Empirical Evidence of Arsenite Oxidase Gene as an Indicator Accounting for Arsenic Phytoextraction by Pteris vittata
Abstract
1. Introduction
2. Materials and Methods
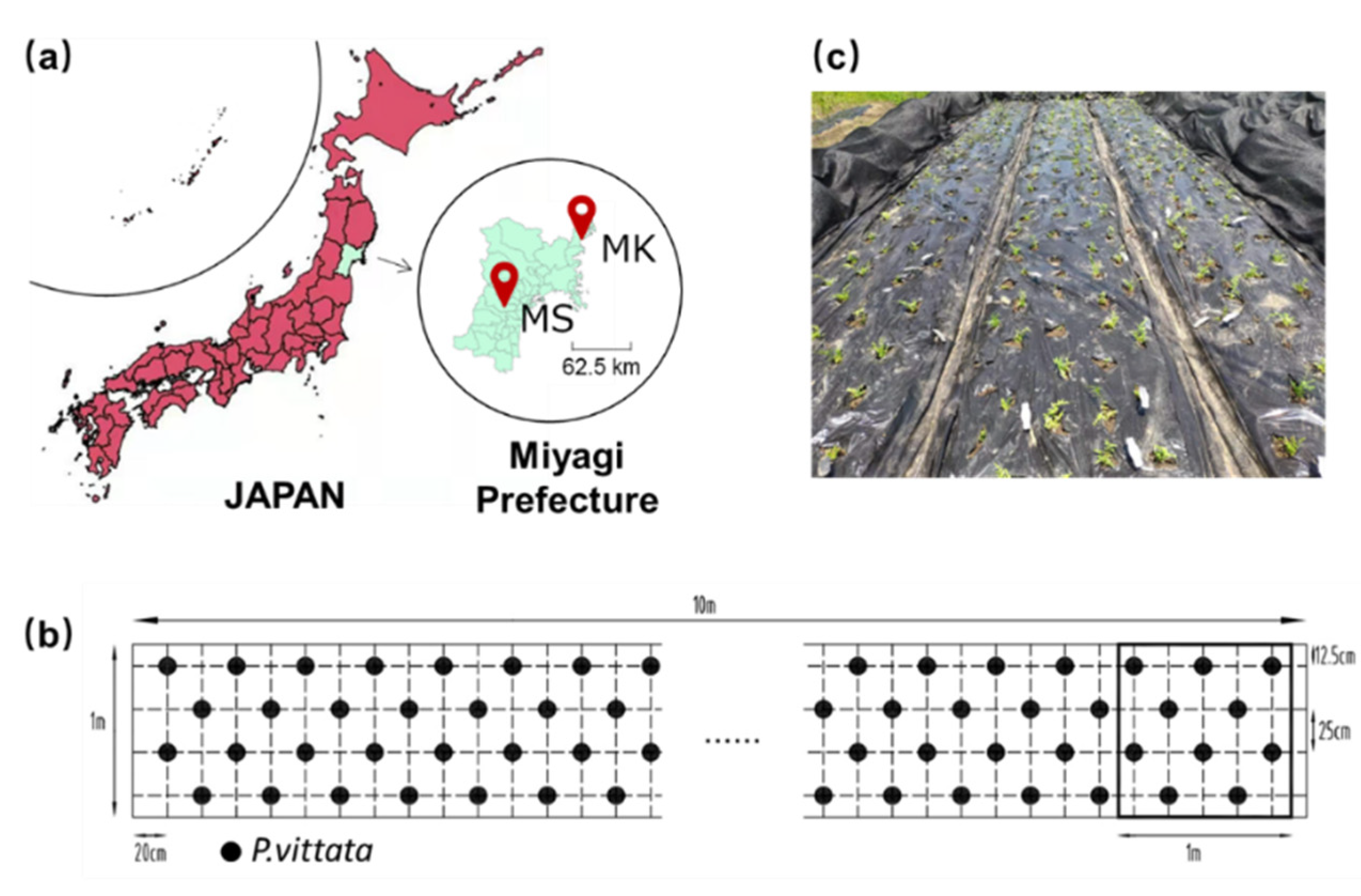
2.1. Site Location and Field Design
2.2. Plant and Microbe Materials and Inoculation Experiment
2.3. Plant Sampling and Analysis
2.4. Soil Sampling and As Content Analysis
2.5. Relative Abundance of Arsenite Oxidase Genes (aioA-like Genes) to 16S rRNA Genes Analysis
2.6. Statistical Analysis
3. Results and Discussions
3.1. Soil As Content in Two Sites
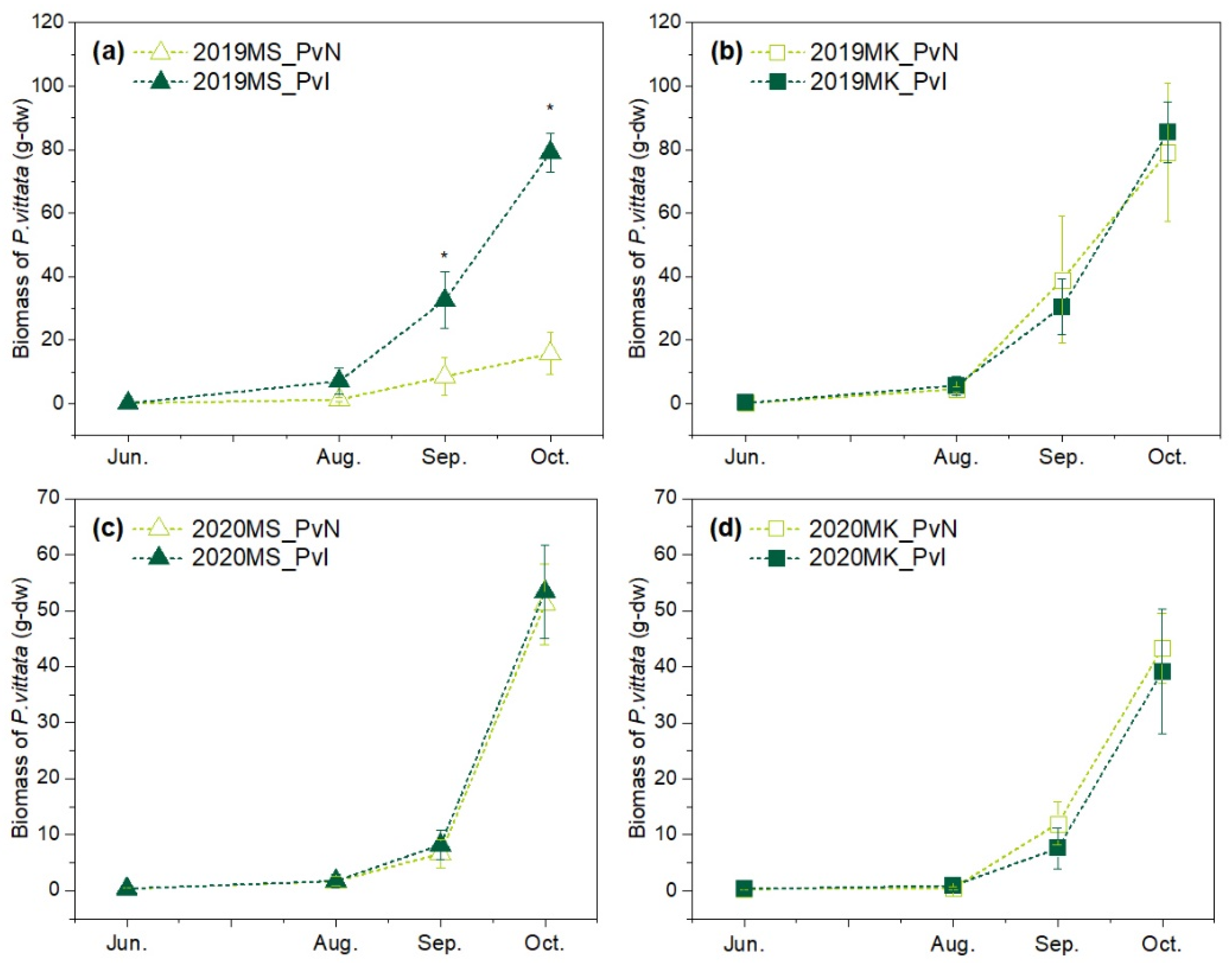
3.2. Growth of P. vittata in Two Sites
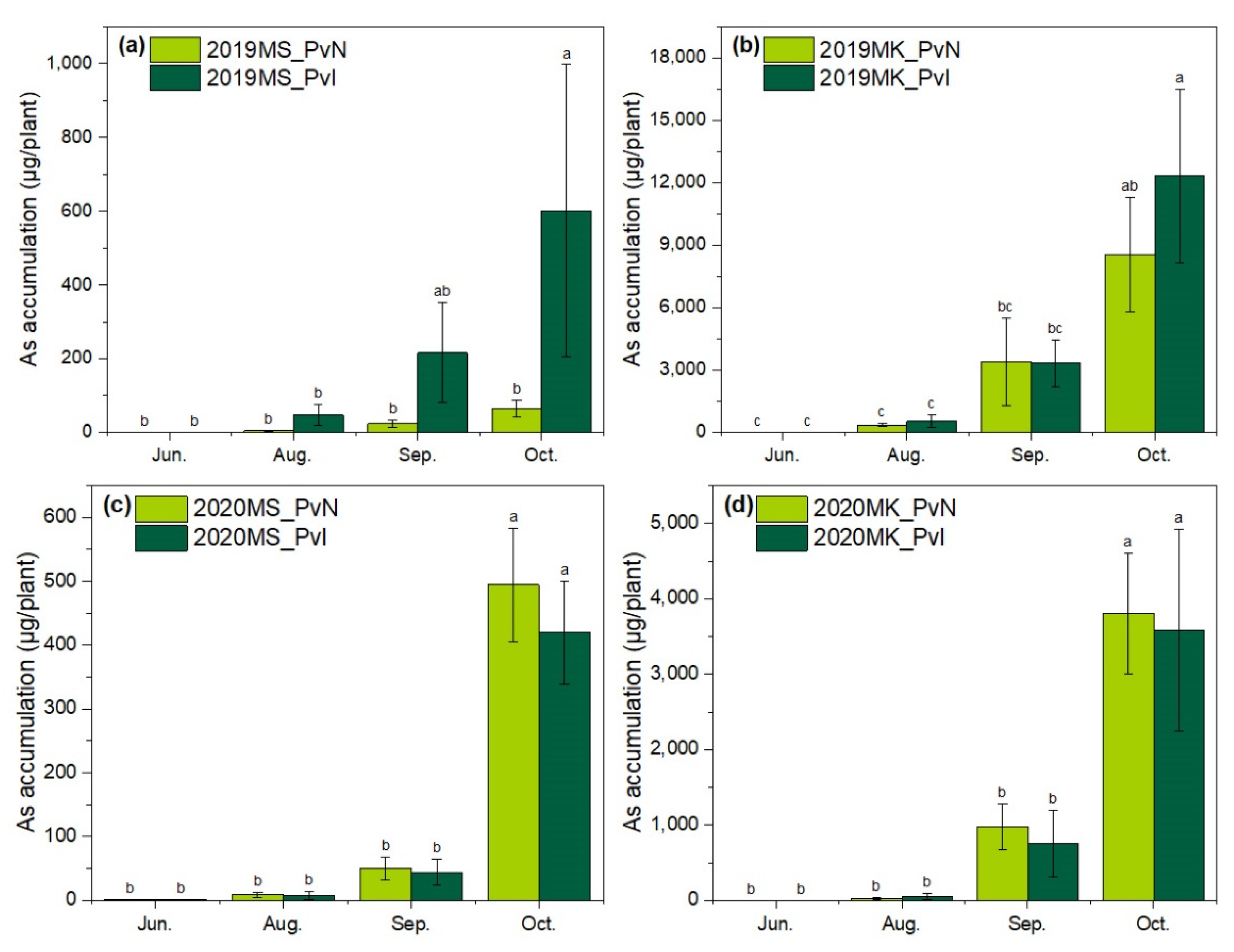
3.3. As Accumulation by P. vittata in Two Sites
3.4. Arsenite Oxidase Genes in the Rhizosphere of P. vittata
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Sharma, A.K.; Tjell, J.C.; Sloth, J.J.; Holm, P.E. Review of arsenic contamination, exposure through water and food and low cost mitigation options for rural areas. Appl. Geochem. 2014, 41, 11–33. [Google Scholar] [CrossRef]
- Smith, C.J.; Livingston, S.D.; Doolittle, D.J. An international literature survey of "IARC Group I carcinogens" reported in mainstream cigarette smoke. Food Chem. Toxicol. 1997, 35, 1107–1130. [Google Scholar] [CrossRef]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef]
- Zhao, F.J.; McGrath, S.P.; Meharg, A.A. Arsenic as a Food Chain Contaminant: Mechanisms of Plant Uptake and Metabolism and Mitigation Strategies. Annu. Rev. Plant Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef] [PubMed]
