Environmental Pollution to Blame for Depressive Disorder?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients/Study Population
2.2. Healthy Volunteers
2.3. Collection of Serum Samples
2.4. Sample Treatment
2.5. Data Analysis
2.6. Reagents and Chemicals
2.7. GC-MS Conditions
2.8. Statistical Analysis
3. Results
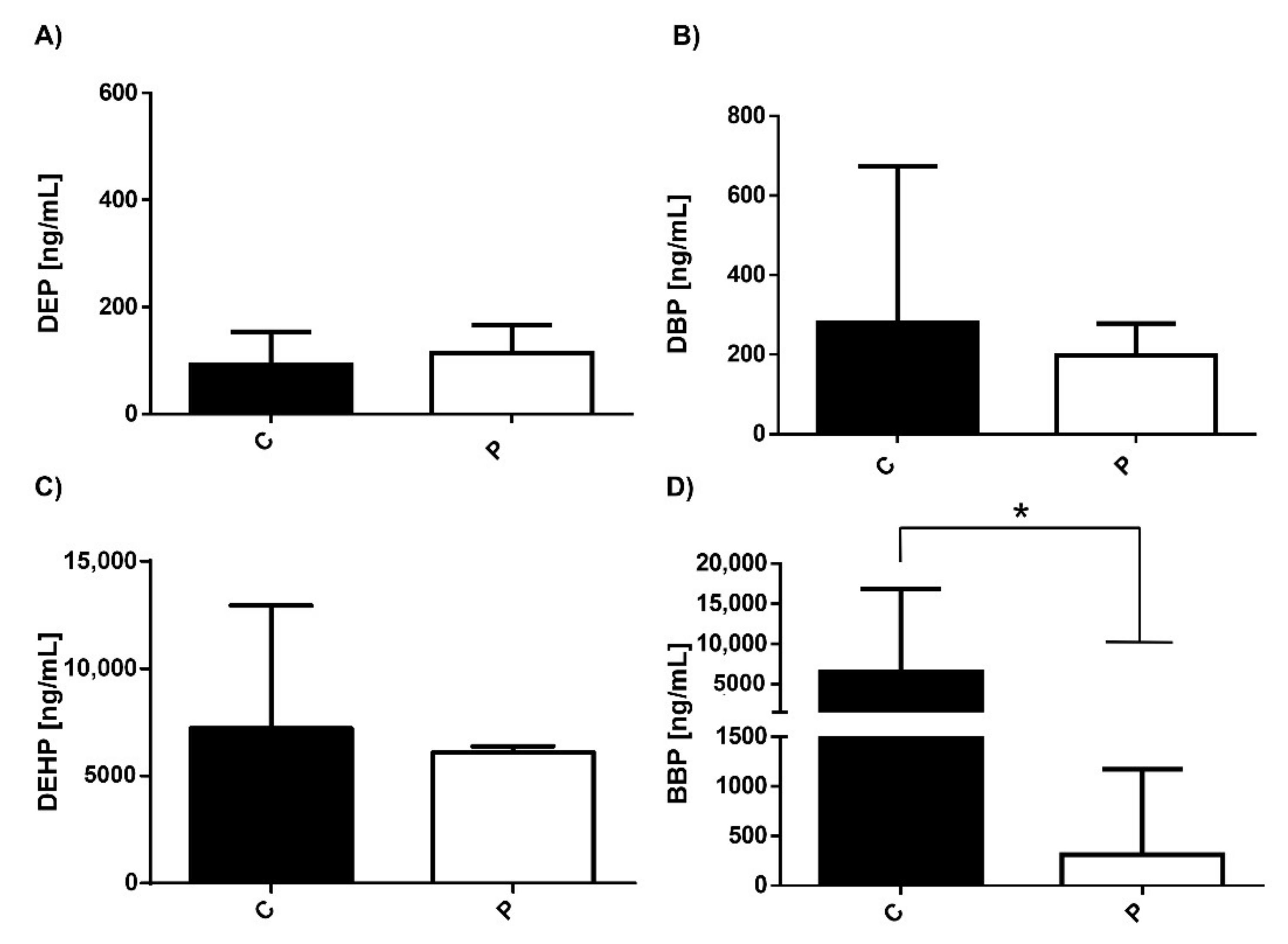
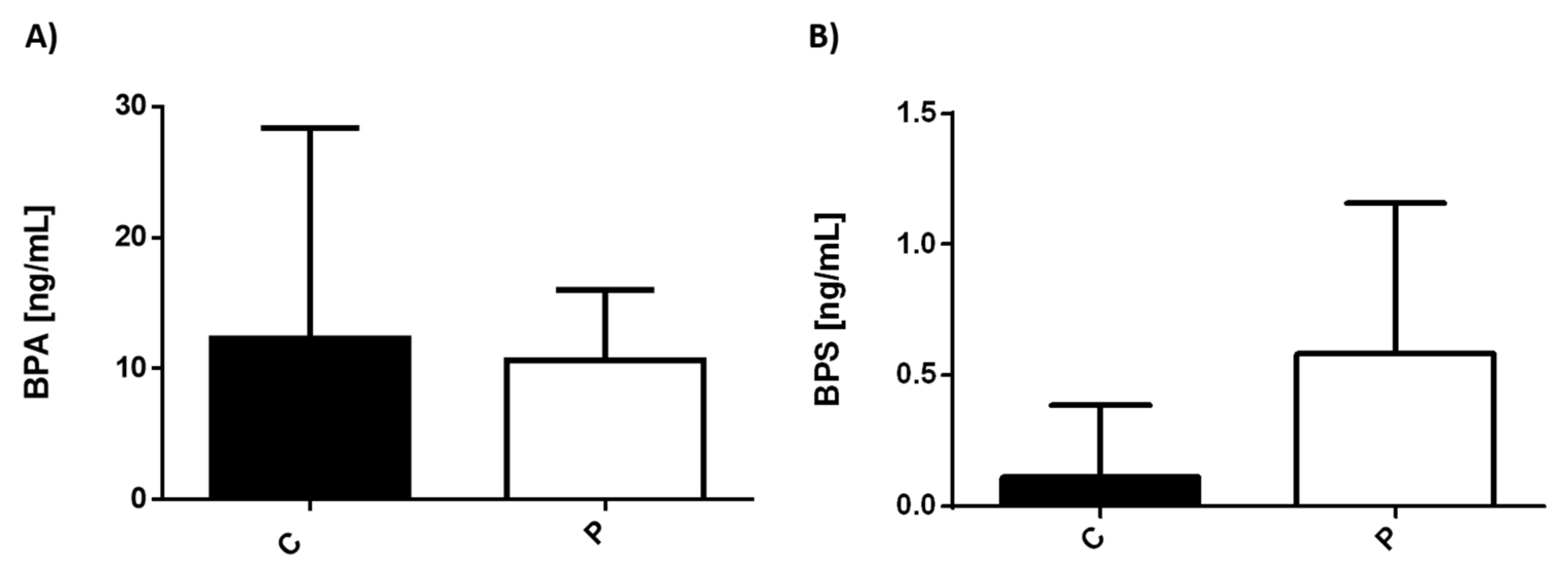
3.1. Serum Levels of Environmental Pollutants in Healthy Individuals and Patients with MDD
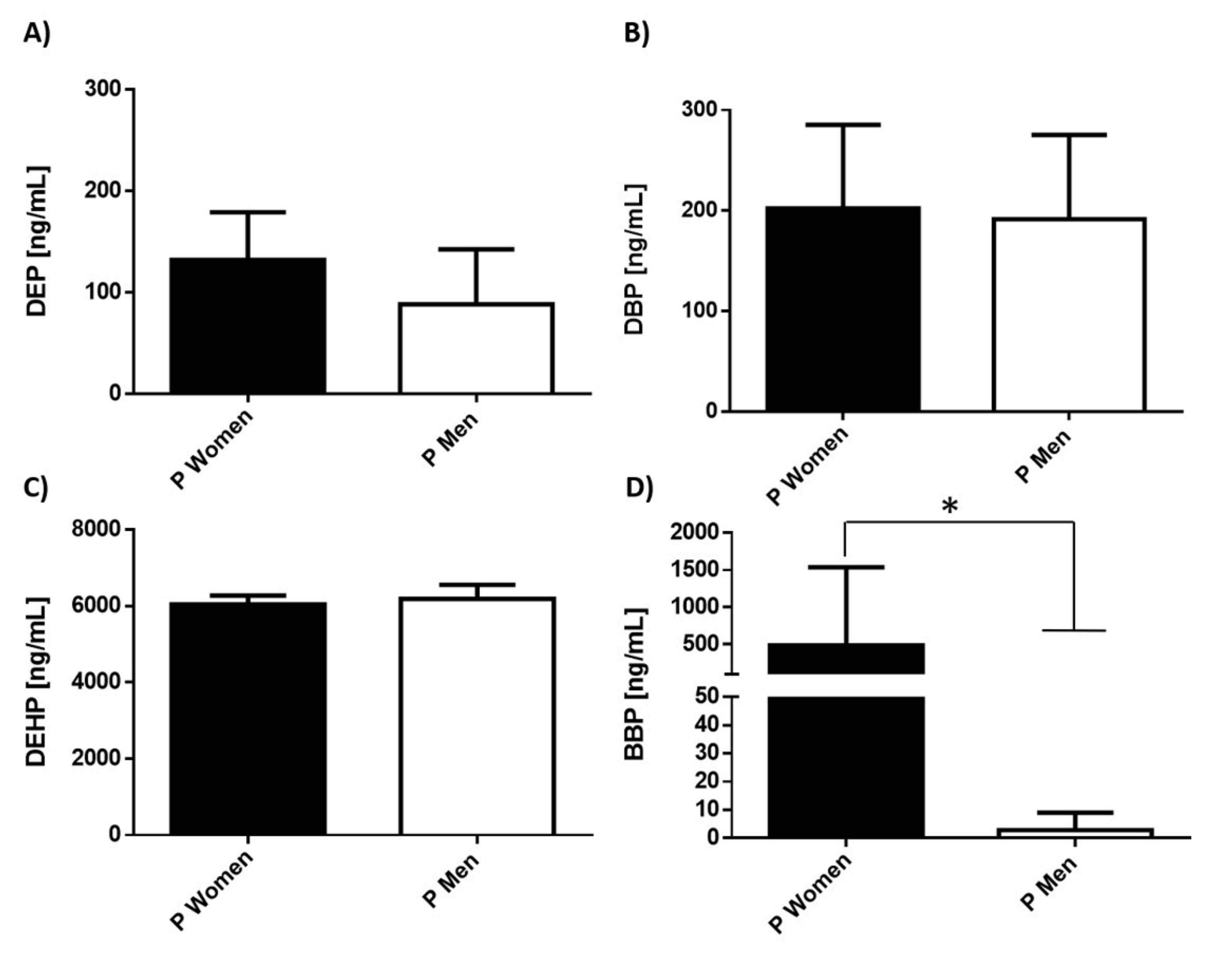
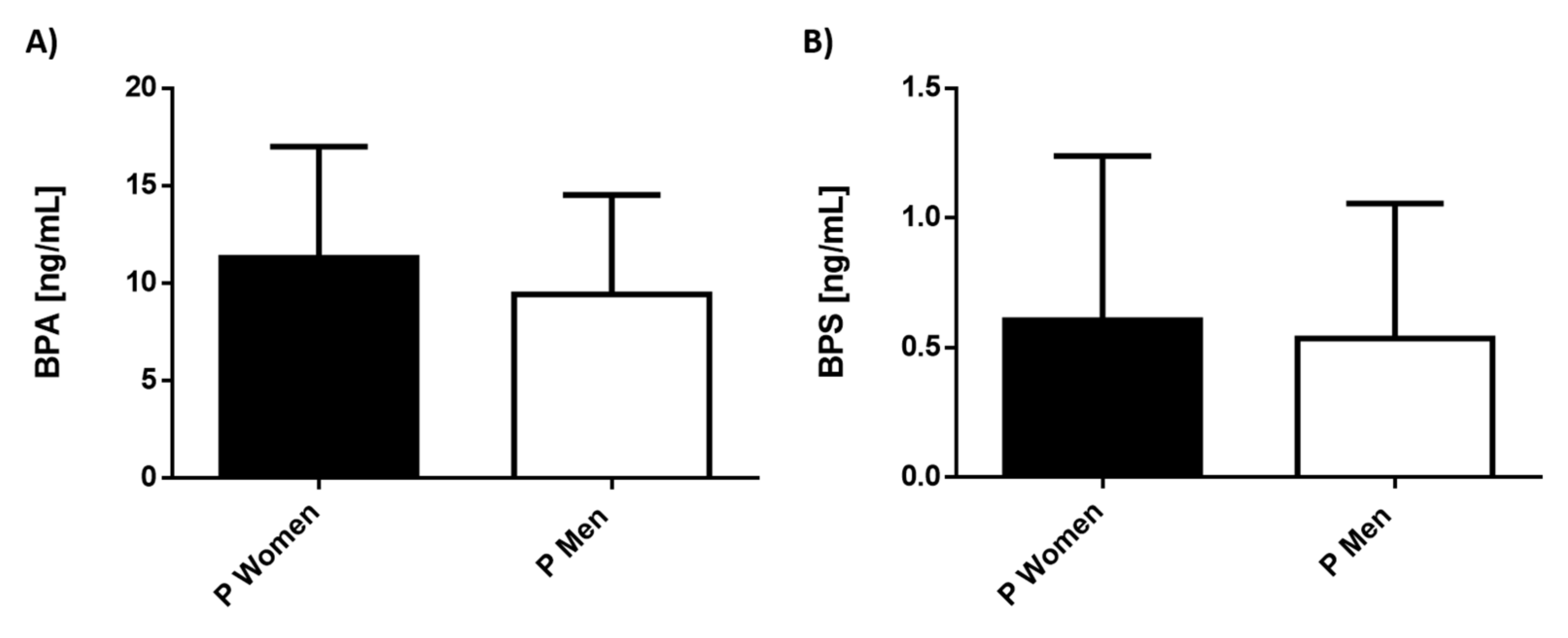
3.2. Serum Levels of Environmental Pollutants in Patients with MDD Separated by Gender
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Institutes of Health (US). Biological Sciences Curriculum Study. NIH Curriculum Supplement Series [Internet]. Information about Mental Illness and the Brain. 2007. Available online: https://www.ncbi.nlm.nih.gov/books/NBK20369/ (accessed on 31 December 2021).
