Assessment of the Microbiological Quality of Ready-to-Eat Salads—Are There Any Reasons for Concern about Public Health?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Samples Preparation
2.2.2. Microbiological Analysis
2.2.3. Statistical Analysis
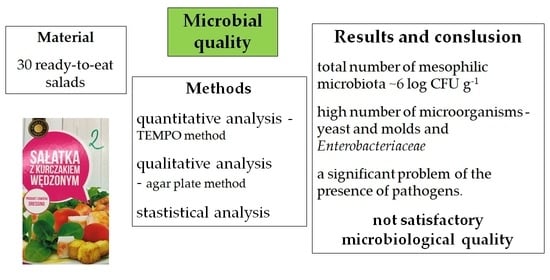
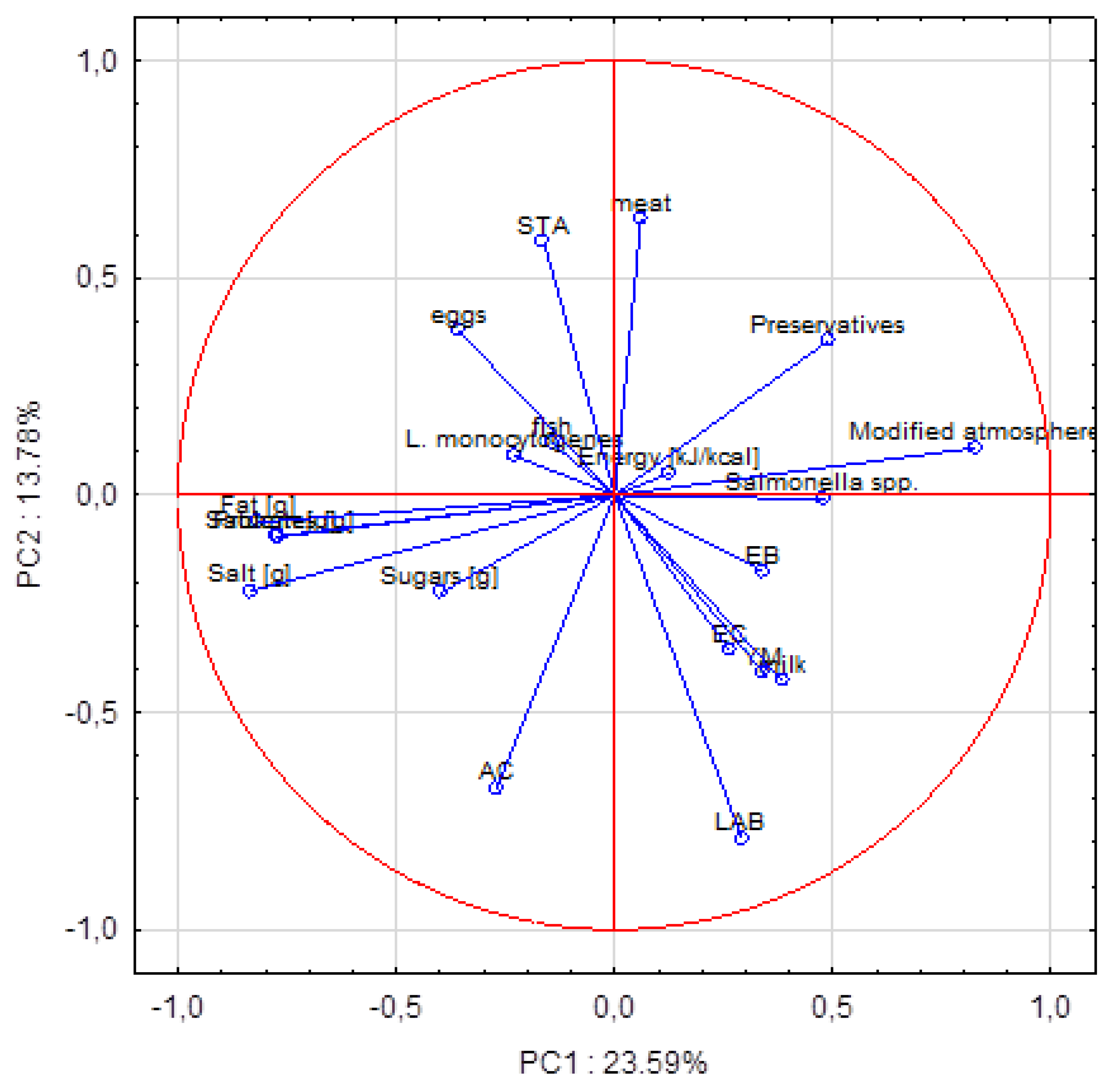
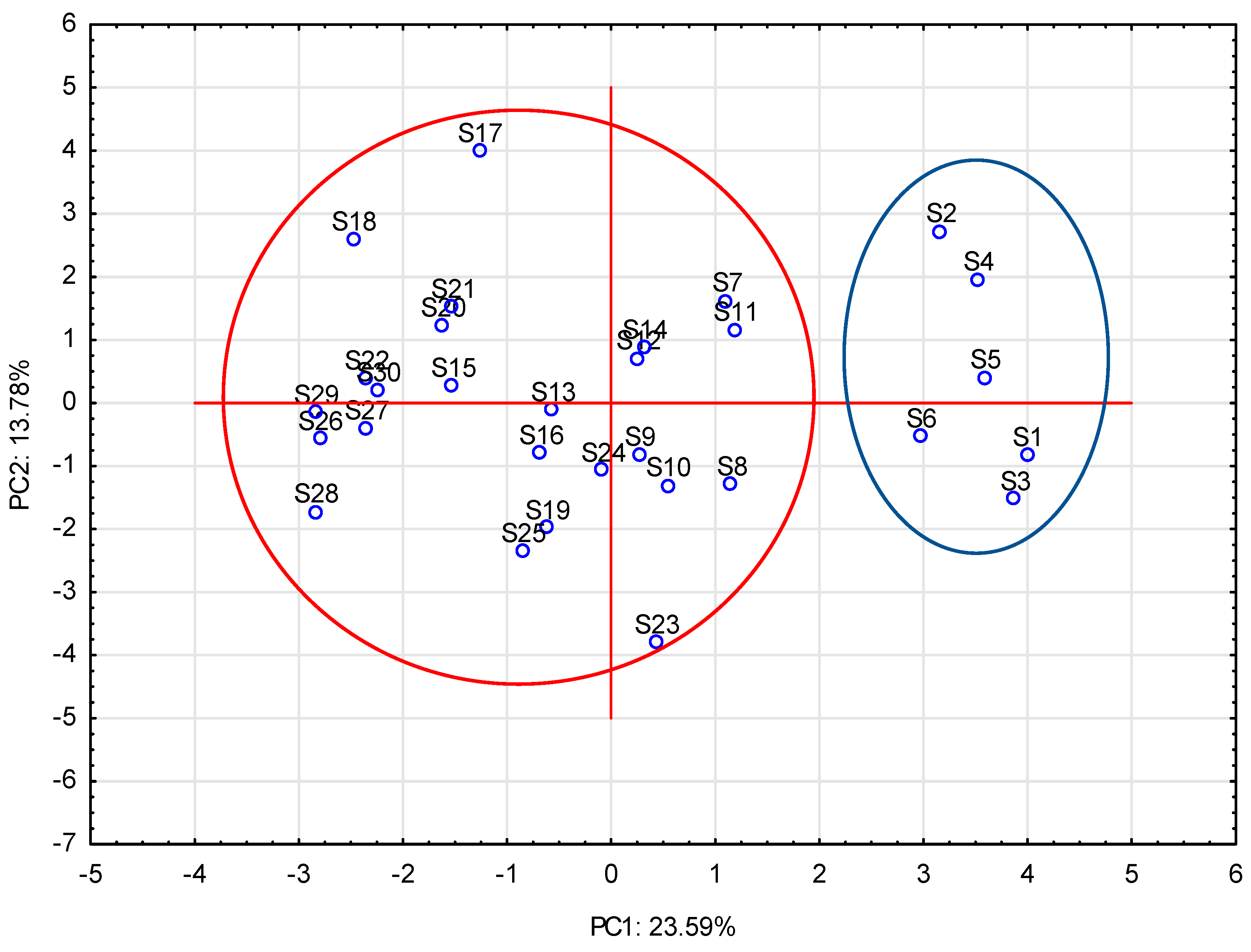
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tomasi, N.; Pinton, R.; Dalla Costa, L.; Cortella, G.; Terzano, R.; Mimmo, T.; Scampicchio, M.; Cesco, S. New ‘solutions’ for floating cultivation system of ready-to-eat salad: A review. Trends Food Sci. Technol. 2015, 46, 267–276. [Google Scholar] [CrossRef]
- Maffei, D.F.; Alvarenga, V.O.; Sant’Ana, A.S.; Franco, B.D. Assessing the effect of washing practices employed in Brazilian processing plants on the quality of ready-to-eat vegetables. LWT-Food Sci. Technol. 2016, 69, 474–481. [Google Scholar] [CrossRef]
- Castro-Ibáñez, I.; Gil, M.I.; Allende, A. Ready-to-eat vegetables: Current problems and potential solutions to reduce microbial risk in the production chain. LWT-Food Sci. Technol. 2017, 85, 284–292. [Google Scholar] [CrossRef]