- Bakhat, H.F.; Zia, Z.; Fahad, S.; Abbas, S.; Hammad, H.M.; Shahzad, A.N.; Abbas, F.; Alharby, H.; Shahid, M. Arsenic uptake, accumulation and toxicity in rice plants: Possible remedies for its detoxification: A review. Environ. Sci. Pollut. Res. 2017, 24, 9142–9158. [Google Scholar] [CrossRef]
- Environmental Quality Standards for Soil Pollution [MOE]. Available online: https://www.env.go.jp/en/water/soil/sp.html (accessed on 31 January 2022).
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Salt, D.E.; Blaylock, M.; Kumar, N.; Dushenkov, V.; Ensley, B.D.; Chet, I.; Raskin, I. PHYTOREMEDIATION—A NOVEL STRATEGY FOR THE REMOVAL OF TOXIC METALS FROM THE ENVIRONMENT USING PLANTS. Bio-Technol. 1995, 13, 468–474. [Google Scholar] [CrossRef]
- Bhargava, A.; Carmona, F.F.; Bhargava, M.; Srivastava, S. Approaches for enhanced phytoextraction of heavy metals. J. Environ. Manag. 2012, 105, 103–120. [Google Scholar] [CrossRef]
- Leitenmaier, B.; Kupper, H. Compartmentation and complexation of metals in hyperaccumulator plants. Front. Plant Sci. 2013, 4, 374. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Niazi, N.K.; Singh, B.; Van Zwieten, L.; Kachenko, A.G. Phytoremediation of an arsenic-contaminated site using Pteris vittata L. and Pityrogramma calomelanos var. austroamericana: A long-term study. Environ. Sci. Pollut. Res. 2012, 19, 3506–3515. [Google Scholar] [CrossRef]
- Danh, L.T.; Truong, P.; Mammucari, R.; Foster, N. A Critical Review of the Arsenic Uptake Mechanisms and Phytoremediation Potential of Pteris vittata. Int. J. Phytoremediation 2014, 16, 429–453. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hatayama, M.; Inoue, C. Characterization of As efflux from the roots of As hyperaccumulator Pteris vittata L. Planta 2011, 234, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Paez-Espino, D.; Tamames, J.; de Lorenzo, V.; Canovas, D. Microbial responses to environmental arsenic. Biometals 2009, 22, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ho, Y.-N.; Makita, R.; Inoue, C.; Chien, M.-F. A multifunctional rhizobacterial strain with wide application in different ferns facilitates arsenic phytoremediation. Sci. Total Environ. 2020, 712, 134504. [Google Scholar] [CrossRef]
- Yang, C.; Ho, Y.-N.; Makita, R.; Inoue, C.; Chien, M.-F. Cupriavidus basilensis strain r507, a toxic arsenic phytoextraction facilitator, potentiates the arsenic accumulation by Pteris vittata. Ecotoxicol. Environ. Saf. 2020, 190, 110075. [Google Scholar] [CrossRef]
- Yang, C.; Ho, Y.-N.; Inoue, C.; Chien, M.-F. Long-term effectiveness of microbe-assisted arsenic phytoremediation by Pteris vittata in field trials. Sci. Total Environ. 2020, 740, 140137. [Google Scholar] [CrossRef]
- Matsunami, H.; Matsuda, K.; Yamasaki, S.-i.; Kimura, K.; Ogawa, Y.; Miura, Y.; Yamaji, I.; Tsuchiya, N. Rapid simultaneous multi-element determination of soils and environmental samples with polarizing energy dispersive X-ray fluorescence (EDXRF) spectrometry using pressed powder pellets. Soil Sci. Plant Nutr. 2010, 56, 530–540. [Google Scholar] [CrossRef]
- Tsuchiya, N.; Inoue, C.; Yamada, R.; Yamazaki, S.I.; Hirano, N.; Okamoto, A.; Ogawa, Y.; Watanabe, T.; Nara, F.; Watanabe, N. Risk assessments of arsenic in tsunami sediments from Iwate, Miyagi and Fukushima Prefectures, Northeast Japan, by the 2011 off the Pacific coast of Tohoku Earthquake. Chishitsugaku Zasshi 2012, 118, 419–430. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Arao, T.; Ishikawa, S.; Murakami, M.; Abe, K.; Maejima, Y.; Makino, T. Heavy metal contamination of agricultural soil and countermeasures in Japan. Paddy Water Environ. 2010, 8, 247–257. [Google Scholar] [CrossRef]
- Katsumi, M. Geological approach to soil contamination caused by natural processes. Chigaku Zasshi 2007, 116, 877–891. (In Japanese) [Google Scholar] [CrossRef]
- Huang, Y.; Miyauchi, K.; Inoue, C.; Endo, G. Development of suitable hydroponics system for phytoremediation of arsenic-contaminated water using an arsenic hyperaccumulator plant Pteris vittata. Biosci. Biotechnol. Biochem. 2016, 80, 614–618. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Li, Y.P.; Hassan, M.J.; Li, Z.; Peng, Y. Indole-3-acetic acid improves drought tolerance of white clover via activating auxin, abscisic acid and jasmonic acid related genes and inhibiting senescence genes. Bmc Plant Biol. 2020, 20, 150. [Google Scholar] [CrossRef]
- Komaresofla, B.R.; Alikhani, H.A.; Etesami, H.; Khoshkholgh-Sima, N.A. Improved growth and salinity tolerance of the halophyte Salicornia sp. by co-inoculation with endophytic and rhizosphere bacteria. Appl. Soil Ecol. 2019, 138, 160–170. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Zahir, Z.A. Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J. Appl. Microbiol. 2004, 96, 473–480. [Google Scholar] [CrossRef]
- Singh, N.; Ma, L.Q. Arsenic speciation, and arsenic and phosphate distribution in arsenic hyperaccumulator Pteris vittata L. and non-hyperaccumulator Pteris ensiformis L. Environ. Pollut. 2006, 141, 238–246. [Google Scholar] [CrossRef]
