The Safety and Efficacy of Calcitonin Gene-Related Peptide (CGRP) Monoclonal Antibodies for the Preventive Treatment of Migraine: A Protocol for Multiple-Treatment Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol Register
2.2. Ethics
2.3. Review Question
- Population: adult migraine patients;
- Intervention: monoclonal antibody against CGRP;
- Comparison: monoclonal antibody against CGRP and/or placebo;
- Outcome: MMD, MHD, HIT-6, TriD and adverse events.
2.4. Inclusion Criteria
- -
- Real-world studies (for the real-world meta-analysis) and RCTs (for the network meta-analysis);
- -
- Articles in English or Spanish;
- -
- Chronic and/or episodic migraine adult patients (18 years or older) who have received any migraine treatment with monoclonal antibodies for migraine (erenumab, galcanezumab, fremanezumab and eptinezumab);
- -
- Articles with any of the following outcomes: MMD, MHD, HIT-6, TriD and adverse events;
- -
- Any monoclonal antibody against CGRP and/or placebo will be accepted as comparisons.
2.5. Exclusion Criteria
- -
- Articles without complete measurement (value and dispersion) at baseline and after exposure, not completed even after contacting the authors;
- -
- Articles with a follow up length of less than three months;
- -
- Patients without chronic or episodic migraine;
- -
- Animal studies, letters and comments, review articles and editorials.
2.6. Information Sources and Search Strategy
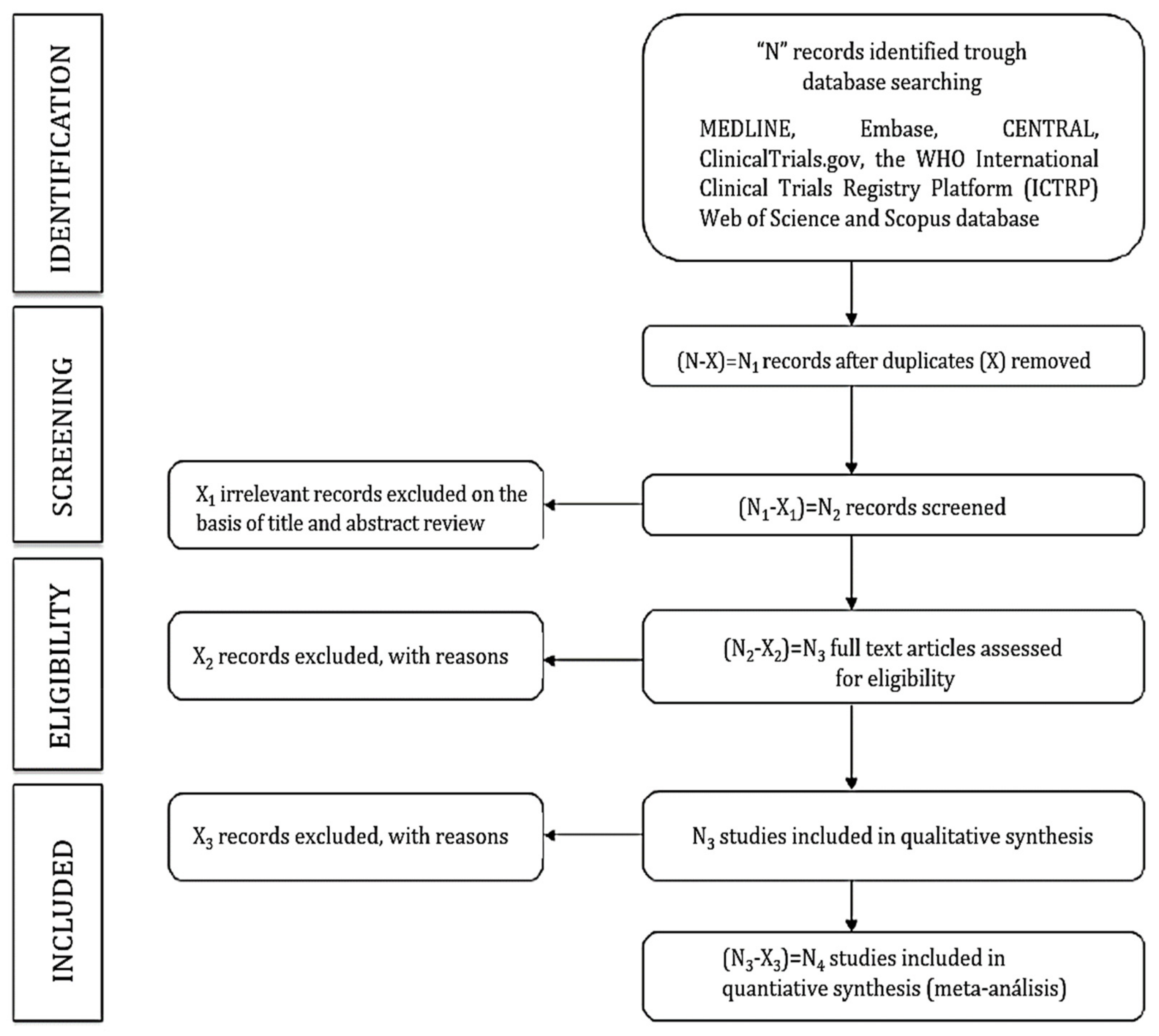
2.7. Study Selection
2.8. Assessment of Reporting Biases
2.9. Grading the Quality of Evidence
2.10. Statistical Analysis
2.10.1. Real-World Meta-Analysis
2.10.2. Network Meta-Analysis
2.11. Heterogeneity Analysis
2.12. Publication Bias
2.13. Subgroup Analysis
2.14. Sensitivity Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Charles, A. The pathophysiology of migraine: Implications for clinical management. Lancet Neurol. 2018, 17, 174–182. [Google Scholar] [CrossRef]
- Buse, D.C.; Greisman, J.D.; Baigi, K.; Lipton, R.B. Migraine Progression: A Systematic Review. Headache 2019, 59, 306–338. [Google Scholar] [CrossRef] [PubMed]
- Borkum, J.M. Migraine Triggers and Oxidative Stress: A Narrative Review and Synthesis. Headache 2016, 56, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Pescador Ruschel, M.A.; De Jesus, O. Migraine Headache; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kelman, L. The triggers or precipitants of the acute migraine attack. Cephalalgia 2007, 27, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Nyholt, D.R.; Borsook, D.; Griffiths, L.R. Migrainomics—identifying brain and genetic markers of migraine. Nat. Rev. Neurol. 2017, 13, 725–741. [Google Scholar] [CrossRef]
- Younger, D.S. Epidemiology of Migraine. Neurol. Clin. 2016, 34, 849–861. [Google Scholar] [CrossRef]
