Advancing Behavioral Intervention and Theory Development for Mobile Health: The HeartSteps II Protocol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Design
2.2. Aims
2.3. HeartSteps Intervention Components
2.3.1. Fitbit
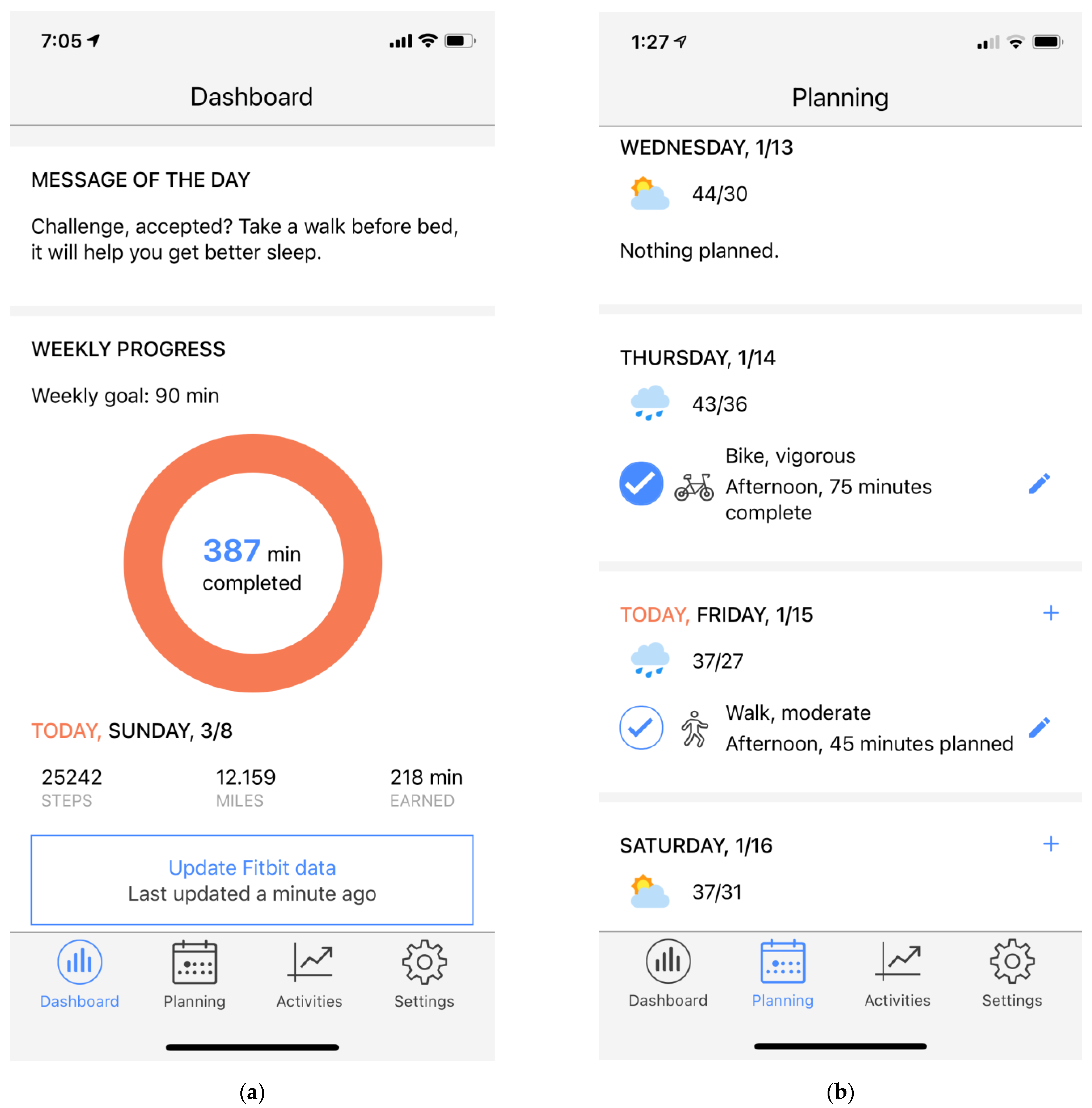
2.3.2. HeartSteps App
2.3.3. HeartSteps Clock Face
2.4. HearSteps Intervention Design: Randomization, Availability and Proximal Outcomes
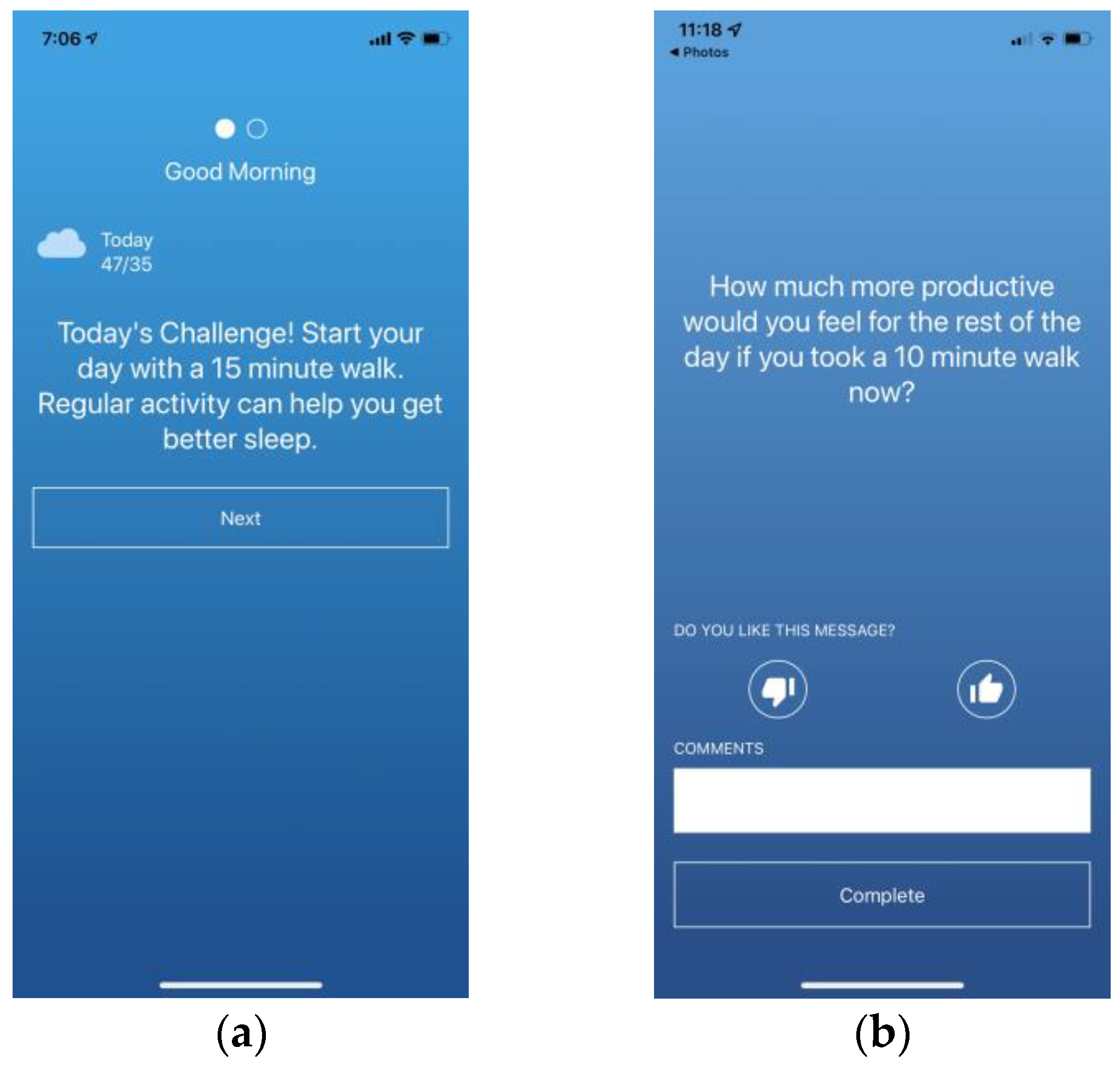
2.4.1. Motivational Messages
2.4.2. Walking Suggestions
2.4.3. Anti-Sedentary Suggestions
2.5. Measures
2.5.1. Baseline and Follow Up
2.5.2. Ecological Momentary Assessment (EMA)
Daily Questionnaires
Weekly Questionnaires
Activity Questionnaires
EMA Bursts
2.5.3. Passive Measures
The Fitbit Activity Tracker
HeartSteps Use Logs
3. Participants and Procedures
3.1. Recruitment
3.2. Power Calculations
3.3. Eligibility
3.4. Initial Screening, Orientation, and Run-in Procedures
3.5. Participants
3.6. Treatment Fidelity (Monitoring, Contacting Protocol, Adherence)
3.6.1. Monitoring Data Collection
No Fitbit Data
No Interaction with the HeartSteps Application
Morning Survey Non-Compliance
3.7. Burst Adherence Protocol
3.8. Data Storage, Security and Privacy
4. Modeling and Data Analysis
4.1. Analyses of Micro-Randomized Intervention Data
4.2. Modeling Framework Development
4.2.1. Computational Modeling with Dynamic Bayesian Networks
4.2.2. Modeling with Continuous Dynamical Systems
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holman, H.R. The Relation of the Chronic Disease Epidemic to the Health Care Crisis. ACR Open Rheumatol. 2020, 2, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spring, B.; Schneider, K.L.; McFadden, H.G.; Vaughn, J.; Kozak, A.T.; Smith, M.L.; Moller, A.C.; Epstein, L.H.; DeMott, A.; Hedeker, D.; et al. Multiple Behavior Changes in Diet and Activity: A Randomized Controlled Trial Using Mobile Technology. Arch. Intern. Med. 2012, 172, 