Analysis of Massaciuccoli Peat after Maturation in Sodium Chloride Water of Undulna Thermae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
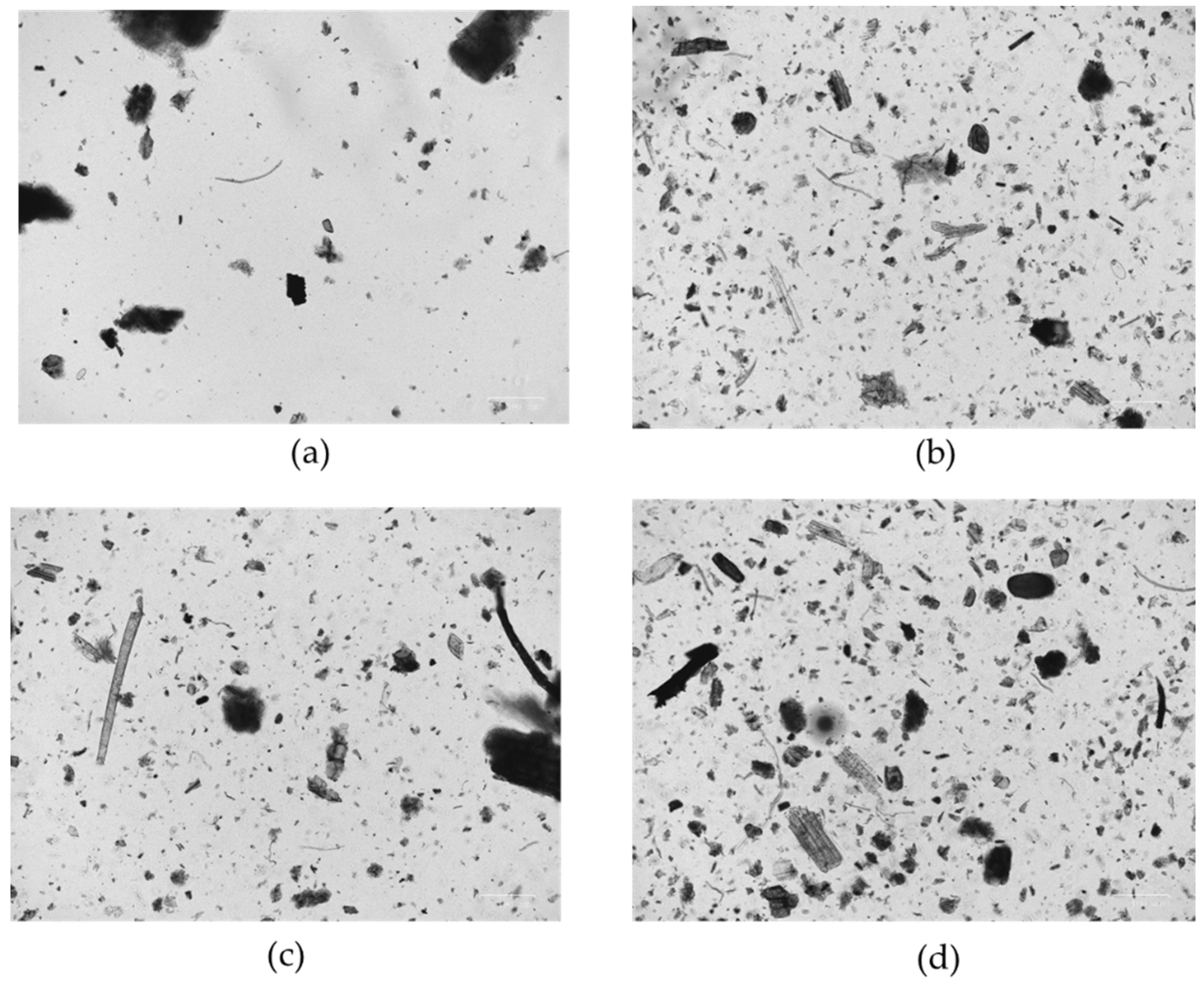
- Basic peat before being introduced into the maturation cycle;
- Peat matured for 6 months;
- Peat matured for 16 months;
- Peat matured for 36 months and ready to use on the patient.
