Why Physical Activity Should Be Considered in Clinical Trials for COVID-19 Vaccines: A Focus on Risk Groups
Abstract
:1. Introduction
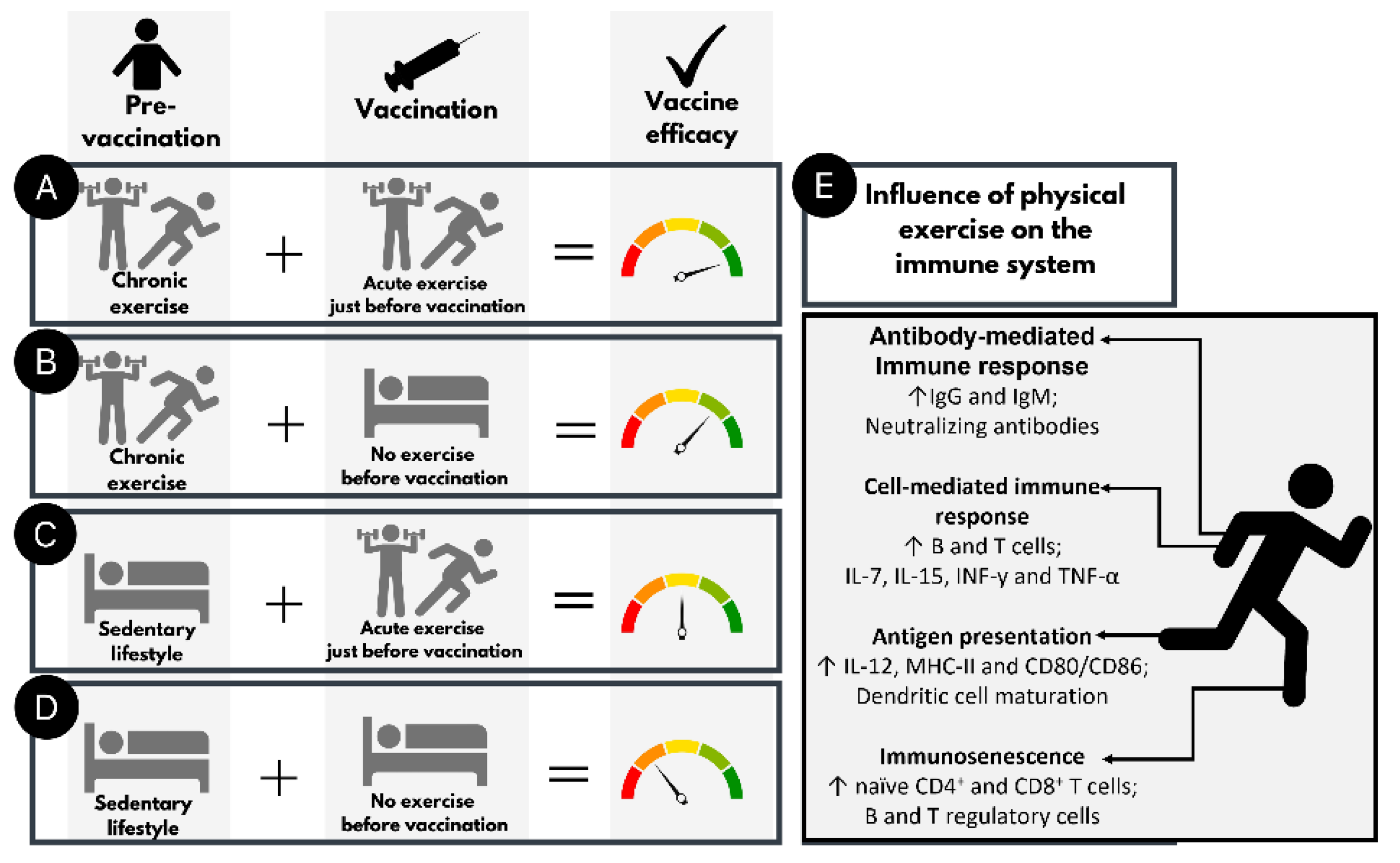
2. Physical Activity and Immune Response
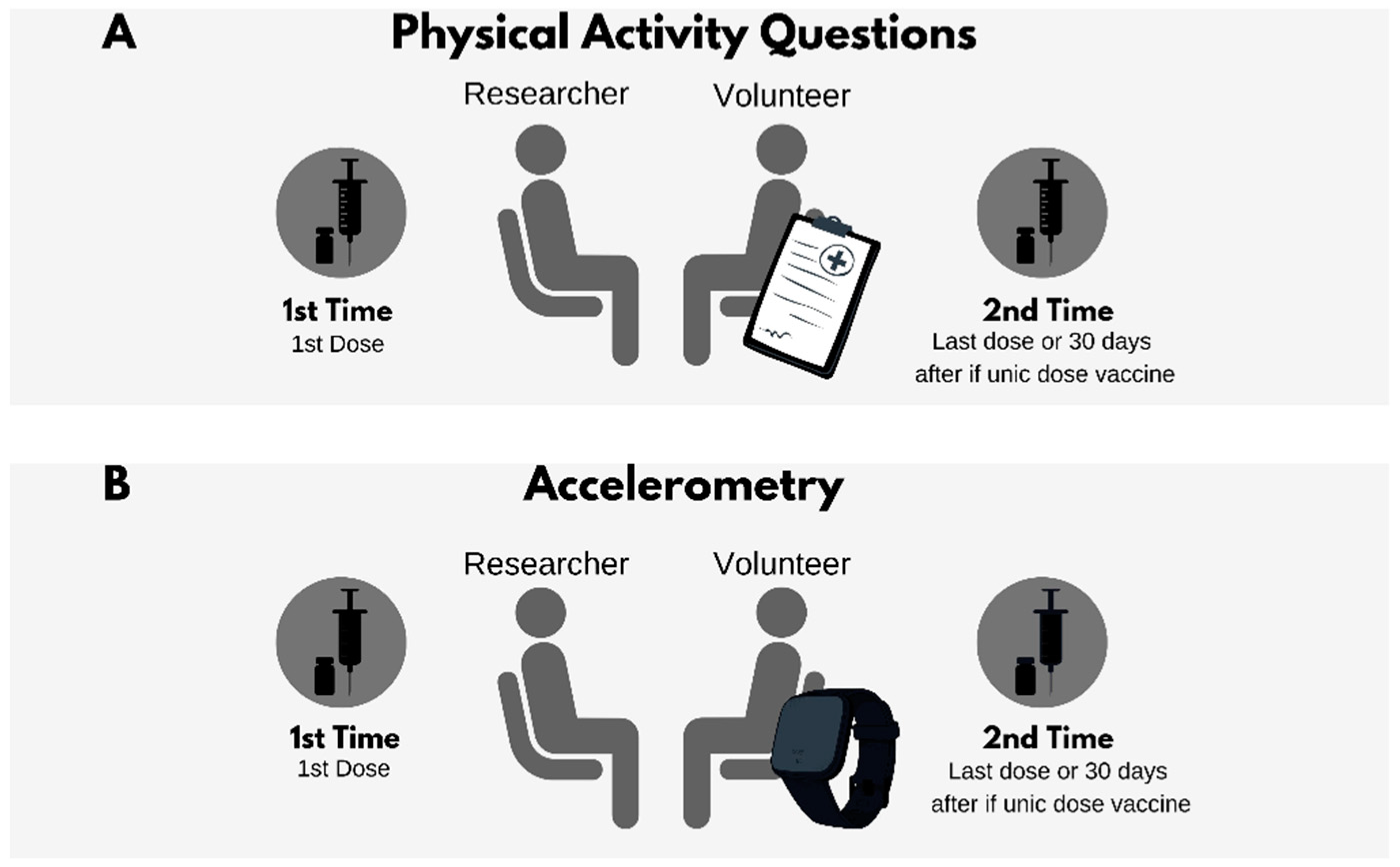
3. COVID-19 Vaccine Candidates and Physical Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO; UNICEF; The World Bank. State of the World’s Vaccines and Immunization, 3rd ed.; World Health Organization: Geneva, Switzerland, 2009.