789–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murawski, B.; Plotnikoff, R.C.; Rayward, A.T.; Oldmeadow, C.; Vandelanotte, C.; Brown, W.J.; Duncan, M.J. Efficacy of an m-Health Physical Activity and Sleep Health Intervention for Adults: A Randomized Waitlist-Controlled Trial. Am. J. Prev. Med. 2019, 57, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, S.A.; Williams, A.J.; Morrissey, K.; Price, L.; Harrison, J. Mobile health interventions to promote physical activity and reduce sedentary behaviour in the workplace: A systematic review. Digit. Health 2019, 5, 2055207619839883. [Google Scholar] [CrossRef] [Green Version]
- Glanz, K.; Rimer, B.K.; Viswanath, K. Health Behavior: Theory, Research, and Practice; John Wiley and Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Spruijt-Metz, D.; Hekler, E.B.; Saranummi, N.; Intille, S.S.; Korhonen, I.; Nilsen, W.; Rivera, D.; Spring, B.; Michie, S.; Asch, D.; et al. Building new computational models to support health behavior change and maintenance: New opportunities in behavioral research. Transl. Behav. Med. 2015, 5, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Hekler, E.B.; Michie, S.; Pavel, M.; Rivera, D.; Collins, L.; Jimison, H.B.; Garnett, C.; Parral, S.; Spruijt-Metz, D. Advancing Models and Theories for Digital Behavior Change Interventions. Am. J. Prev. Med. 2016, 51, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Nahum-Shani, I.; Hekler, E.B.; Spruijt-Metz, D. Building health behavior models to guide the development of just-in-time adaptive interventions: A pragmatic framework. Health Psychol. 2015, 34, 1209–1219. [Google Scholar] [CrossRef]
- Hekler, E.; Tiro, J.A.; Hunter, C.M.; Nebeker, C. Precision Health: The Role of the Social and Behavioral Sciences in Advancing the Vision. Ann. Behav. Med. 2020, 54, 805–826. [Google Scholar] [CrossRef]
- Hekler, E.; King, A.C. Toward an open mechanistic science of behavior change. Health Psychol. 2020, 39, 841–845. [Google Scholar] [CrossRef]