2.2. Morphological and Biochemical Analysis
2.3. Statistical Analysis
3. Results
3.1. Morphological Analysis
3.2. Total Protein, Thiol Protein, and Lipid Content in Peat in Relation to the Maturation Period
4. Discussion
4.1. Components of the Peat
4.2. Possible Therapeutic Effect of These Components
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maraver, F.; Armijo, F.; Fernandez-Toran, M.; Armijo, O.; Ejeda, J.; Vazquez, I.; Corvillo, I.; Torres-Piles, S. Peloids as Thermotherapeutic Agents. Int. J. Environ. Res. Public Health 2021, 18, 1965. [Google Scholar] [CrossRef]
- Agostini, G.; Cervadoro, E. Il peloide organico delle Terme della Versilia. Clin. Term. 2000, 49, 75–90. [Google Scholar]
- Menozzi, B.I. Lineamenti paleoambientali del bacino del lago di Massaciuccoli (Toscana nord-occidentale, Italia). Atti. Soc. Tosc. Sci. Nat. Mem. 2002, 109, 177–187. [Google Scholar]
- Van Rensburg, C.E.J. The antiinflammatory properties of humic substances: A mini review. Phyther. Res. 2015, 29, 791–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trofimova, E.S.; Zykova, M.V.; Ligacheva, A.A.; Sherstoboev, E.Y.; Zhdanov, V.V.; Belousov, M.V.; Yusubov, M.S.; Krivoshchekov, S.V.; Danilets, M.G.; Dygai, A.M. Effects of Humic Acids Isolated from Peat of Various Origin on in Vitro Production of Nitric Oxide: A Screening Study. Bull. Exp. Biol. Med. 2016, 161, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Trofimova, E.; Zykova, M.V.; Ligacheva, A.A.; Sherstoboev, E.; Zhdanov, V.V.; Belousov, M.V.; Yusubov, M.S.; Krivoshchekov, S.; Danilets, M.G.; Dygai, A. Influence of Humic Acids Extracted from Peat by Different Methods on Functional Activity of Macrophages in Vitro. Bull. Exp. Biol. Med. 2017, 45, 392–745. [Google Scholar] [CrossRef]
- Steinberg, C.E.W.; Meinelt, T.; Timofeyev, M.; Bittner, M.; Menzel, R. Humic substances. Environ. Sci. Pollut. Res. 2007, 15, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Fabrizi, G.; Morganti, P. A new peloid mask of etruscan origin. J. Appl. Cosmetol. 1997, 15, 109–113. [Google Scholar]
- Rondanelli, M.; Opizzi, A.; Perna, S.; Faliva, M.A.; Buonocore, D.; Pezzoni, G.; Michelotti, A.; Marchetti, R.; Marzatico, F. Efficacia significativa del trattamento di due settimane con associazione di torba del Massaciuccoli ed acqua clorurato-sodica delle terme di Undulna sulla lipodistrofia a localizzazione ginoide in un gruppo di donne sovrappeso. Ann. Ig 2012, 24, 369. [Google Scholar]
- Pedrinazzi, C.; Pedrinazzi, G.M.; Gregotti, C.; Battistini, E.; D’Errigo, M.L.; Andreoli, S.; Richelimi, P. Analisi delle variazoni dei rilievi di densitometria ossea in corso di crenoterapia mediante lorba e acqua salsobromoiodica (fonte Undulna) per la prevenzione dell’osteoporosi. Clin. Term. 2009, 56, 119–128. [Google Scholar]
- Pedrinazzi, C.; Andreoli, S.; Battistini, E.; D’Errigo, M.L.; Gregotti, C.; Richelmi, P. Efficacia di una maschera di torba e acqua termale salsobromoiodica nel trattamento della dermatite seborroica del viso. J. Plast. Dermatol. 2009, 5, 294. [Google Scholar]