- Ye, L.; Wang, L.Y.; Jing, C.Y. Biotransformation of adsorbed arsenic on iron minerals by coexisting arsenate-reducing and arsenite-oxidizing bacteria. Environ. Pollut. 2020, 256, 113471. [Google Scholar] [CrossRef]
- Wang, X.; Rathinasabapathi, B.; de Oliveira, L.M.; Guilherme, L.R.G.; Ma, L.N.Q. Bacteria-Mediated Arsenic Oxidation and Reduction in the Growth Media of Arsenic Hyperaccumulator Pteris vittata. Environ. Sci. Technol. 2012, 46, 11259–11266. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, D.; Mhamdi, R. Microbial Inoculants and Their Impact on Soil Microbial Communities: A Review. Biomed Res. Int. 2013, 2013, 863240. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Shahzad, S.M.; Khalid, A.; Arif, M.S.; Riaz, M.; Ashraf, M.; Iqbal, Z.; Yasmeen, T. Co-inoculation integrated with P-enriched compost improved nodulation and growth of Chickpea (Cicer arietinum L.) under irrigated and rainfed farming systems. Biol. Fertil. Soils 2014, 50, 1–12. [Google Scholar] [CrossRef]
- Maila, M.P.; Randima, P.; Cloete, T.E. Multispecies and monoculture rhizoremediation of polycyclic aromatic hydrocarbons (PAHs) from the soil. Int. J. Phytoremediation 2005, 7, 87–98. [Google Scholar] [CrossRef]


| 2019 | 2020 | |||||||
|---|---|---|---|---|---|---|---|---|
| MS | MK | MS | MK | |||||
| Initial As content (mg/kg) | 8.66 | ±0.67 | 63.45 | ±0.69 | 8.53 | ±0.72 | 62.50 | ±11.93 |
| Harvest As content (mg/kg) | 6.50 | ±1.36 | 72.54 | ±8.34 | 9.64 | ±1.15 | 71.83 | ±13.89 |
| 2019 | 2020 | |||||||