- Leidner, A.J.; Murthy, N.; Chesson, H.W.; Biggerstaff, M.; Stoecker, C.; Harris, A.M.; Acosta, A.; Dooling, K.; Bridges, C.B. Cost-effectiveness of adult vaccinations: A systematic review. Vaccine 2019, 37, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Brüssow, H. Efforts towards a COVID-19 vaccine. Environ. Microbiol. 2020, 22, 4071–4084. [Google Scholar] [CrossRef] [PubMed]
- Awadasseid, A.; Wu, Y.; Tanaka, Y.; Zhang, W. Current advances in the development of SARS-CoV-2 vaccines. Int. J. Biol. Sci. 2021, 17, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Kis, Z.; Shattock, R.; Shah, N.; Kontoravdi, C. Emerging technologies for low-cost, rapid vaccine manufacture. Biotechnol. J. 2019, 14, e1800376. [Google Scholar] [CrossRef] [Green Version]
- Hume, H.K.C.; Lua, L.H.L. Platform technologies for modern vaccine manufacturing. Vaccine 2017, 35, 4480–4485. [Google Scholar] [CrossRef]
- Khan, N. New Virus Discovered by Chinese Scientists Investigating Pneumonia Outbreak. Available online: https://www.wsj.com/articles/new-virus-discovered-by-chinese-scientists-investigating-pneumonia-outbreak-11578485668 (accessed on 8 June 2021).
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-Infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, Y.; Sun, C.; Wang, X.; Guo, Y.; Qiu, S.; Ma, K. A systematic review of asymptomatic infections with COVID-19. J. Microbiol. Immunol. Infect. 2021, 54, 12–16. [Google Scholar] [CrossRef]
- Ragab, D.; Eldin, H.S.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 cytokine storm: What we know so far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Da Silveira, M.P.; da Silva Fagundes, K.K.; Bizuti, M.R.; Starck, E.; Rossi, R.C.; de Resende, E.S.D.T. Physical exercise as a tool to help the immune system against COVID-19: An integrative review of the current literature. Clin. Exp. Med. 2021, 21, 15–28. [Google Scholar] [CrossRef]
- Ingram, J.; Maciejewski, G.; Hand, C.J. Changes in diet, sleep, and physical activity are associated with differences in negative mood during COVID-19 lockdown. Front. Psychol. 2020, 11, 588604. [Google Scholar] [CrossRef] [PubMed]
- Werneck, A.O.; Collings, P.J.; Barboza, L.L.; Stubbs, B.; Silva, D.R. Associations of sedentary behaviors and physical activity with social isolation in 100,839 school students: The Brazilian Scholar Health Survey. Gen. Hosp. Psychiatry 2019, 59, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roso, M.B.; Knott-Torcal, C.; Matilla-Escalante, D.C.; Garcimartin, A.; Sampedro-Nunez, M.A.; Davalos, A.; Marazuela, M. COVID-19 lockdown and changes of the dietary pattern and physical activity habits in a cohort of patients with type 2 diabetes mellitus. Nutrients 2020, 12, 2327. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roso, M.B.; de Carvalho Padilha, P.; Matilla-Escalante, D.C.; Brun, P.; Ulloa, N.; Acevedo-Correa, D.; Peres, W.A.F.; Martorell, M.; Carrilho, T.R.B.; de Oliveira Cardoso, L.; et al. Changes of physical activity and ultra-processed food consumption in adolescents from different countries during Covid-19 pandemic: An observational study. Nutrients 2020, 12, 2289. [Google Scholar] [CrossRef] [PubMed]
- Damiot, A.; Pinto, A.J.; Turner, J.E.; Gualano, B. Immunological implications of physical inactivity among older adults during the COVID-19 pandemic. Gerontology 2020, 66, 431–438. [Google Scholar] [CrossRef]
- Sallis, R.; Young, D.R.; Tartof, S.Y.; Sallis, J.F.; Sall, J.; Li, Q.; Smith, G.N.; Cohen, D.A. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: A study in 48,440 adult patients. Br. J. Sports Med. 2021, 55, 1099–1105. [Google Scholar] [CrossRef]