- Klasnja, P.; Hekler, E.B.; Shiffman, S.; Boruvka, A.; Almirall, D.; Tewari, A.; Murphy, S.A. Microrandomized trials: An experimental design for developing just-in-time adaptive interventions. Health Psychol. 2015, 34, 1220–1228. [Google Scholar] [CrossRef]
- Klasnja, P.; Smith, S.; Seewald, N.; Lee, A.J.; Hall, K.; Luers, B.; Hekler, E.B.; Murphy, S.A. Efficacy of Contextually Tailored Suggestions for Physical Activity: A Micro-randomized Optimization Trial of HeartSteps. Ann. Behav. Med. 2019, 53, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Greenewald, K.; Klasnja, P.; Murphy, S. Personalized heartsteps: A reinforcement learning algorithm for optimizing physical activity. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2020, 4, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Deci, E.L.; Ryan, R.M. Motivation, personality, and development within embedded social contexts: An overview of self-determination theory. In Oxford Library of Psychology. The Oxford Handbook of Human Motivation; Ryan, R.M., Ed.; Oxford University Press: Oxford, UK, 2012; pp. 85–107. [Google Scholar]
- Deci, E.L.; Ryan, R.M. Self-determination theory. In Handbook of Theories of Social Psychology; Van Lange, A.K.P., Higgins, E., Eds.; Sage: London, UK, 2012; Volume 1, pp. 416–436. [Google Scholar]
- Abraham, C.; Michie, S. A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008, 27, 379–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michie, S.; Ashford, S.; Sniehotta, F.; Dombrowski, S.U.; Bishop, A.; French, D. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: The CALO-RE taxonomy. Psychol. Health 2011, 26, 1479–1498. [Google Scholar] [CrossRef]
- Achtziger, A.; Gollwitzer, P.M.; Sheeran, P. Implementation Intentions and Shielding Goal Striving From Unwanted Thoughts and Feelings. Pers. Soc. Psychol. Bull. 2008, 34, 381–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagger, M.S.; Luszczynska, A. Implementation Intention and Action Planning Interventions in Health Contexts: State of the Research and Proposals for the Way Forward. Appl. Psychol. Health Well-Being 2014, 6, 1–47. [Google Scholar] [CrossRef] [Green Version]