- World Health Organization. Headache Disorders. 2016. Available online: https://www.who.int/news-room/fact-sheets/detail/headache-disorders (accessed on 14 October 2021).
- Barral, E.; Buonanotte, F. Catastrofización ante el dolor y abuso de analgésicos en pacientes con migraña crónica [Pain catastrophizing and medication overuse in patients with chronic migraine]. Rev. Neurol. 2020, 70, 282–286. [Google Scholar]
- Mayans, L.; Walling, A. Acute Migraine Headache: Treatment Strategies. Am. Fam Physician 2018, 97, 243–251. [Google Scholar]
- Gomez, R. HEART Score for Predicting Adverse Outcomes in Patients with Chest Pain. Am. Fam Physician 2018, 98, 72–75. [Google Scholar]
- Magán, P.; Escuredo, M.T.; Estrada, S. Tratamiento de la migraña aguda y crónica y aspectos preventivos. FMC 2017, 24, 157–165. [Google Scholar] [CrossRef]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies—successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Carmine Belin, A.; Ran, C.; Edvinsson, L. Calcitonin Gene-Related Peptide (CGRP) and Cluster Headache. Brain Sci. 2020, 10, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavridis, T.; Deligianni, C.I.; Karagiorgis, G.; Daponte, A.; Breza, M.; Mitsikostas, D.D. Monoclonal Antibodies Targeting CGRP: From Clinical Studies to Real-World Evidence-What Do We Know So Far? Pharmaceuticals 2021, 14, 700. [Google Scholar] [CrossRef] [PubMed]
- Vandervorst, F.; Van Deun, L.; Van Dycke, A.; Paemeleire, K.; Reuter, U.; Schoenen, J.; Versijpt, J. CGRP monoclonal antibodies in migraine: An efficacy and tolerability comparison with standard prophylactic drugs. J. Headache Pain 2021, 22, 128. [Google Scholar] [CrossRef]
- Caronna, E.; Gallardo, V.J.; Alpuente, A.; Torres-Ferrus, M.; Pozo-Rosich, P. Anti-CGRP monoclonal antibodies in chronic migraine with medication overuse: Real-life effectiveness and predictors of response at 6 months. J. Headache Pain 2021, 22, 120. [Google Scholar] [CrossRef]
- Cohen, J.M.; Ning, X.; Kessler, Y.; Rasamoelisolo, M.; Campos, V.R.; Seminerio, M.J.; Krasenbaum, L.J.; Shen, H.; Stratton, J. Immunogenicity of biologic therapies for migraine: A review of current evidence. J. Headache Pain 2021, 22, 3. [Google Scholar] [CrossRef]
- Haanes, K.A.; Edvinsson, L.; Sams, A. Understanding side-effects of anti-CGRP and anti-CGRP receptor antibodies. J. Headache Pain 2020, 21, 26. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Guan, J.; Xiao, H.; Luo, W.; Tong, R. Erenumab safety and efficacy in migraine: A systematic review and meta-analysis of randomized clinical trials. Medicine 2019, 98, e18483. [Google Scholar] [CrossRef]
- Lattanzi, S.; Brigo, F.; Trinka, E.; Vernieri, F.; Corradetti, T.; Dobran, M.; Silvestrini, M. Erenumab for Preventive Treatment of Migraine: A Systematic Review and Meta-Analysis of Efficacy and Safety. Drugs 2019, 79, 417–431. [Google Scholar] [CrossRef]