- Flanagan, K.L.; Best, E.; Crawford, N.W.; Giles, M.; Koirala, A.; Macartney, K.; Russell, F.; Teh, B.W.; Wen, S.C. Progress and pitfalls in the quest for effective SARS-CoV-2 (COVID-19) vaccines. Front. Immunol. 2020, 11, 579250. [Google Scholar] [CrossRef]
- Walsh, N.P.; Gleeson, M.; Pyne, D.B.; Nieman, D.C.; Dhabhar, F.S.; Shephard, R.J.; Oliver, S.J.; Bermon, S.; Kajeniene, A. Position statement. Part two: Maintaining immune health. Exerc. Immunol. Rev. 2011, 17, 64–103. [Google Scholar]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar]
- Bortolini, M.J.; Silva, M.V.; Alonso, F.M.; Medeiros, L.A.; Carvalho, F.R.; Costa, L.F.; Silva, N.M.; Penha-Silva, N.; Mineo, T.W.; Mineo, J.R. Strength and aerobic physical exercises are able to increase survival of Toxoplasma gondii-infected C57BL/6 mice by interfering in the IFN-gamma expression. Front. Physiol. 2016, 7, 641. [Google Scholar] [CrossRef] [Green Version]
- Schebeleski-Soares, C.; Occhi-Soares, R.C.; Franzoi-de-Moraes, S.M.; de Oliveira Dalalio, M.M.; Almeida, F.N.; de Ornelas Toledo, M.J.; de Araujo, S.M. Preinfection aerobic treadmill training improves resistance against Trypanosoma cruzi infection in mice. Appl. Physiol. Nutr. Metab. 2009, 34, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Terra, R.; Alves, P.J.; da Silva, S.A.G.; Salerno, V.P.; Dutra, P.M. Exercise improves the Th1 response by modulating cytokine and NO production in BALB/c mice. Int. J. Sports Med. 2013, 34, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, W. Roles and molecular mechanisms of physical exercise in cancer prevention and treatment. J. Sport Health Sci. 2020, 10, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Benatti, F.B.; Pedersen, B.K. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat. Rev. Rheumatol. 2015, 11, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Nyenhuis, S.M.; Greiwe, J.; Zeiger, J.S.; Nanda, A.; Cooke, A. Exercise and Fitness in the Age of Social Distancing during the COVID-19 Pandemic. J. Allergy Clin. Immunol. Pract. 2020, 8, 2152–2155. [Google Scholar] [CrossRef]
- Fernandes, P.; de Mendonca Oliveira, L.; Bruggemann, T.R.; Sato, M.N.; Olivo, C.R.; Arantes-Costa, F.M. Physical exercise induces immunoregulation of TREG, M2, and pDCs in a lung allergic inflammation model. Front. Immunol. 2019, 10, 854. [Google Scholar] [CrossRef]
- Kohut, M.L.; Cooper, M.M.; Nickolaus, M.S.; Russell, D.R.; Cunnick, J.E. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M557–M562. [Google Scholar] [CrossRef] [Green Version]
- Stenger, T.; Ledo, A.; Ziller, C.; Schub, D.; Schmidt, T.; Enders, M.; Gärtner, B.C.; Sester, M.; Meyer, T. Timing of vaccination after training: Immune response and side effects in athletes. Med. Sci. Sports Exerc. 2020, 52, 1603–1609. [Google Scholar] [CrossRef]
- Edwards, K.M.; Burns, V.E.; Allen, L.M.; McPhee, J.S.; Bosch, J.A.; Carroll, D.; Drayson, M.; Ring, C. Eccentric exercise as an adjuvant to influenza vaccination in humans. Brain Behav. Immun. 2007, 21, 209–217. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef] [Green Version]
- Dugan, H.L.; Henry, C.; Wilson, P.C. Aging and influenza vaccine-induced immunity. Cell. Immunol. 2020, 348, 103998. [Google Scholar] [CrossRef] [PubMed]