- Becker, M.H. The Health Belief Model and Sick Role Behavior. Health Educ. Monogr. 1974, 2, 409–419. [Google Scholar] [CrossRef]
- Maiman, L.A.; Becker, M.H. The Health Belief Model: Origins and Correlates in Psychological Theory. Health Educ. Monogr. 1974, 2, 336–353. [Google Scholar] [CrossRef]
- Rothman, A.J.; Salovey, P. Shaping perceptions to motivate healthy behavior: The role of message framing. Psychol. Bull. 1997, 121, 3–19. [Google Scholar] [CrossRef]
- Updegraff, J.A.; Rothman, A.J. Health Message Framing: Moderators, Mediators, and Mysteries. Soc. Pers. Psychol. Compass 2013, 7, 668–679. [Google Scholar] [CrossRef]
- Liao, P.; Dempsey, W.; Sarker, H.; Hossain, S.M.; Al’Absi, M.; Klasnja, P.; Murphy, S. Just-in-time but not too much: Determining treatment timing in mobile health. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2018, 2, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Gosling, S.D.; Rentfrow, P.J.; Swann, W.B., Jr. A very brief measure of the Big-Five personality domains. J. Res. Pers. 2003, 37, 504–528. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Marcus, B.H.; Eaton, C.A.; Rossi, J.S.; Harlow, L.L. Self-Efficacy, Decision-Making, and Stages of Change: An Integrative Model of Physical Exercise1. J. Appl. Soc. Psychol. 1994, 24, 489–508. [Google Scholar] [CrossRef]
- Markland, D.; Tobin, V. A Modification to the Behavioural Regulation in Exercise Questionnaire to Include an Assessment of Amotivation. J. Sport Exerc. Psychol. 2004, 26, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Ajzen, I. Construction of a Standard Questionnaire for the Theory of Planned Behavior; 2002. Available online: https://people.umass.edu/aizen/pdf/tpb.measurement.pdf (accessed on 6 January 2022).