- Vogelzangs, N.; Duivis, H.E.; Beekman, A.T.; Kluft, C.; Neuteboom, J.; Hoogendijk, W.; Smit, J.H.; de Jonge, P.; Penninx, B.W. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl. Psychiatry 2012, 2, e79. [Google Scholar] [CrossRef] [Green Version]
- Pochigaeva, K.; Druzhkova, T.; Yakovlev, A.; Onufriev, M.; Grishkina, M.; Chepelev, A.; Guekht, A.; Gulyaeva, N. Hair cortisol as a marker of hypothalamic-pituitary-adrenal Axis activity in female patients with major depressive disorder. Metab. Brain Dis. 2017, 32, 577–583. [Google Scholar] [CrossRef]
- Vreeburg, S.A.; Hoogendijk, W.J.; van Pelt, J.; Derijk, R.H.; Verhagen, J.C.; van Dyck, R.; Smit, J.H.; Zitman, F.G.; Penninx, B.W. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from a large cohort study. Arch. Gen. Psychiatry 2009, 66, 617–626. [Google Scholar] [CrossRef] [Green Version]
- De Villiers, A.S.; Russell, V.A.; Carstens, M.E.; Searson, J.A.; van Zyl, A.M.; Lombard, C.J.; Taljaard, J.J. Noradrenergic function and hypothalamic-pituitary-adrenal axis activity in adolescents with major depressive disorder. Psychiatry Res. 1989, 27, 101–109. [Google Scholar] [CrossRef]
- Okasha, T.A.; Sabry, W.M.; Hashim, M.A.; Abdeen, M.S.; Abdelhamid, A.M. Vitamin D serum level in major depressive disorder and schizophrenia. Middle East Curr. Psychiatry 2020, 27, 34. [Google Scholar] [CrossRef]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Depression in Parents, Parenting, and Children: Opportunities to Improve Identification, Treatment, and Prevention; England, M.J., Sim, L.J., Eds.; National Research Council: Washington, DC, USA, 2009. [Google Scholar]
- Shoaff, J.R.; Coull, B.; Weuve, J.; Bellinger, D.C.; Calafat, A.M.; Schantz, S.L.; Korrick, S.A. Association of Exposure to Endocrine-Disrupting Chemicals During Adolescence with Attention-Deficit/Hyperactivity Disorder–Related Behaviors. JAMA Netw. Open 2020, 3, e2015041. [Google Scholar] [CrossRef]
- De Cock, M.; Maas, Y.G.; van de Bor, M. Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Review. Acta Paediatr. 2012, 101, 811–818. [Google Scholar] [CrossRef]
- Liu, T.; Jia, Y.; Zhou, L.; Wang, Q.; Sun, D.; Xu, J.; Wu, J.; Chen, H.; Xu, F.; Ye, L. Effects of Di-(2-ethylhexyl) Phthalate on the Hypothalamus-Uterus in Pubertal Female Rats. Int. J. Environ. Res. Public Health 2016, 13, 1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konieczna, A.; Rutkowska, A.; Rachon, D. Health risk of exposure to Bisphenol A (BPA). Rocz. Państwowego Zakładu Hig. 2015, 66, 5–11. [Google Scholar]
- Thoene, M.; Dzika, E.; Gonkowski, S.; Wojtkiewicz, J. Bisphenol S in Food Causes Hormonal and Obesogenic Effects Comparable to or Worse than Bisphenol A: A Literature Review. Nutrients 2020, 12, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.; Haggerty, D.K.; Rappolee, D.A.; Ruden, D.M. Phthalate Exposure and Long-Term Epigenomic Consequences: A Review. Front. Genet. 2020, 11, 405. [Google Scholar] [CrossRef]
- Segovia-Mendoza, M.; Nava-Castro, K.E.; Palacios-Arreola, M.I.; Garay-Canales, C.; Morales-Montor, J. How microplastic components influence the immune system and impact on children health: Focus on cancer. Birth Defects Res. 2020, 112, 1341–1361. [Google Scholar] [CrossRef]
- Geens, T.; Goeyens, L.; Covaci, A. Are potential sources for human exposure to bisphenol-A overlooked? Int. J. Hyg. Environ. Health 2011, 214, 339–347. [Google Scholar] [CrossRef]
- Koniecki, D.; Wang, R.; Moody, R.P.; Zhu, J. Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ. Res. 2011, 111, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Acconcia, F.; Pallottini, V.; Marino, M. Molecular Mechanisms of Action of BPA. Dose Response 2015, 13, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaguer, P.; Delfosse, V.; Grimaldi, M.; Bourguet, W. Structural and functional evidences for the interactions between nuclear hormone receptors and endocrine disruptors at low doses. Comptes Rendus Biol. 2017, 340, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Archer, M.; Suzanne, J. Relationship between Estrogen, Serotonin and Depression. Menopause 1997, 6, 71–78. [Google Scholar] [CrossRef]