- Alasad, Y.W.; Asha, M.Z. Monoclonal antibodies as a preventive therapy for migraine: A meta-analysis. Clin. Neurol. Neurosurg. 2020, 195, 105900. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Li, G.G.; Nie, H.; Feng, Y.Y.; Guo, G.Y.; Guo, W.L.; Tang, Z.P. Efficacy and safety of calcitonin-gene-related peptide binding monoclonal antibodies for the preventive treatment of episodic migraine -an updated systematic review and meta-analysis. BMC Neurol. 2020, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Zhao, J.; Han, Q.; Liu, L.; Shen, X. The efficacy and safety of calcitonin gene-related peptide monoclonal antibody for episodic migraine: A meta-analysis. Neurol Sci. 2018, 39, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Guo, J.; Li, Z.; Sun, H.; Yang, X.; Yang, D.; Zhao, H. Network meta-analysis on efficacy and safety of different anti-CGRP monoclonal antibody regimens for prophylaxis and treatment of episodic migraine. Neurol Res. 2021, 43, 932–949. [Google Scholar] [CrossRef]
- Soni, P.; Chawla, E. Efficacy and safety of anti-calcitonin gene-related peptide monoclonal antibodies for treatment of chronic migraine: A systematic review and network meta-analysis. Clin. Neurol. Neurosurg. 2021, 209, 106893. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Song, J.; You, C. Efficacy and Safety of Monoclonal Antibody Against Calcitonin Gene-Related Peptide or Its Receptor for Migraine: A Systematic Review and Network Meta-analysis. Front. Pharmacol. 2021, 12, 649143. [Google Scholar] [CrossRef]
- Masoud, A.T.; Hasan, M.T.; Sayed, A.; Edward, H.N.; Amer, A.M.; Naga, A.E.; Elfil, M.; Alghamdi, B.S.; Perveen, A.; Ashraf, G.M.; et al. Efficacy of calcitonin gene-related peptide (CGRP) receptor blockers in reducing the number of monthly migraine headache days (MHDs): A network meta-analysis of randomized controlled trials. J. Neurol Sci. 2021, 427, 117505. [Google Scholar] [CrossRef]
- Chodankar, D. Introduction to real-world evidence studies. Perspect. Clin. Res. 2021, 12, 171–174. [Google Scholar] [CrossRef]
- Li, T.; Puhan, M.A.; Vedula, S.S.; Singh, S.; Dickersin, K. Network meta-analysis-highly attractive but more methodological research is needed. BMC Med. 2011, 9, 79. [Google Scholar] [CrossRef] [Green Version]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; The PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [Green Version]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: Aproposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Catalá-López, F.; Moher, D. La extensión de la declaración PRISMA para revisiones sistemáticas que incorporan metaanálisis en red: PRISMA-NMA. Med. Clin. 2016, 147, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.A.; Stewart, L.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane. 2021. Available online: www.training.cochrane.org/handbook (accessed on 30 January 2022).