- Spilioti, E.; Vargiami, M.; Letsiou, S.; Gardikis, K.; Sygouni, V.; Koutsoukos, P.; Chinou, I.; Kassi, E.; Moutsatsou, P. Biological properties of mud extracts derived from various spa resorts. Environ. Geochem. Health 2016, 39, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Di Monte, D.; Ross, D.; Bellomo, G.; Eklöw, L.; Orrenius, S. Alterations in intracellular thiol homeostasis during the metabolism of menadione by isolated rat hepatocytes. Arch. Biochem. Biophys. 1984, 235, 334–342. [Google Scholar] [CrossRef]
- Rumin, J.; Bonnefond, H.; Saint-Jean, B.; Rouxel, C.; Sciandra, A.; Bernard, O.; Cadoret, J.-P.; Bougaran, G. The use of fluorescent Nile red and BODIPY for lipid measurement in microalgae. Biotechnol. Biofuels 2015, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Giusti, P.; Cima, L.; Tinello, A.; Cozzi, F.; Targa, L.; Lazzarin, P.; Todesco, S. Stress hormones liberated by fangotherapy. ACTH and beta-endorphin levels under heat stress. Fortschr. Med. 1990, 108, 601–603. [Google Scholar]
- Veniale, F.; Barberis, E.; Carcangiu, G.; Morandi, N.; Setti, M.; Tamanini, M.; Tessier, D. Formulation of muds for pelotherapy: Effects of “maturation” by different mineral waters. Appl. Clay Sci. 2004, 25, 135–148. [Google Scholar] [CrossRef]
- Beer, A.-M.; Grozeva, A.; Sagorchev, P.; Lukanov, J. Comparative Study of the Thermal Properties of Mud and Peat Solutions Applied in Clinical Practice. Biomed. Eng./Biomed. Tech. 2003, 48, 301–305. [Google Scholar] [CrossRef]
- Messina, B.; Tirri, G.; Fraioli, A.; Grassi, M.; De Bernardi, M. Medicina termale e termalismo. Caleidosc. Ital. 1999, 132, 1–89. [Google Scholar]
- Agostini, G.; Agostini, S.; Di Russo, P.P.; Martini, P. Acqua minerale salsobromoiodica di Villa Undulna, Terme della Versilia. Clin. Term. 2000, 49, 223–230. [Google Scholar]
- Veniale, F.; Bettero, A.; Jobstraibizer, P.; Setti, M. Thermal muds: Perspectives of innovations. Appl. Clay Sci. 2007, 36, 141–147. [Google Scholar] [CrossRef]
- Centini, M.; Tredici, M.R.; Biondi, N.; Buonocore, A.; Facino, R.M.; Anselmi, C. Thermal mud maturation: Organic matter and biological activity. Int. J. Cosmet. Sci. 2015, 37, 339–347. [Google Scholar] [CrossRef]
- Cima, L.; Giusti, P.; Tinello, A.; Cozzi, F.; Menozzi, L.; Todesco, S. Risposte neuro-endocrine all’applicazione di fanghi di diversa maturazione del bacino termale euganeo. Clin. Term. 1992, 45, 77–81. [Google Scholar]
- Ansorg, R. Untersuchungen zur Antimikrobiellen Wirksamkeit von Natuerlichen und Kuenstlichen Huminsaeuren. Arzneim-Fosch/Drug Res. 1978, 28, 2195–2198. [Google Scholar]
- Van Rensburg, C.E.J.; Van Straten, A.; Dekker, J. An in vitro investigation of the antimicrobial activity of oxifulvic acid. J. Antimicrob. Chemother. 2000, 46, 853–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, J.; Ghosh, S. Therapeutic Potential of Fulvic Acid in Chronic Inflammatory Diseases and Diabetes. J. Diabetes Res. 2018, 2018, 5391014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aeschbacher, M.; Graf, C.; Schwarzenbach, R.P.; Sander, M. Antioxidant Properties of Humic Substances. Environ. Sci. Technol. 2012, 46, 4916–4925. [Google Scholar] [CrossRef]