- Raffo, A.; Senatore, M.; Moneta, E.; Paoletti, F.; Peparaio, M.; Saggia Civitelli, E. Impact of different temperature abuse scenarios on sensory quality and off-odour formation in ready-to-eat salad leaves. Int. J. Food Sci. Technol. 2021, 56, 2345–2356. [Google Scholar] [CrossRef]
- Castrica, M.; Andoni, E.; Curone, G.; Copelotti, E.; Massacci, F.R.; Terio, V.; Colombo, S.; Balzaretti, C.M. Prevalence of Listeria monocytogenes and Salmonella spp. in Different Ready to Eat Foods from Large Retailers and Canteens over a 2-Year Period in Northern Italy. Int. J. Environ. Res. Public Health 2021, 18, 10568. [Google Scholar] [CrossRef]
- COMMISSION REGULATION (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02005R2073-20140601&from=DA (accessed on 2 December 2021).
- COMMISSION REGULATION (EC) No 1441/2007 of 5 December 2007 Amending Regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuffs. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:322:0012:0029:EN:PDF (accessed on 2 December 2021).
- World Health Organization. Salmonella (Non-Typhoidal). Available online: https://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal) (accessed on 10 November 2021).
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 5500. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Technical Report. Eleventh External Quality Assessment Scheme for Salmonella Typing. Stockholm, October 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/eleventh-external-quality-assessment-scheme-salmonella-typing (accessed on 10 November 2021).
- World Health Organization. Listeriosis. Available online: https://www.who.int/news-room/fact-sheets/detail/listeriosis (accessed on 10 November 2021).
- European Centre for Disease Prevention and Control. Technical Report. Seventh External Quality Assessment Scheme for Listeria monocytogenes Typing. Stockholm, June 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/listeria-monocytogenes-typing-seventh-external-quality-assessment-scheme (accessed on 10 November 2021).