- Guichelaar, T.; Hoeboer, J.; Widjojoatmodjo, M.N.; Reemers, S.S.; van Els, C.A.; Otten, R.; van Remmerden, Y.; Boes, J.; Luytjes, W. Impaired immune response to vaccination against infection with human respiratory syncytial virus at advanced age. J. Virol. 2014, 88, 9744–9750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gershon, A.A.; Brooks, D.; Stevenson, D.D.; Chin, W.K.; Oldstone, M.B.A.; Gershon, M.D. High constitutive interleukin 10 level interferes with the immune response to varicella-zoster virus in elderly recipients of live attenuated zoster vaccine. J. Infect. Dis. 2019, 219, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Beyer, W.E.; Nauta, J.J.; Palache, A.M.; Giezeman, K.M.; Osterhaus, A.D. Immunogenicity and safety of inactivated influenza vaccines in primed populations: A systematic literature review and meta-analysis. Vaccine 2011, 29, 5785–5792. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.K.; McElhaney, J.E. Age and frailty in COVID-19 vaccine development. Lancet 2021, 396, 1942–1944. [Google Scholar] [CrossRef]
- Ledo, A.; Schub, D.; Ziller, C.; Enders, M.; Stenger, T.; Gartner, B.C.; Schmidt, T.; Meyer, T.; Sester, M. Elite athletes on regular training show more pronounced induction of vaccine-specific T-cells and antibodies after tetravalent influenza vaccination than controls. Brain Behav. Immun. 2020, 83, 135–145. [Google Scholar] [CrossRef]
- Bachi, A.L.; Suguri, V.M.; Ramos, L.R.; Mariano, M.; Vaisberg, M.; Lopes, J.D. Increased production of autoantibodies and specific antibodies in response to influenza virus vaccination in physically active older individuals. Results Immunol. 2013, 3, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Wong, G.C.L.; Narang, V.; Lu, Y.; Camous, X.; Nyunt, M.S.Z.; Carre, C.; Tan, C.; Xian, C.H.; Chong, J.; Chua, M.; et al. Hallmarks of improved immunological responses in the vaccination of more physically active elderly females. Exerc. Immunol. Rev. 2019, 25, 20–33. [Google Scholar]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [Green Version]
- Whitham, M.; Febbraio, M.A. The ever-expanding myokinome: Discovery challenges and therapeutic implications. Nat. Rev. Drug Discov. 2016, 15, 719–729. [Google Scholar] [CrossRef]
- Crescioli, C. Targeting age-dependent functional and metabolic decline of human skeletal muscle: The geroprotective role of exercise, myokine IL-6, and vitamin D. Int. J. Mol. Sci. 2020, 21, 1010. [Google Scholar] [CrossRef] [Green Version]
- Riechman, S.E.; Balasekaran, G.; Roth, S.M.; Ferrell, R.E. Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J. Appl. Physiol. 2004, 97, 2214–2219. [Google Scholar] [CrossRef]
- Haugen, F.; Norheim, F.; Lian, H.; Wensaas, A.J.; Dueland, S.; Berg, O.; Funderud, A.; Skalhegg, B.S.; Raastad, T.; Drevon, C.A. IL-7 is expressed and secreted by human skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2010, 298, C807–C816. [Google Scholar] [CrossRef] [Green Version]
- Raeber, M.E.; Zurbuchen, Y.; Impellizzieri, D.; Boyman, O. The role of cytokines in T-cell memory in health and disease. Immunol. Rev. 2018, 283, 176–193. [Google Scholar] [CrossRef]
- Katharina, A.; Christian, P.; Karsten, K. Current knowledge and new challenges in exercise immunology. Ger. J. Sports Med. 2019, 70, 250–260. [Google Scholar] [CrossRef]
- Ho, N.I.; Huis In’t Veld, L.G.M.; Raaijmakers, T.K.; Adema, G.J. Adjuvants enhancing cross-oresentation by dendritic cells: The key to more effective vaccines? Front. Immunol. 2018, 9, 2874. [Google Scholar] [CrossRef]
- Edwards, K.M.; Pung, M.A.; Tomfohr, L.M.; Ziegler, M.G.; Campbell, J.P.; Drayson, M.T.; Mills, P.J. Acute exercise enhancement of pneumococcal vaccination response: A randomised controlled trial of weaker and stronger immune response. Vaccine 2012, 30, 6389–6395. [Google Scholar] [CrossRef] [Green Version]