- Rhodes, R.E.; Rebar, A.L. Conceptualizing and Defining the Intention Construct for Future Physical Activity Research. Exerc. Sport Sci. Rev. 2017, 45, 209–216. [Google Scholar] [CrossRef]
- Cerin, E.; Saelens, B.E.; Sallis, J.F.; Frank, L.D. Neighborhood Environment Walkability Scale: Validity and development of a short form. Med. Sci. Sports Exerc. 2006, 38, 1682. [Google Scholar] [CrossRef] [Green Version]
- Shankar, A.; McMunn, A.; Banks, J.; Steptoe, A. Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychol. 2011, 30, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Kapteyn, A.; Angrisani, M.; Bennett, D.; Bruine de Bruin, W.; Darling, J.; Gutsche, T.; Liu, Y.; Meijer, E.; Perez-Arce, F.; Schaner, S.; et al. Tracking the Effect of the COVID-19 Pandemic on the Lives of American Households. Surv. Res. Methods 2020, 14, 179–186. [Google Scholar] [CrossRef]
- Stoyanov, S.R.; Hides, L.; Kavanagh, D.J.; Zelenko, O.; Tjondronegoro, D.; Mani, M. Mobile App Rating Scale: A New Tool for Assessing the Quality of Health Mobile Apps. JMIR mHealth uHealth 2015, 3, e27. [Google Scholar] [CrossRef] [Green Version]
- Boruvka, A.; Almirall, D.; Witkiewitz, K.; Murphy, S.A. Assessing Time-Varying Causal Effect Moderation in Mobile Health. J. Am. Stat. Assoc. 2018, 113, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, G.A. Using Generalized Estimating Equations for Longitudinal Data Analysis. Organ. Res. Methods 2004, 7, 127–150. [Google Scholar] [CrossRef]
- Goldstein, H. Multilevel Models in Education and Social Research; Oxford University Press: Oxford UK, 1987. [Google Scholar]
- Murphy, K.P. Dynamic Bayesian Networks: Representation, Inference and Learning; University of California: Berkeley, CA, USA, 2002. [Google Scholar]
- Murphy, K.P. Machine Learning: A Probabilistic Perspective; MIT Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Riley, W.T.; Martin, C.; Rivera, D.E.; Hekler, E.B.; Adams, M.A.; Buman, M.; Pavel, M.; King, A.C. Development of a dynamic computational model of social cognitive theory. Transl. Behav. Med. 2016, 6, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Rivera, D.E.; Hekler, E.B.; Savage, J.S.; Downs, D.S. Intensively Adaptive Interventions Using Control Systems Engineering: Two Illustrative Examples. In Optimization of Behavioral, Biobehavioral, and Biomedical Interventions; Springer International Publishing: Cham, Switzerland, 2018; pp. 121–173. [Google Scholar]
- Martin, C.A.; Rivera, D.E.; Hekler, E.B.; Riley, W.T.; Buman, M.P.; Adams, M.A.; Magann, A.B. Development of a Control-Oriented Model of Social Cognitive Theory for Optimized mHealth Behavioral Interventions. IEEE Trans. Control Syst. Technol. 2020, 28, 331–346. [Google Scholar] [CrossRef]
- Hekler, E.B.; Rivera, D.E.; Martin, C.A.; Phatak, S.S.; Freigoun, M.T.; Korinek, E.; Klasnja, P.; Adams, M.A.; Buman, M.P. Tutorial for Using Control Systems Engineering to Optimize Adaptive Mobile Health Interventions. J. Med. Internet Res. 2018, 20, e214. [Google Scholar] [CrossRef]
- Korinek, E.V.; Phatak, S.S.; Martin, C.A.; Freigoun, M.T.; Rivera, D.E.; Adams, M.A.; Klasnja, P.; Buman, M.; Hekler, E.B. Adaptive step goals and rewards: A longitudinal growth model of daily steps for a smartphone-based walking intervention. J. Behav. Med. 2017, 41, 74–86. [Google Scholar] [CrossRef]