- EFSA BIOHAZ Panel. Scientific Opinion on the Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16, 5134. [Google Scholar] [CrossRef]
- Bernardo, R.; Duarte, A.; Tavares, L.; Barreto, A.S.; Henriques, A.R. Listeria monocytogenes Assessment in a Ready-to-Eat Salad Shelf-Life Study Using Conventional Culture-Based Methods, Genetic Profiling, and Propidium Monoazide Quantitative PCR. Foods 2021, 10, 235. [Google Scholar] [CrossRef]
- Koushki, M.; Koohy-Kamaly, P.; Sohrabvandi, S.; Mehrabi, S. Assessment of the Microbial Quality of Industrial Ready-to-Eat Salads Containing Meat Products. Curr. Res. Nutr. Food Sci. 2021, 9, 662–670. [Google Scholar] [CrossRef]
- Okafor-Elenwo, E.J.; Imade, O.S. Ready-to-eat vegetable salads served in Nigerian restaurants: A potential source of multidrug-resistant bacteria. J. Appl. Microbiol. 2020, 129, 1402–1409. [Google Scholar] [CrossRef]
- Zhou, S.Y.D.; Wei, M.Y.; Giles, M.; Neilson, R.; Zheng, F.; Zhang, Q.; Zhu, Y.G.; Yang, X.R. Prevalence of antibiotic resistome in ready-to-eat salad. Front. Public Health 2020, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- COMMISSION REGULATION (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the Hygiene of Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R0852&from=en (accessed on 2 December 2021).
- Xylia, P.; Botsaris, G.; Skandamis, P.; Tzortzakis, N. Expiration Date of Ready-to-Eat Salads: Effects on Microbial Load and Biochemical Attributes. Foods 2021, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- ISO 6579-1:2017/AMD 1:2020; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp.—Amendment 1: Broader Range of Incubation Temperatures, Amendment to the Status of Annex D, and Correction of the Composition of MSRV and SC. Available online: https://www.iso.org/standard/76671.html (accessed on 2 December 2021).
- ISO 11290-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method. Available online: https://www.iso.org/standard/60313.html (accessed on 2 December 2021).
- Bovo, F.; De Cesare, A.; Manfreda, G.; Bach, S.; Delaquis, P. Fate of Salmonella enterica in a mixed ingredient salad containing lettuce, cheddar cheese, and cooked chicken meat. J. Food Prot. 2015, 78, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Feroz, F.; Senjuti, J.D.; Noor, R. Determination of microbial growth and survival in salad vegetables through in vitro challenge test. Int. J. Nutr. Food Sci. 2013, 2, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.; Abadias, M.; Usall, J.; Torres, R.; Teixidó, N.; Viñas, I. Application of modified atmosphere packaging as a safety approach to fresh-cut fruits and vegetables—A review. Trends Food Sci. Technol. 2015, 46, 13–26. [Google Scholar] [CrossRef]
- Lokerse, R.F.A.; Maslowska-Corker, K.A.; Van de Wardt, L.C.; Wijtzes, T. Growth capacity of Listeria monocytogenes in ingredients of ready-to-eat salads. Food Control 2016, 60, 338–345. [Google Scholar] [CrossRef]
- Söderqvist, K.; Lambertz, S.T.; Vågsholm, I.; Boqvist, S. Foodborne bacterial pathogens in retail prepacked ready-to-eat mixed ingredient salads. J. Food Prot. 2016, 79, 978–985. [Google Scholar] [CrossRef]
- Söderqvist, K.; Lambertz, S.T.; Vågsholm, I.; Fernström, L.L.; Alsanius, B.; Mogren, L.; Boqvist, S. Fate of Listeria monocytogenes, Pathogenic Yersinia enterocolitica, and Escherichia coli O157:H7 gfp+ in Ready-to-Eat Salad during Cold Storage: What Is the Risk to Consumers? J. Food Prot. 2017, 80, 204–212. [Google Scholar] [CrossRef]