- Ma, Z.-Q.; Violani, E.; Villa, F.; Picotti, G.B.; Maggi, A. Estrogenic control of monoamine oxidase A activity in human neuroblastoma cells expressing physiological concentrations of estrogen receptor. Eur. J. Pharmacol. 1995, 284, 171–176. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, O.T.; Martinez-Mota, L.; Herrera-Perez, J.J.; Jimenez-Rubio, G. Role of Estradiol in the Expression of Genes Involved in Serotonin Neurotransmission: Implications for Female Depression. Curr. Neuropharmacol. 2019, 17, 459–471. [Google Scholar] [CrossRef]
- Bethea, C.L.; Smith, A.W.; Centeno, M.L.; Reddy, A.P. Long-term ovariectomy decreases serotonin neuron number and gene expression in free ranging macaques. Neuroscience 2011, 192, 675–688. [Google Scholar] [CrossRef] [Green Version]
- Hiroi, R.; Handa, R.J. Estrogen receptor-beta regulates human tryptophan hydroxylase-2 through an estrogen response element in the 5′ untranslated region. J. Neurochem. 2013, 127, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, K.; Shih, J.C.; Teng, C.T. Estrogen-related receptors-stimulated monoamine oxidase B promoter activity is down-regulated by estrogen receptors. Mol. Endocrinol. 2006, 20, 1547–1561. [Google Scholar] [CrossRef] [Green Version]
- Schneider, L.S.; Small, G.W.; Clary, C.M. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am. J. Geriatr. Psychiatry 2001, 9, 393–399. [Google Scholar] [CrossRef]
- Schneider, L.S.; Small, G.W.; Hamilton, S.H.; Bystritsky, A.; Nemeroff, C.B.; Meyers, B.S. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am. J. Geriatr. Psychiatry 1997, 5, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Georgieva, M.G.; Atanasov, A.G.; Tzvetkov, N.T. Monoamine Oxidases (MAOs) as Privileged Molecular Targets in Neuroscience: Research Literature Analysis. Front. Mol. Neurosci. 2019, 12, 143. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, P.J.; Rubinow, D.R. Sex hormones and mood in the perimenopause. Ann. N. Y. Acad. Sci. 2009, 1179, 70–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maki, P.M.; Kornstein, S.G.; Joffe, H.; Bromberger, J.T.; Freeman, E.W.; Athappilly, G.; Bobo, W.V.; Rubin, L.H.; Koleva, H.K.; Cohen, L.S.; et al. Guidelines for the Evaluation and Treatment of Perimenopausal Depression: Summary and Recommendations. J. Women’s Health 2019, 28, 117–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, J.L.; Girdler, S.S.; Meltzer-Brody, S.E.; Stika, C.S.; Thurston, R.C.; Clark, C.T.; Prairie, B.A.; Moses-Kolko, E.; Joffe, H.; Wisner, K.L. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: A novel heuristic model. Am. J. Psychiatry 2015, 172, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Segovia-Mendoza, M.; Morales-Montor, J. Immune Tumor Microenvironment in Breast Cancer and the Participation of Estrogen and Its Receptors in Cancer Physiopathology. Front. Immunol. 2019, 10, 348. [Google Scholar] [CrossRef] [Green Version]
- Bereshchenko, O.; Bruscoli, S.; Riccardi, C. Glucocorticoids, Sex Hormones, and Immunity. Front. Immunol. 2018, 9, 1332. [Google Scholar] [CrossRef]
- Pavon, L.; Sandoval-Lopez, G.; Eugenia Hernandez, M.; Loria, F.; Estrada, I.; Perez, M.; Moreno, J.; Avila, U.; Leff, P.; Anton, B.; et al. Th2 cytokine response in Major Depressive Disorder patients before treatment. J. Neuroimmunol. 2006, 172, 156–165. [Google Scholar] [CrossRef]
- Nava-Castro, K.E.; Togno-Peirce, C.; Palacios-Arreola, M.I.; Del Rio-Araiza, V.H.; Hernandez-Bello, R.; Morales Montor, J. Bisphenol A induces protection through modulation of the immune response against the helminth parasite Taenia crassiceps. Parasite Immunol. 2020, 42, e12733. [Google Scholar] [CrossRef]
- Bornehag, C.G.; Nanberg, E. Phthalate exposure and asthma in children. Int. J. Androl. 2010, 33, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.F.; Nielsen, C.H.; Brorson, M.M.; Frederiksen, H.; Hartoft-Nielsen, M.-L.; Rasmussen, Å.K.; Bendtzen, K.; Feldt-Rasmussen, U. Influence of phthalates on in vitro innate and adaptive immune responses. PLoS ONE 2015, 10, e0131168. [Google Scholar] [CrossRef] [PubMed]
- Flores-Ramos, M.; Burrola-Suárez, M.A.; Guiza-Zayas, R.; Enciso-Araujo, J.M.; Islas-Preciado, D.; Estrada, C.E. Evaluation of hormonal and metabolic factors related to depression in reproductive age women. Salud Ment. 2020, 43, 35–41. [Google Scholar] [CrossRef]