- Lehtonen, K.; Hänninen, K.; Ketola, M. Structurally bound lipids in peat humic acids. Org. Geochem. 2001, 32, 33–43. [Google Scholar] [CrossRef]
- Agostini, G.; Fedeli, E.; Cortesi, N.; Martinelli, M.; Curri, S.B. Costituenti lipidici di una torba italiana. Clin. Term. 1983, XXXIV, 135. [Google Scholar]
- Iversen, L.; Kragballe, K. Arachidonic acid metabolism in skin health and disease. Prostaglandins Other Lipid Mediat. 2000, 63, 25–42. [Google Scholar] [CrossRef]
- Guidoni, M.; Scherer, M.D.C.; Figueira, M.; Schmitt, E.; De Almeida, L.; Scherer, R.; Bogusz, S.; Fronza, M. Fatty acid composition of vegetable oil blend and in vitro effects of pharmacotherapeutical skin care applications. Braz. J. Med. Biol. Res. 2019, 52, e8209. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Am, F.; Bmv, S.; Ma, R.; Mrd, L. The use of fatty acids in wound care: An integrative review of the Brazilian literature. Rev. Esc. Enferm. USP 2012, 46, 745–753. [Google Scholar]
- Pilkington, S.M.; Watson, R.E.B.; Nicolaou, A.; Rhodes, L.E. Omega-3 polyunsaturated fatty acids: Photoprotective macronutrients. Exp. Dermatol. 2011, 20, 537–543. [Google Scholar] [CrossRef]


| Ions | Mg/L |
|---|---|
| Na+ | 6500 |
| K+ | 120 |
| Mg2+ | 729 |
| Ca2+ | 109 |
| Sr2+ | 4.50 |
| Al2+ | 0.61 |
| Mn2+ | 0.56 |
| Fe2+ | 0.83 |
| Ba2+ | 0.32 |
| HCO3− | 307 |
| SO42− | 1462 |
| Cl− | 12,400 |
| Br− | 56 |
| SiO(OH)3 | 7.96 |
| Total Proteins 1 | Thiol Proteins 1 | Total Lipids 1 | ||
|---|---|---|---|---|
| 1 | 106 | 17.49 | 718.6 | |
| Basic peat | 2 | 134 | 19.73 | 746.9 |
| 3 | 118 | 18.18 | 769.5 | |
| 1 | 174 | 24.11 | 878.5 | |
| Peat matured for 6 months | 2 | 170 | 26.19 | 965.0 |
| 3 | 182 | 24.55 | 948.1 | |
| 1 | 121 | 18.55 | 847.5 | |
| Peat matured for 16 months | 2 | 165 | 20.06 | 882.5 |
| 3 | 139 | 18.18 | 872.5 | |
| 1 | 181 | 15.11 | 788.3 | |
| Peat matured for 36 months | 2 | 174 | 18.12 | 870.1 |
| 3 | 171 | 17.39 | 854.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pasqua, L.G.; Berardo, C.; Raffo, L.; Ferrigno, A.; Guffanti, E.; Vairetti, M. Analysis of Massaciuccoli Peat after Maturation in Sodium Chloride Water of Undulna Thermae. Int. J. Environ. Res. Public Health 2022, 19, 2169. https://doi.org/10.3390/ijerph19042169
Di Pasqua LG, Berardo C, Raffo L, Ferrigno A, Guffanti E, Vairetti M. Analysis of Massaciuccoli Peat after Maturation in Sodium Chloride Water of Undulna Thermae. International Journal of Environmental Research and Public Health. 2022; 19(4):2169. https://doi.org/10.3390/ijerph19042169
Chicago/Turabian StyleDi Pasqua, Laura Giuseppina, Clarissa Berardo, Lorenzo Raffo, Andrea Ferrigno, Enrico Guffanti, and Mariapia Vairetti. 2022. "Analysis of Massaciuccoli Peat after Maturation in Sodium Chloride Water of Undulna Thermae" International Journal of Environmental Research and Public Health 19, no. 4: 2169. https://doi.org/10.3390/ijerph19042169
APA StyleDi Pasqua, L. G., Berardo, C., Raffo, L., Ferrigno, A., Guffanti, E., & Vairetti, M. (2022). Analysis of Massaciuccoli Peat after Maturation in Sodium Chloride Water of Undulna Thermae. International Journal of Environmental Research and Public Health, 19(4), 2169. https://doi.org/10.3390/ijerph19042169