- Tsironi, T.; Dermesonlouoglou, E.; Giannoglou, M.; Gogou, E.; Katsaros, G.; Taoukis, P. Shelf-life prediction models for ready-to-eat fresh cut salads: Testing in real cold chain. Int. J. Food Microbiol. 2017, 240, 131–140. [Google Scholar] [CrossRef]
- Caggiano, G.; Diella, G.; Trerotoli, P.; Lopuzzo, M.; Triggiano, F.; Ricci, M.; Marcotrigiano, V.; Montagna, M.T.; De Giglio, O. A Pilot Survey on Hygienic–Sanitary Characteristics of Ready-To-Eat Sauces and Pesto. Int. J. Environ. Res. Public Health 2020, 17, 5005. [Google Scholar] [CrossRef]
- Szymczak, B.; Szymczak, M.; Trafiałek, J. Prevalence of Listeria species and L. monocytogenes in ready-to-eat foods in the West Pomeranian region of Poland: Correlations between the contamination level, serogroups, ingredients, and producers. Food Microbiol. 2020, 91, 103532. [Google Scholar] [CrossRef] [PubMed]
- Chaves, R.D.; Ruiz Martinez, C.R.; Bortolossi Rezende, A.C.; Dias Rocha, M.; Oteiza, J.M.; de Souza Sant’Ana, A. Salmonella and Listeria monocytogenes in ready-to-eat leafy vegetables. In Food Hygiene and Toxicology in Ready-to-Eat Foods, 1st ed.; Kotzekidou, P., Ed.; Academic Press: Cambridge, MA, USA, 2016; Chapter 8; pp. 123–149. [Google Scholar] [CrossRef]
- Neri, D.; Antoci, S.; Iannetti, L.; Ciorba, A.B.; D’Aurelio, R.; Del Matto, I.; Di Leonardo, M.; Giovannini, A.; Prencipe, V.A.; Pomilio, F.; et al. EU and US control measures on Listeria monocytogenes and Salmonella spp. in certain ready-to-eat meat products: An equivalence study. Food Control 2019, 96, 98–103. [Google Scholar] [CrossRef]
- Berthold-Pluta, A.; Garbowska, M.; Stefańska, I.; Pluta, A. Microbiological quality of selected ready-to-eat leaf vegetables, sprouts and non-pasteurized fresh fruit-vegetable juices including the presence of Cronobacter spp. Food Microbiol. 2017, 65, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Jeddi, M.Z.; Yunesian, M.; Gorji, M.E.H.; Noori, N.; Pourmand, M.R.; Khaniki, G.R.J. Microbial evaluation of fresh, minimally-processed vegetables and bagged sprouts from chain supermarkets. J. Health Popul. Nutr. 2014, 32, 391. Available online: https://pubmed.ncbi.nlm.nih.gov/25395902/ (accessed on 2 December 2021). [PubMed]
- Campos, J.; Mourão, J.; Pestana, N.; Peixe, L.; Novais, C.; Antunes, P. Microbiological quality of ready-to-eat salads: An underestimated vehicle of bacteria and clinically relevant antibiotic resistance genes. Int. J. Food Microbiol. 2013, 166, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Leff, J.W.; Fierer, N. Bacterial communities associated with the surfaces of fresh fruits and vegetables. PLoS ONE 2013, 8, e59310. [Google Scholar] [CrossRef] [Green Version]
- Faour-Klingbeil, D.; Todd, E.C.; Kuri, V. Microbiological quality of ready-to-eat fresh vegetables and their link to food safety environment and handling practices in restaurants. LWT 2016, 74, 224–233. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, M.A.; De Souza, V.M.; Bergamini, A.M.M.; De Martinis, E.C.P. Microbiological quality of ready-to-eat minimally processed vegetables consumed in Brazil. Food Control 2011, 22, 1400–1403. [Google Scholar] [CrossRef]
- Abakari, G.; Cobbina, S.J.; Yeleliere, E. Microbial quality of ready-to-eat vegetable salads vended in the central business district of Tamale, Ghana. Int. J. Food Contam. 2018, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Sant’Ana, A.S.; Barbosa, M.S.; Destro, M.T.; Landgraf, M.; Franco, B.D. Growth potential of Salmonella spp. and Listeria monocytogenes in nine types of ready-to-eat vegetables stored at variable temperature conditions during shelf-life. Int. J. Food Microbiol. 2012, 157, 52–58. [Google Scholar] [CrossRef]