- Rivera, D.E.; Pew, M.D.; Collins, L. Using engineering control principles to inform the design of adaptive interventions: A conceptual introduction. Drug Alcohol Depend. 2007, 88, S31–S40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenman, A. Model on Demand: Algorithms, Analysis and Applications; Citeseer: Linköping, Sweden, 1999. [Google Scholar]
- Braun, M.W.; Rivera, D.E.; Stenman, A. A ’Model-on-Demand’ identification methodology for non-linear process systems. Int. J. Control 2001, 74, 1708–1717. [Google Scholar] [CrossRef]
- Wang, Q.; Spall, J.C. Discrete simultaneous perturbation stochastic approximation for resource allocation in public health. In Proceedings of the 2014 American Control Conference, San Francisco, CA, USA, 29 June–1 July 2011; pp. 3639–3644. [Google Scholar]

| Measures | Answer Categories (Number of Items) |
|---|---|
| Demographic Information * | Age, Race, Marital Status, Household Members, Employment Status, Level of Education (6 items) |
| Autonomy to Decide Schedule * | Strongly Disagree to Strongly agree (3 items) (5-point Likert) |
| Mobile Phone Usage * | More than 30 times per day; Between 10 and 30 times per day; Between 5 and 9 times per day; 3 or 4 times per day; 1 or 2 per day; Less frequently than once per day (1 item) |
| Ten-Item Personality Inventory (TIPI) [25] * | Disagree strongly to Agree Strongly (10 items) (7-point Likert scale) |
| Stress (Perceived Stress Scale—PSS) [26] ^ | Never to very often (5 items) (5-point Likert scale) |
| Routine * | Definitely a morning type; Rather more a morning type than an evening type; Rather more an evening-type than a morning-type; Definitely an evening type (1 item) |
| Self-efficacy for Physical Activity [27] ^ | Not at all confident to extremely confident (5 items) (5-point Likert) |
| Motivation for Physical Activity [28] ^ | Not true for me to very true for me (19 items) (5-point Likert) |
| Value of Physical Activity [29] * | Extremely worthless to Extremely valuable (1 item) (5-point Likert) |
| Intention in Physical Activity [30] * | Not at all to very much (1 item) (5-point Likert) |
| Perceived Effects of Physical Activity ^ | How long does it take before you notice the positive (3 items)/negative (3 items) effects of physical activity on; emotional well-being, physical heath, mood |
| Social Support in Physical Activity ^ | Never true, sometimes true, always true, not applicable (6 items) |
| Neighborhood Walkability [31] * | Strongly disagree to strongly agree (6 items) (4-point Likert) |
| Social Isolation Index [32] * | Rarely or never, once a month, several times a month, at least once a week (6 items) |
| Physical Activity During COVID-19 Outbreak [33] + | Yes, no, decline to answer (3 items) |
| App Usability (Mobile App Rating Scale (MARS) [34] + | Item specific answer categories (20 items) |
| Morning Questionnaire (Once Daily, Available after 6:00 a.m.) | ||||
|---|---|---|---|---|
| Item | Answer Categories | * Current | Pro-Spective | Retro-Spective |
| How well rested do you feel this morning? | Not at all—Very (5-pt. Likert) | X | ||
| How busy is your day going to be today? | Not at all—Very (5-pt. Likert) | X | ||
| If I walk or exercise today, it’s because it’s important to my life | Not at all true—Very true (5-pt. Likert) | X | ||
| If I walk or exercise today, it’s because other people think I should | Not at all true—Very true (5-pt. Likert) | X | ||