- Findikli, E.; Kurutas, E.B.; Camkurt, M.A.; Karaaslan, M.F.; Izci, F.; Fındıklı, H.A.; Kardaş, S.; Dag, B.; Altun, H. Increased Serum G Protein-coupled Estrogen Receptor 1 Levels and Its Diagnostic Value in Drug Naïve Patients with Major Depressive Disorder. Clin. Psychopharmacol. Neurosci. 2017, 15, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.J.; Reidy, J.A.; Herbert, A.R.; Preau, J.L.; Needham, L.L.; Calafat, A.M. Detection of Phthalate Metabolites in Human Amniotic Fluid. Bull. Environ. Contam. Toxicol. 2004, 72, 1226–1231. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Kannan, K. A Review of Biomonitoring of Phthalate Exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose Response Publ. Int. Hormesis Soc. 2015, 13, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Main, K.M.; Mortensen, G.K.; Kaleva, M.M.; Boisen, K.A.; Damgaard, I.N.; Chellakooty, M.; Schmidt, I.M.; Suomi, A.M.; Virtanen, H.E.; Petersen, D.V.; et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ. Health Perspect. 2006, 114, 270–276. [Google Scholar] [CrossRef]
- Repouskou, A.; Papadopoulou, A.K.; Panagiotidou, E.; Trichas, P.; Lindh, C.; Bergman, A.; Gennings, C.; Bornehag, C.G.; Ruegg, J.; Kitraki, E.; et al. Long term transcriptional and behavioral effects in mice developmentally exposed to a mixture of endocrine disruptors associated with delayed human neurodevelopment. Sci. Rep. 2020, 10, 9367. [Google Scholar] [CrossRef] [PubMed]
- Kajta, M.; Wojtowicz, A.K. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol. Rep. 2013, 65, 1632–1639. [Google Scholar] [CrossRef]
- Inoue, H.; Yuki, G.; Yokota, H.; Kato, S. Bisphenol A glucuronidation and absorption in rat intestine. Drug Metab. Dispos. 2003, 31, 140–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genuis, S.J.; Beesoon, S.; Birkholz, D.; Lobo, R.A. Human Excretion of Bisphenol A: Blood, Urine, and Sweat (BUS) Study. J. Environ. Public Health 2012, 2012, 185731. [Google Scholar] [CrossRef] [Green Version]
- Volkel, W.; Colnot, T.; Csanady, G.A.; Filser, J.G.; Dekant, W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem. Res. Toxicol. 2002, 15, 1281–1287. [Google Scholar] [CrossRef]
- Hauser, R.; Calafat, A. Phthalates and human health. Occup. Environ. Med. 2005, 62, 806–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsberg, G.; Rice, D.C. Does rapid metabolism ensure negligible risk from bisphenol A? Environ. Health Perspect. 2009, 117, 1639–1643. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. 20), 22–33. [Google Scholar]
- Sheehan, D.V.; Sheehan, K.H.; Shytle, R.D.; Janavs, J.; Bannon, Y.; Rogers, J.E.; Milo, K.M.; Stock, S.L.; Wilkinson, B. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). J. Clin. Psychiatry 2010, 71, 313–326. [Google Scholar] [CrossRef]
- Lecrubier, Y.; Sheehan, D.V.; Weiller, E.; Amorim, P.; Bonora, I.; Sheehan, K.; Harnett Janavs, J.; Dunbar, G.C. The MINI International Neuropsychiatric Interview (MINI). A Short Diagnostic Structured Interview: Reliability and Validity According to the CIDI. Eur. Psychiatry 1997, 12, 224–231. [Google Scholar] [CrossRef]
- Carrozzino, D.; Patierno, C.; Fava, G.A.; Guidi, J. The Hamilton Rating Scales for Depression: A Critical Review of Clinimetric Properties of Different Versions. Psychother. Psychosom. 2020, 89, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J. Personal. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, A.; Rudnitskaya, A.; Blackburn, G.J.; Mohd Fauzi, N.; Pitt, A.R.; Spickett, C.M. A comparison of five lipid extraction solvent systems for lipidomic studies of human LDL. J. Lipid Res. 2013, 54, 1812–1824. [Google Scholar] [CrossRef] [Green Version]
- Amador-Munoz, O.; Marriott, P.J. Quantification in comprehensive two-dimensional gas chromatography and a model of quantification based on selected summed modulated peaks. J. Chromatogr. A 2008, 1184, 323–340. [Google Scholar] [CrossRef]
- Lyche, J.L.; Gutleb, A.C.; Bergman, A.; Eriksen, G.S. Reproductive and developmental toxicity of phthalates. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 225–249. [Google Scholar] [CrossRef]
- Reddy, B.S.; Rozati, R.; Reddy, B.V.; Raman, N.V. Association of phthalate esters with endometriosis in Indian women. BJOG 2006, 113, 515–520. [Google Scholar] [CrossRef]