- Caponigro, V.; Ventura, M.; Chiancone, I.; Amato, L.; Parente, E.; Piro, F. Variation of microbial load and visual quality of ready-to-eat salads by vegetable type, season, processor and retailer. Food Microbiol. 2010, 27, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Pothakos, V.; Snauwaert, C.; De Vos, P.; Huys, G.; Devlieghere, F. Monitoring psychrotrophic lactic acid bacteria contamination in a ready-to-eat vegetable salad production environment. Int. J. Food Microbiol. 2014, 185, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Abadias, M.; Usall, J.; Anguera, M.; Solsona, C.; Viñas, I. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. Int. J. Food Microbiol. 2008, 123, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, P.; Wojdyło, A.; Oszmiański, J. Zagrożenia powstające w żywności minimalnie przetworzonej i skuteczne metody ich eliminacji. Żywn. Nauka Technol. Jakość 2014, 2, 5–18. [Google Scholar] [CrossRef]
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef]
- Aung, K.T.; Chen, H.J.; Chau, M.L.; Yap, G.; Lim, X.F.; Humaidi, M.; Chua, C.; Yeo, G.; Yap, H.M.; Oh, J.Q.; et al. Salmonella in retail food and wild birds in Singapore—Prevalence, antimicrobial resistance, and sequence types. Int. J. Environ. Res. Public Health 2019, 16, 4235. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, M.; Chikindas, M.L. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef]
- Doijad, S.P.; Barbuddhe, S.B.; Garg, S.; Poharkar, K.V.; Kalorey, D.R.; Kurkure, N.V.; Rawool, D.B.; Chakraborty, T. Biofilm-forming abilities of Listeria monocytogenes serotypes isolated from different sources. PLoS ONE 2015, 10, e0137046. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Wong, C.; Chung, H.J.; Yuk, H.G. Biofilm formation of Listeria monocytogenes and its resistance to quaternary ammonium compounds in a simulated salmon processing environment. Food Control 2019, 98, 200–208. [Google Scholar] [CrossRef]
- Churchill, K.J.; Sargeant, J.M.; Farber, J.M.; O’Connor, A.M. Prevalence of Listeria monocytogenes in Select Ready-to-Eat Foods-Deli Meat, Soft Cheese, and Packaged Salad: A Systematic Review and Meta-Analysis. J. Food Prot. 2019, 82, 344–357. [Google Scholar] [CrossRef]
- EFSA. Analysis of the baseline survey on the prevalence of Listeria monocytogenes in certain ready-to-eat (RTE) foods in the EU, 2010–2011 Part A: Listeria monocytogenes prevalence estimates. EFSA J. 2013, 11, 3241. [Google Scholar] [CrossRef]
- Gurler, Z.; Pamuk, S.; Yildirim, Y.; Ertas, N. The microbiological quality of ready-to-eat salads in Turkey: A focus on Salmonella spp. and Listeria monocytogenes. Int. J. Food Microbiol. 2015, 196, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Koseki, S.; Mizuno, Y.; Kawasaki, S.; Yamamoto, K. A survey of iceberg lettuce for the presence of Salmonella, Escherichia coli O157:H7, and Listeria monocytogenes in Japan. J. Food Prot. 2011, 74, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Althaus, D.; Hofer, E.; Corti, S.; Julmi, A.; Stephan, R. Bacteriological survey of ready-to-eat lettuce, fresh-cut fruit, and sprouts collected from the Swiss market. J. Food Prot. 2012, 75, 1338–1341. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization; World Health Organization. Microbiological Hazards in Fresh Fruits and Vegetables. Meeting Report. Microbiological Risk Assessment Series No. 14. 2008, Rome, 1–28. Available online: https://www.fao.org/3/i0452e/i0452e.pdf (accessed on 2 December 2021).