| How committed do you feel this morning in being physically active today | Not at all—Very (5-pt. Likert) | X | ||
| Which of these best describes how you feel this morning | (Choose one) sad, energetic, stressed, relaxed, fatigued, happy, tense | X | ||
| Weekly questionnaire (once a week, available Sunday) | ||||
| People in my life supported me in my efforts to be more active this past week (not at all true to very true | Not at all true—Very true (5-pt. Likert) | X | ||
| How much are you enjoying physical activities you did this week? | Not at all—Very much (5-pt. Likert) | X | ||
| Over the past week, how socially connected you felt? | Not at all—Extremely (5-pt. Likert) | X | ||
| This week, I walked or exercised because I felt restless, stressed, or in a bad mood | Not at all true—Very true (5-pt. Likert) | X | ||
| How well is physical activity currently fitting into your daily routine | Not at all—Very much (5-pt. Likert) | X | ||
| This past week, I walked or exercised because other people think I should | Not at all true—Very true (5-pt. Likert) | X | ||
| This past week, I walked or exercised because it’s important for my life | Not at all—Very much (5-pt. Likert) | X | ||
| Over the past week, how lonely have you felt? | Not at all—Extremely (5-pt. Likert) | X | ||
| Did any of the following make it difficult for you to be active this week? (Barriers) | (Check all that apply) illness or injury; poor weather; sore muscles; no time/too busy; no place to be active; personal safety; traffic safety; travel, open response category | X | ||
| Is this barrier likely to continue into next week? | (Choose one) Yes, No, I don’t know | X | ||
| How confident are you that you’ll reach your goal | Not at all—Very much (5-pt. Likert) | X | X | |
| Activity Questionnaires (outside of burst weeks, probability of 0.1 after activity is detected) | ||||
| How much did you enjoy the activity you just did? | Not at all—Very much (5-pt. Likert) | X | ||
| How well did this activity fit into your day? | Not at all—Very much (5-pt. Likert) | X | ||
| I did this activity … | (Choose one) alone, with friends, with family, with colleagues, with someone else | X | ||
| I did this activity partly because I wanted to be more active | Not at all true—Very true (5-pt. Likert) | X | ||
| I did this activity because other people thought I should | Not at all true—Very true (5-pt. Likert) | X | ||
| EMA Bursts—once every three months for seven days | ||||
| Activity questionnaires (see above) after completion of each detected activity | ||||
| Walking suggestion questionnaires (see below) five times per day | ||||
| How busy are you right now? | Not at all—Very (5-pt. Likert) | X | ||
| Right now, how relaxed do you feel? | Not at all—Very (5-pt. Likert) | X | ||
| Right now, how tense do you feel? | Not at all—Very (5-pt. Likert) | X | ||
| Right now, how energetic do you feel? | Not at all—Very (5-pt. Likert) | X | ||
| Right now, how fatigued do you feel? | Not at all—Very (5-pt. Likert) | X | ||
| Right now, how happy do you feel? | Not at all—Very (5-pt. Likert) | X | ||
| Right now, how sad do you feel? | Not at all—Very (5-pt. Likert) | X | ||
| Right now, how stressed do you feel? | Not at all—Very (5-pt. Likert) | X | ||
| How committed do you feel right now to being physically active today? | Not at all—Very (5-pt. Likert) | X | ||
| Given what’s going on right now, I will be able to be active in the next hour. | Strongly disagree—Strongly agree (5-pt. Likert) | X | ||
