometriosis severity. Fertil. Steril. 2020, 114, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Tellum, T.; Nygaard, S.; Skovholtek Qvigstad, E.; Lieng, M. Development of a clinical prediction model for diagnosing adenomy-osis. Fertil. Steril. 2018, 110, 957–964.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazot, M.; Daraï, E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil. Steril. 2018, 109, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Larsen, S.; Lundorf, E.; Forman, A.; Dueholm, M. Adenomyosis and junctional zone changes in patients with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 157, 206–211. [Google Scholar] [CrossRef]
- Kishi, Y.; Suginami, H.; Kuramori, R.; Yabuta, M.; Suginami, R.; Taniguchi, F. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am. J. Obstet. Gynecol. 2012, 207, 114.e1–114.e7. [Google Scholar] [CrossRef]
- Kishi, Y.; Shimada, K.; Fujii, T.; Uchiyama, T.; Yoshimoto, C.; Konishi, N.; Ohbayashi, C.; Kobayashi, H. Phenotypic characterization of adenomyosis occurring at the inner and outer myometrium. PLoS ONE 2017, 12, e0189522. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Koshiba, A.; Kuroboshi, H.; Mori, T.; Ogi, H.; Itoh, K.; Nakashima, M.; Kitawaki, J. Biological differences be-tween intrinsic and extrinsic adenomyosis with coexisting deep infiltrating endometriosis. Reprod. Biomed. Online 2019, 39, 343–353. [Google Scholar] [CrossRef]
- Rasmussen, C.K.; Hansen, E.S.; Dueholm, M. Two- and three-dimensional ultrasonographic features related to histopathology of the uterine endometrial-myometrial junctional zone. Acta Obstet. Gynecol. Scand. 2019, 98, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Benagiano, G.; Habiba, M.; Brosens, I. The pathophysiology of uterine adenomyosis: An update. Fertil. Steril. 2012, 98, 572–579. [Google Scholar] [CrossRef]
- Tellum, T.; Nygaard, S.; Lieng, M. Noninvasive Diagnosis of Adenomyosis: A Structured Review and Meta-analysis of Diagnostic Accuracy in Imaging. J. Minim. Invasive Gynecol. 2020, 27, 408–418.e3. [Google Scholar] [CrossRef] [PubMed]
- Leone, F.P.; Timmerman, D.; Bourne, T.; Valentin, L.; Epstein, E.; Goldstein, S.R.; Marret, H.; Parsons, A.K.; Gull, B.; Istre, O.; et al. Terms, definitions and measurements to describe the sonographic features of the endome-trium and intrauterine lesions: A consensus opinion from the International Endometrial Tumor Analysis (IETA) group. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2010, 35, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Exacoustos, C.; Morosetti, G.; Conway, F.; Camilli, S.; Martire, F.G.; Lazzeri, L.; Piccione, E.; Zupi, E. New Sonographic Classification of Adenomyosis: Do Type and Degree of Adenomyosis Correlate to Severity of Symptoms? J. Minim. Invasive Gynecol. 2020, 27, 1308–1315. [Google Scholar] [CrossRef] [PubMed]

| Reference | Descriptor |
|---|---|
| Microscopic field of view | |
| Novak and Woodruff, 1979 | >1 high-power field. |
| Parazzini et al., 1997 | >0.5 of low-power field |
| Zaloudek and Hendrickson, 2002 | >0.5 of low-power field |
| Gompel and Silverberg, 1985 | medium-power field (×100 lens) |
| Owolabi and Strickler, 1977 | >low-power field |
| Uterine wall thickness | |
| Hendrickson and Kempson, 1980 | >1/4 of the total thickness |
| Shaikh and Khan, 1990 | >1/3 to 1/4 of the total thickness |
| Measurement in mm | |
| Bergholt et al., 2001 | recommend 3 mm as cut-off |
| Levgur et al., 2000 | ≥2.5 mm |
| Reference | Categories/Basis of Classification |
|---|---|
| Histopathology | |
| Bird et al. (1972) Sammour et al. (2002) | Depth of invasion within the mometrium Degree of involvement: Number of glands per low power field |
| Siegler and Camilien (1994) Hulka et al. (2002) Vercellini et al. (2006) | Depth of penetration Degree of involvement Configuration: Diffuse, discrete. |
| Levgur et al. (2000) | Depth of invasion |
| Ultrasound based | |
| Lazzeri et al. (2018) | Configuration Depth of lesion (affecting Junctional zone, inner, outer myometrium) |
| MRI based | |
| Grodts et al. (2008) | JZ hyperplasia Adenomyosis Adenomyoma |
| Kishi et al. (2012) | Subtype I: Intrinsic: Inner uterine layer Subtype II: Extrinsic: Outer uterine layer (normal JZ) Subtype III: Solitary adenomyosis no connection to the JZ or to the serosa. Subtype IV: Indeterminate |
| Kobayashi and Matsubara (2020) | Depth of lesion Configuration Size of lesion Localization of lesion Concomitant pathology |
| Bazot and Daraï (2018) | A Internal adenomyosis Configuration B Adenomyomas Consistency C External adenomyosis Location Coexisting endometriosis |
| Grimbizis et al. (2014) | Configuration Polypoid forms Special (rare forms) |
| Features at surgery | |
| Pistofidis et al. (2014) | Consistency |
| Imaging based | |
| Gordt et al. (2018) | Extent Localization Configuration Consistency Size |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habiba, M.; Benagiano, G. Classifying Adenomyosis: Progress and Challenges. Int. J. Environ. Res. Public Health 2021, 18, 12386. https://doi.org/10.3390/ijerph182312386
Habiba M, Benagiano G. Classifying Adenomyosis: Progress and Challenges. International Journal of Environmental Research and Public Health. 2021; 18(23):12386. https://doi.org/10.3390/ijerph182312386
Chicago/Turabian StyleHabiba, Marwan, and Giuseppe Benagiano. 2021. "Classifying Adenomyosis: Progress and Challenges" International Journal of Environmental Research and Public Health 18, no. 23: 12386. https://doi.org/10.3390/ijerph182312386
APA StyleHabiba, M., & Benagiano, G. (2021). Classifying Adenomyosis: Progress and Challenges. International Journal of Environmental Research and Public Health, 18(23), 12386. https://doi.org/10.3390/ijerph182312386