|---|---|---|---|---|---|---|---|---|
| MS | MK | MS | MK | |||||
| PvN | PvI | PvN | PvI | PvN | PvI | PvN | PvI | |
| Biomass (kg/m2) | NA | 0.50 | 0.52 | 0.82 | 0.36 | 0.48 | 0.28 | 0.44 |
| Average As concentration (mg/kg) | 4.69 | 7.91 | 119.13 | 150.8 | 9.56 | 8.59 | 94.77 | 96.89 |
| Removal amount of As (mg/m2) | NA | 3.96 | 61.95 | 123.66 | 3.44 | 4.12 | 26.54 | 42.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, N.; Yang, C.; Shimomura, S.; Inoue, C.; Chien, M.-F. Empirical Evidence of Arsenite Oxidase Gene as an Indicator Accounting for Arsenic Phytoextraction by Pteris vittata. Int. J. Environ. Res. Public Health 2022, 19, 1796. https://doi.org/10.3390/ijerph19031796
Han N, Yang C, Shimomura S, Inoue C, Chien M-F. Empirical Evidence of Arsenite Oxidase Gene as an Indicator Accounting for Arsenic Phytoextraction by Pteris vittata. International Journal of Environmental Research and Public Health. 2022; 19(3):1796. https://doi.org/10.3390/ijerph19031796
Chicago/Turabian StyleHan, Ning, Chongyang Yang, Shunya Shimomura, Chihiro Inoue, and Mei-Fang Chien. 2022. "Empirical Evidence of Arsenite Oxidase Gene as an Indicator Accounting for Arsenic Phytoextraction by Pteris vittata" International Journal of Environmental Research and Public Health 19, no. 3: 1796. https://doi.org/10.3390/ijerph19031796
APA StyleHan, N., Yang, C., Shimomura, S., Inoue, C., & Chien, M.-F. (2022). Empirical Evidence of Arsenite Oxidase Gene as an Indicator Accounting for Arsenic Phytoextraction by Pteris vittata. International Journal of Environmental Research and Public Health, 19(3), 1796. https://doi.org/10.3390/ijerph19031796