| Inclusion Criteria |
|---|
| BMI between 25–45 kg/m2 |
| 18–65 years of age |
| Competent to give informed consent |
| Own either an iPhone with iOS 6 or above or an Android phone with Version 7 or above. |
| Willing to participate in the study protocols including |
| Regularly carrying a mobile phone |
| Using the HeartSteps application using the HeartSteps application, |
| Answering phone-based questionnaires |
| Wear the Fitbit Versa activity tracker at least 8 h a day. |
| Fluent in English |
| Residing in Southern California |
| Exclusion Criteria |
| Mentally incapable of giving informed consent, |
| Psychiatric disorder that limits the patients’ ability to follow study protocol, including psychosis and dementia. |
| Non-English speaking |
| Orthopedic problems that prevent participation in a walking program |
| Significant peripheral neuropathy |
| Vigorous Activity that spans at least three days and leads to a total of at least 1500 MET min or 7 or more days of any combo of exercises that exceeds a total of 3000 MET min (Based on IPAQ scoring) |
| Demographics | Participants: |
|---|---|
| Gender | |
| Male | 23 |
| Female | 72 |
| Age | |
| 18–20 | 2 |
| 21–34 | 37 |
| 35–44 | 25 |
| 45–65 | 31 |
| Race/Ethnicity | |
| American Indian/Alaska Native | 2 |
| Asian | 19 |
| Black or African American | 4 |
| White | 48 |
| Middle Eastern | 2 |
| Hispanic/Latinx | 39 |
| More Than One Race | 5 |
| Other | 10 |
| Prefer not to respond | 5 |
| Level of education | |
| Some High school | 1 |
| High school graduate/diploma/GED | 5 |
| Some College or 2 year Degree | 27 |
| Bachelor’s degree (BS/BA/AB) | 31 |
| Some Graduate School | 5 |
| Graduate degree | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spruijt-Metz, D.; Marlin, B.M.; Pavel, M.; Rivera, D.E.; Hekler, E.; De La Torre, S.; El Mistiri, M.; Golaszweski, N.M.; Li, C.; Braga De Braganca, R.; et al. Advancing Behavioral Intervention and Theory Development for Mobile Health: The HeartSteps II Protocol. Int. J. Environ. Res. Public Health 2022, 19, 2267. https://doi.org/10.3390/ijerph19042267
Spruijt-Metz D, Marlin BM, Pavel M, Rivera DE, Hekler E, De La Torre S, El Mistiri M, Golaszweski NM, Li C, Braga De Braganca R, et al. Advancing Behavioral Intervention and Theory Development for Mobile Health: The HeartSteps II Protocol. International Journal of Environmental Research and Public Health. 2022; 19(4):2267. https://doi.org/10.3390/ijerph19042267
Chicago/Turabian StyleSpruijt-Metz, Donna, Benjamin M. Marlin, Misha Pavel, Daniel E. Rivera, Eric Hekler, Steven De La Torre, Mohamed El Mistiri, Natalie M. Golaszweski, Cynthia Li, Rebecca Braga De Braganca, and et al. 2022. "Advancing Behavioral Intervention and Theory Development for Mobile Health: The HeartSteps II Protocol" International Journal of Environmental Research and Public Health 19, no. 4: 2267. https://doi.org/10.3390/ijerph19042267
APA StyleSpruijt-Metz, D., Marlin, B. M., Pavel, M., Rivera, D. E., Hekler, E., De La Torre, S., El Mistiri, M., Golaszweski, N. M., Li, C., Braga De Braganca, R., Tung, K., Kha, R., & Klasnja, P. (2022). Advancing Behavioral Intervention and Theory Development for Mobile Health: The HeartSteps II Protocol. International Journal of Environmental Research and Public Health, 19(4), 2267. https://doi.org/10.3390/ijerph19042267