- Tyl, R.W.; Myers, C.B.; Marr, M.C.; Fail, P.A.; Seely, J.C.; Brine, D.R.; Barter, R.A.; Butala, J.H. Reproductive toxicity evaluation of dietary butyl benzyl phthalate (BBP) in rats. Reprod. Toxicol. 2004, 18, 241–264. [Google Scholar] [CrossRef]
- Harris, C.A.; Henttu, P.; Parker, M.G.; Sumpter, J.P. The estrogenic activity of phthalate esters in vitro. Environ. Health Perspect. 1997, 105, 802–811. [Google Scholar] [CrossRef]
- Lovekamp-Swan, T.; Davis, B.J. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ. Health Perspect. 2003, 111, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, M.N.; Marczylo, E.L.; Guerrero-Bosagna, C.; Rüegg, J. Marked for Life: Epigenetic Effects of Endocrine Disrupting Chemicals. Annu. Rev. Environ. Resour. 2017, 42, 105–160. [Google Scholar] [CrossRef]
- Frederiksen, H.; Jorgensen, N.; Andersson, A.M. Correlations between phthalate metabolites in urine, serum, and seminal plasma from young Danish men determined by isotope dilution liquid chromatography tandem mass spectrometry. J. Anal. Toxicol. 2010, 34, 400–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, B.S.; Rozati, R.; Reddy, S.; Kodampur, S.; Reddy, P.; Reddy, R. High plasma concentrations of polychlorinated biphenyls and phthalate esters in women with endometriosis: A prospective case control study. Fertil. Steril. 2006, 85, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Wittassek, M.; Koch, H.M.; Angerer, J.; Bruning, T. Assessing exposure to phthalates—The human biomonitoring approach. Mol. Nutr. Food Res. 2011, 55, 7–31. [Google Scholar] [CrossRef]
- Hernandez-Diaz, S.; Mitchell, A.A.; Kelley, K.E.; Calafat, A.M.; Hauser, R. Medications as a potential source of exposure to phthalates in the U.S. population. Environ. Health Perspect. 2009, 117, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.H.; Shen, Y.X.; Li, L.; Fan, T.T.; Wang, Y.; Wei, N. Phthalate exposure and high blood pressure in adults: A cross-sectional study in China. Environ. Sci. Pollut. Res. Int. 2018, 25, 15934–15942. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Romero, E.; Scheringer, M. A review of phthalate pharmacokinetics in human and rat: What factors drive phthalate distribution and partitioning? Drug Metab. Rev. 2019, 51, 314–329. [Google Scholar] [CrossRef] [Green Version]
- Min, A.; Liu, F.; Yang, X.; Chen, M. Benzyl butyl phthalate exposure impairs learning and memory and attenuates neurotransmission and CREB phosphorylation in mice. Food Chem. Toxicol. 2014, 71, 81–89. [Google Scholar] [CrossRef]
- Sicińska, P.; Kik, K.; Bukowska, B. Human Erythrocytes Exposed to Phthalates and Their Metabolites Alter Antioxidant Enzyme Activity and Hemoglobin Oxidation. Int. J. Mol. Sci. 2020, 21, 4480. [Google Scholar] [CrossRef]
- Hlisníková, H.; Petrovicova, I.; Kolena, B.; Sidlovska, M.; Sirotkin, A. Effects and Mechanisms of Phthalates’ Action on Reproductive Processes and Reproductive Health: A Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 6811. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Y.; Wang, R.; Wang, Y.; Ruan, Q.; Lu, Y. Perinatal exposure to di-(2-ethylhexyl) phthalate affects anxiety- and depression-like behaviors in mice. Chemosphere 2015, 124, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yuan, K.; Li, L.; Liu, S.; Li, S.; Hu, G.; Lian, Q.Q.; Ge, R.S. In Utero Exposure to Diethylhexyl Phthalate Affects Rat Brain Development: A Behavioral and Genomic Approach. Int. J. Environ. Res. Public Health 2015, 12, 13696–13710. [Google Scholar] [CrossRef]
- Xu, Y.; Agrawal, S.; Cook, T.J.; Knipp, G.T. Di-(2-ethylhexyl)-phthalate affects lipid profiling in fetal rat brain upon maternal exposure. Arch. Toxicol. 2007, 81, 57–62. [Google Scholar] [CrossRef]
- Derghal, A.; Djelloul, M.; Trouslard, J.; Mounien, L. An Emerging Role of micro-RNA in the Effect of the Endocrine Disruptors. Front. Neurosci. 2016, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Babenko, O.; Kovalchuk, I.; Metz, G.A. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci. Biobehav. Rev. 2015, 48, 70–91. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Holahan, M.R. Reduced hippocampal dendritic spine density and BDNF expression following acute postnatal exposure to di(2-ethylhexyl) phthalate in male Long Evans rats. PLoS ONE 2014, 9, e109522. [Google Scholar]