- Booth, A.; Clarke, M.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. An international registry of systematic-review protocols. Lancet 2011, 377, 108–109. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.; Elbers, R.G.; Blencowe, N.; Boutron, I.; Cates, C.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norrise, S.; Falck-Ytterf, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence pro les and summary of ndings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Goldet, G.; Howick, J. Understanding GRADE: An introduction. JBEM 2013, 6, 50–54. [Google Scholar] [CrossRef]
- Sutton, A.J.; Abrams, K.R.; Jones, D.R.; Sheldon, T.A.; Song, F. Methods for Meta-Analysis in Medical Research; John Wiley & Sons, Ltd.: Chichester, UK, 2000. [Google Scholar]
- Veroniki, A.A.; Bender, R.; Glasziou, P.; Straus, S.E.; Tricco, A.C. The number needed to treat in pairwise and network meta-analysis and its graphical representation. J. Clin. Epidemiol. 2019, 111, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P.A. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Stettler, C.; Allemann, S.; Wandel, S.; Kastrati, A.; Morice, M.C.; Schömig, A.; Pfisterer, M.E.; Stone, G.W.; Leon, M.B.; De Lezo, J.S.; et al. Drug eluting and bare metal stents in people with and without diabetes: Collaborative network meta-analysis. BMJ 2008, 337, a1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Egger, M.; Smith, G.D. Investigating and dealing with publication and other biases in meta-analysis. Br. Med. J. 2001, 323, 101–105. [Google Scholar] [CrossRef] [PubMed]
| Population Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reference | Country | Sample Size | Mean Age | Pathology and Duration | Previous and/or Concomitant OnabotulinumtoxinA | |||
| First author and year of publication | Country in which the study data were collected | Number of participants and percentage of women | Age (years) of the participants range or mean ± SD | Episodic Migraine or Chronic Migraine Years of migraine duration | Previous or actual administration of onabotulinumtoxinA | |||
| Intervention characteristics | Outcome | |||||||
| Monoclonal antibody against CGRP | Dose | Length | MMD | MHD | HIT-6 | Tri-D | Adverse Effects | |
| erenumab, galcanezumab, fremanezumab and eptinezumab | Dose administered and frequency | Length (months) of treatment and follow-up | Baseline and post exposure mean ± SD | Baseline and post exposure mean ± SD | Baseline and post exposure mean ± SD | Baseline and post exposure mean ± SD | Presence of any type of adverse effect and/or their grade. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Bravo-Rodrigo, J.; Pascual-Morena, C.; Flor-García, A.; Saz-Lara, A.; Sequí-Dominguez, I.; Álvarez-Bueno, C.; Barreda-Hernández, D.; Cavero-Redondo, I. The Safety and Efficacy of Calcitonin Gene-Related Peptide (CGRP) Monoclonal Antibodies for the Preventive Treatment of Migraine: A Protocol for Multiple-Treatment Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 1753. https://doi.org/10.3390/ijerph19031753
Fernández-Bravo-Rodrigo J, Pascual-Morena C, Flor-García A, Saz-Lara A, Sequí-Dominguez I, Álvarez-Bueno C, Barreda-Hernández D, Cavero-Redondo I. The Safety and Efficacy of Calcitonin Gene-Related Peptide (CGRP) Monoclonal Antibodies for the Preventive Treatment of Migraine: A Protocol for Multiple-Treatment Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(3):1753. https://doi.org/10.3390/ijerph19031753
Chicago/Turabian StyleFernández-Bravo-Rodrigo, Jaime, Carlos Pascual-Morena, Amparo Flor-García, Alicia Saz-Lara, Irene Sequí-Dominguez, Celia Álvarez-Bueno, Dolores Barreda-Hernández, and Iván Cavero-Redondo. 2022. "The Safety and Efficacy of Calcitonin Gene-Related Peptide (CGRP) Monoclonal Antibodies for the Preventive Treatment of Migraine: A Protocol for Multiple-Treatment Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 3: 1753. https://doi.org/10.3390/ijerph19031753
APA StyleFernández-Bravo-Rodrigo, J., Pascual-Morena, C., Flor-García, A., Saz-Lara, A., Sequí-Dominguez, I., Álvarez-Bueno, C., Barreda-Hernández, D., & Cavero-Redondo, I. (2022). The Safety and Efficacy of Calcitonin Gene-Related Peptide (CGRP) Monoclonal Antibodies for the Preventive Treatment of Migraine: A Protocol for Multiple-Treatment Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(3), 1753. https://doi.org/10.3390/ijerph19031753