- Ranadive, S.M.; Cook, M.; Kappus, R.M.; Yan, H.; Lane, A.D.; Woods, J.A.; Wilund, K.R.; Iwamoto, G.; Vanar, V.; Tandon, R.; et al. Effect of acute aerobic exercise on vaccine efficacy in older adults. Med. Sci. Sports Exerc. 2014, 46, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Pascoe, A.R.; Singh, M.A.F.; Edwards, K.M. The effects of exercise on vaccination responses: A review of chronic and acute exercise interventions in humans. Brain Behav. Immun. 2014, 39, 33–41. [Google Scholar] [CrossRef]
- De Araujo, A.L.; Silva, L.C.; Fernandes, J.R.; Matias Mde, S.; Boas, L.S.; Machado, C.M.; Garcez-Leme, L.E.; Benard, G. Elderly men with moderate and intense training lifestyle present sustained higher antibody responses to influenza vaccine. Age 2015, 37, 105. [Google Scholar] [CrossRef] [Green Version]
- Shamsi, M.M.; Najedi, S.; Hassan, Z.M.; Isanejad, A.; Mahdavi, M. Short term exercise training enhances cell-mediated responses to HSV-1 vaccine in mice. Microb. Pathog. 2017, 110, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.P.; Turner, J.E. Debunking the myth of exercise-induced immune suppression: Redefining the impact of exercise on immunological health across the lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.E.; Spielmann, G.; Wadley, A.J.; Aldred, S.; Simpson, R.J.; Campbell, J.P. Exercise-induced B cell mobilisation: Preliminary evidence for an influx of immature cells into the bloodstream. Physiol. Behav. 2016, 164, 376–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poinsatte, K.; Smith, E.E.; Torres, V.O.; Ortega, S.B.; Huebinger, R.M.; Cullum, C.M.; Monson, N.L.; Zhang, R.; Stowe, A.M. T and B cell subsets differentially correlate with amyloid deposition and neurocognitive function in patients with amnestic mild cognitive impairment after one year of physical activity. Exerc. Immunol. Rev. 2019, 25, 34–49. [Google Scholar] [PubMed]
- MacDonald, K.P.; Munster, D.J.; Clark, G.J.; Dzionek, A.; Schmitz, J.; Hart, D.N. Characterization of human blood dendritic cell subsets. Blood 2002, 100, 4512–4520. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Liu, C.; Kim, G.J.; Liu, Y.J.; Hwu, P.; Wang, G. Plasmacytoid dendritic cells synergize with myeloid dendritic cells in the induction of antigen-specific antitumor immune responses. J. Immunol. 2007, 178, 1534–1541. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Tan, J.; Li, X.; Li, H.; Wu, W.; Wu, Y.; Zhang, J.; Gu, L. Myeloid and plasmacytoid dendritic cell combined vaccines loaded with heat-treated tumor cell lysates enhance antitumor activity in murine lung cancer. Oncol. Lett. 2021, 21, 90. [Google Scholar] [CrossRef]
- Fu, C.; Peng, P.; Loschko, J.; Feng, L.; Pham, P.; Cui, W.; Lee, K.P.; Krug, A.B.; Jiang, A. Plasmacytoid dendritic cells cross-prime naive CD8 T cells by transferring antigen to conventional dendritic cells through exosomes. Proc. Natl. Acad. Sci. USA 2020, 117, 23730–23741. [Google Scholar] [CrossRef]
- Nickel, T.; Emslander, I.; Sisic, Z.; David, R.; Schmaderer, C.; Marx, N.; Schmidt-Trucksass, A.; Hoster, E.; Halle, M.; Weis, M.; et al. Modulation of dendritic cells and toll-like receptors by marathon running. Eur. J. Appl. Physiol. 2012, 112, 1699–1708. [Google Scholar] [CrossRef]
- Brown, F.F.; Campbell, J.P.; Wadley, A.J.; Fisher, J.P.; Aldred, S.; Turner, J.E. Acute aerobic exercise induces a preferential mobilisation of plasmacytoid dendritic cells into the peripheral blood in man. Physiol. Behav. 2018, 194, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, D.R.; Gomes, B.B.M.; Murta, E.F.C.; Michelin, M.A. Bone marrow-derived dendritic cells under influence of experimental breast cancer and physical activity. Oncol. Lett. 2017, 13, 1406–1410. [Google Scholar] [CrossRef] [Green Version]