- Smith, C.A.; Farmer, K.; Lee, H.; Holahan, M.R.; Smith, J.C. Altered Hippocampal Lipid Profile Following Acute Postnatal Exposure to Di(2-Ethylhexyl) Phthalate in Rats. Int. J. Environ. Res. Public Health 2015, 12, 13542–13559. [Google Scholar] [CrossRef] [Green Version]
- Kubo, K.; Arai, O.; Ogata, R.; Omura, M.; Hori, T.; Aou, S. Exposure to bisphenol A during the fetal and suckling periods disrupts sexual differentiation of the locus coeruleus and of behavior in the rat. Neurosci. Lett. 2001, 304, 73–76. [Google Scholar] [CrossRef]
- Yang, M.; Ryu, J.H.; Jeon, R.; Kang, D.; Yoo, K.Y. Effects of bisphenol A on breast cancer and its risk factors. Arch. Toxicol. 2009, 83, 281–285. [Google Scholar] [CrossRef]
- Parada, H., Jr.; Gammon, M.D.; Ettore, H.L.; Chen, J.; Calafat, A.M.; Neugut, A.I.; Santella, R.M.; Wolff, M.S.; Teitelbaum, S.L. Urinary concentrations of environmental phenols and their associations with breast cancer incidence and mortality following breast cancer. Environ. Int. 2019, 130, 104890. [Google Scholar] [CrossRef] [PubMed]
- Cobellis, L.; Colacurci, N.; Trabucco, E.; Carpentiero, C.; Grumetto, L. Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed. Chromatogr. 2009, 23, 1186–1190. [Google Scholar] [CrossRef]
- Ven den Bosch, M.; Meyer-Lindenberg, A. Environmental Exposures and Depression: Biological Mechanisms and Epidemiological Evidence. Annu. Rev. Public Health 2019, 40, 239–259. [Google Scholar] [CrossRef] [Green Version]
- Braun, J.M.; Kalkbrenner, A.E.; Calafat, A.M.; Yolton, K.; Ye, X.; Dietrich, K.N.; Lanphear, B.P. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 2011, 128, 873–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preciados, M.; Yoo, C.; Roy, D. Estrogenic Endocrine Disrupting Chemicals Influencing NRF1 Regulated Gene Networks in the Development of Complex Human Brain Diseases. Int. J. Mol. Sci. 2016, 17, 2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Patients n = 14 | Healthy Volunteers n = 53 | |
|---|---|---|
| Age (years) | 34.1 ± 9.1 | 34.1 ± 9.1 |
| Sex (male/female) | 5/9 | 7/46 |
| BMI (kg/m2) | 24.8 ± 2.5 | 24.8 ± 2.5 |
| Education (years) | 10.6 ± 4.93 | 14 ± 5.6 |
| Family history (yes/no) | 5/9 | NA |
| First episode | 6 | NA |
| Recurrent episode | 8 | NA |
| Compound | Linear Range (pg) | Slope | Intercept | r2 | Monitored Ions |
|---|---|---|---|---|---|
| BPA-TMS | 0.1–10 | 0.0078 | 0.0375 | 0.9902 | 357, 358, 372 |
| BPS-TMS | 0.1–10 | 0.0025 | 0.004 | 0.9856 | 394, 379 |
| DEP | 10–10,000 | 0.002 | 0.2031 | 0.9948 | 149, 177, 76 |
| DBP | 10–10,000 | 0.0045 | 0.4721 | 0.9998 | 149, 205, 223 |
| BBP | 10–10,000 | 0.0019 | 0.0548 | 0.9999 | 149, 91, 206 |
| DEHP | 10–10,000 | 0.0027 | 0.2162 | 0.9995 | 149, 176, 279 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segovia-Mendoza, M.; Palacios-Arreola, M.I.; Pavón, L.; Becerril, L.E.; Nava-Castro, K.E.; Amador-Muñoz, O.; Morales-Montor, J. Environmental Pollution to Blame for Depressive Disorder? Int. J. Environ. Res. Public Health 2022, 19, 1737. https://doi.org/10.3390/ijerph19031737
Segovia-Mendoza M, Palacios-Arreola MI, Pavón L, Becerril LE, Nava-Castro KE, Amador-Muñoz O, Morales-Montor J. Environmental Pollution to Blame for Depressive Disorder? International Journal of Environmental Research and Public Health. 2022; 19(3):1737. https://doi.org/10.3390/ijerph19031737
Chicago/Turabian StyleSegovia-Mendoza, Mariana, Margarita Isabel Palacios-Arreola, Lenin Pavón, Luis Enrique Becerril, Karen Elizabeth Nava-Castro, Omar Amador-Muñoz, and Jorge Morales-Montor. 2022. "Environmental Pollution to Blame for Depressive Disorder?" International Journal of Environmental Research and Public Health 19, no. 3: 1737. https://doi.org/10.3390/ijerph19031737
APA StyleSegovia-Mendoza, M., Palacios-Arreola, M. I., Pavón, L., Becerril, L. E., Nava-Castro, K. E., Amador-Muñoz, O., & Morales-Montor, J. (2022). Environmental Pollution to Blame for Depressive Disorder? International Journal of Environmental Research and Public Health, 19(3), 1737. https://doi.org/10.3390/ijerph19031737