- Estebanez, B.; Jimenez-Pavon, D.; Huang, C.J.; Cuevas, M.J.; Gonzalez-Gallego, J. Effects of exercise on exosome release and cargo in in vivo and ex vivo models: A systematic review. J. Cell. Physiol. 2021, 236, 3336–3353. [Google Scholar] [CrossRef]
- Brahmer, A.; Neuberger, E.; Esch-Heisser, L.; Haller, N.; Jorgensen, M.M.; Baek, R.; Mobius, W.; Simon, P.; Kramer-Albers, E.M. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. J. Extracell. Vesicles 2019, 8, 1615820. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Harris, N.L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 2004, 4, 478–485. [Google Scholar] [CrossRef]
- Lundin, A.; Bok, C.M.; Aronsson, L.; Bjorkholm, B.; Gustafsson, J.A.; Pott, S.; Arulampalam, V.; Hibberd, M.; Rafter, J.; Pettersson, S. Gut flora, Toll-like receptors and nuclear receptors: A tripartite communication that tunes innate immunity in large intestine. Cell. Microbiol. 2008, 10, 1093–1103. [Google Scholar] [CrossRef]
- Hu, G.; Gong, A.Y.; Roth, A.L.; Huang, B.Q.; Ward, H.D.; Zhu, G.; Larusso, N.F.; Hanson, N.D.; Chen, X.M. Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog. 2013, 9, e1003261. [Google Scholar] [CrossRef] [Green Version]
- Bermon, S.; Petriz, B.; Kajeniene, A.; Prestes, J.; Castell, L.; Franco, O.L. The microbiota: An exercise immunology perspective. Exerc. Immunol. Rev. 2015, 21, 70–79. [Google Scholar]
- Oliveira, G.P., Jr.; Porto, W.F.; Palu, C.C.; Pereira, L.M.; Petriz, B.; Almeida, J.A.; Viana, J.; Filho, N.N.A.; Franco, O.L.; Pereira, R.W. Effects of Acute Aerobic Exercise on Rats Serum Extracellular Vesicles Diameter, Concentration and Small RNAs Content. Front. Physiol. 2018, 9, 532. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.; Zhang, W.; et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: Interim analysis of 2 randomized clinical trials. JAMA 2020, 324, 951–960. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Mallapaty, S.; Ledford, H. COVID-vaccine results are on the way—And scientists’ concerns are growing. Nature 2020, 586, 16–17. [Google Scholar] [CrossRef]
- Jamrozik, E.; Selgelid, M.J. COVID-19 human challenge studies: Ethical issues. Lancet Infect. Dis. 2020, 20, e198–e203. [Google Scholar] [CrossRef]
- Callaway, E. COVID vaccine boosters: The most important questions. Nature 2021, 596, 178–180. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortolini, M.J.S.; Petriz, B.; Mineo, J.R.; Resende, R.d.O. Why Physical Activity Should Be Considered in Clinical Trials for COVID-19 Vaccines: A Focus on Risk Groups. Int. J. Environ. Res. Public Health 2022, 19, 1853. https://doi.org/10.3390/ijerph19031853
Bortolini MJS, Petriz B, Mineo JR, Resende RdO. Why Physical Activity Should Be Considered in Clinical Trials for COVID-19 Vaccines: A Focus on Risk Groups. International Journal of Environmental Research and Public Health. 2022; 19(3):1853. https://doi.org/10.3390/ijerph19031853
Chicago/Turabian StyleBortolini, Miguel Junior Sordi, Bernardo Petriz, José Roberto Mineo, and Rafael de Oliveira Resende. 2022. "Why Physical Activity Should Be Considered in Clinical Trials for COVID-19 Vaccines: A Focus on Risk Groups" International Journal of Environmental Research and Public Health 19, no. 3: 1853. https://doi.org/10.3390/ijerph19031853
APA StyleBortolini, M. J. S., Petriz, B., Mineo, J. R., & Resende, R. d. O. (2022). Why Physical Activity Should Be Considered in Clinical Trials for COVID-19 Vaccines: A Focus on Risk Groups. International Journal of Environmental Research and Public Health, 19(3), 1853. https://doi.org/10.3390/ijerph19031853