- De Sousa, G.B.; Tamagnini, L.M.; Olmos, P.; Gonzalez, R.D. Microbial enumeration in ready-to-eat foods and their relationship to good manufacturing practice. J. Food Saf. 2002, 22, 27–38. [Google Scholar] [CrossRef]
- Bernardo, R.; Barreto, A.S.; Nunes, T.; Henriques, A.R. Estimating Listeria monocytogenes Growth in Ready-to-Eat Chicken Salad Using a Challenge Test for Quantitative Microbial Risk Assessment. Risk Anal. 2020, 40, 2427–2441. [Google Scholar] [CrossRef]
- Caleb, O.J.; Mahajan, P.V.; Al-Said, F.A.J.; Opara, U.L. Modified atmosphere packaging technology of fresh and fresh-cut produce and the microbial consequences—A review. Food Bioprocess Technol. 2013, 6, 303–329. [Google Scholar] [CrossRef]
- Putnik, P.; Kovačević, D.B.; Herceg, K.; Roohinejad, S.; Greiner, R.; Bekhit, A.E.D.A.; Levaj, B. Modelling the shelf-life of minimally-processed fresh-cut apples packaged in a modified atmosphere using food quality parameters. Food Control 2017, 81, 55–64. [Google Scholar] [CrossRef]
- Putnik, P.; Roohinejad, S.; Greiner, R.; Granato, D.; Bekhit, A.E.D.A.; Kovačević, D.B. Prediction and modeling of microbial growth in minimally processed fresh-cut apples packaged in a modified atmosphere: A review. Food Control 2017, 80, 411–419. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M.; Dar, B.N.; Greiner, R.; Roohinejad, S. Microbiological contamination of ready-to-eat vegetable salads in developing countries and potential solutions in the supply chain to control microbial pathogens. Food Control 2018, 85, 235–244. [Google Scholar] [CrossRef]
- European Food Safety Authority. Training in tools to develop Quantitative Risk Assessment using Spanish ready-to-eat food examples. EFSA J. 2020, 18, e181103. [Google Scholar] [CrossRef]
- Ziegler, M.; Kent, D.; Stephan, R.; Guldimann, C. Growth potential of Listeria monocytogenes in twelve different types of RTE salads: Impact of food matrix, storage temperature and storage time. Int. J. Food Microbiol. 2019, 296, 83–92. [Google Scholar] [CrossRef] [PubMed]
| Symbol and Name | Ingredients of Vegetable Origin | Ingredients of Animal Origin |
|---|---|---|
| S1—Mediterranean | Iceberg lettuce, beet leaves, cherry tomatoes, olive oil, black olives | Mediterranean cheese |
| S2—Smoked chicken | Lettuce, cherry tomatoes, dressing, toast | Smoked chicken |
| S3—Mozzarella | Lettuce, chicory, arugula, cocktail tomatoes, olive oil, toast, spices, sunflower oil, salt | Mozzarella cheese |
| S4—Vegetable salmon | Lettuce, cherry tomatoes | Smoked salmon, yoghurt |
| S5—Vegetable with blue cheese | Lettuce, radicchio lettuce, pumpkin seeds, cranberry sauce, cranberry, beetroot concentrate | Blue cheese |
| S6—Vegetable with mozzarella | Lettuce, chicory, radicchio, rocket, red pepper, fennel, garlic, dried tomatoes, sauce | Unripened rennet cheese |
| S7—With eggs and croutons | Cabbage, croutons, cherry tomatoes, salt, pepper, sugar, oregano, garlic | Boiled egg, ham, yoghurt, cream, mayonnaise |
| S8—Sicilian lunch | Cabbage, carrot, pepper, cucumber, sweet corn, red beans, olives, spices | Feta, yogurt, mayonnaise |
| S9—Caribbean lunch | Cabbage, peach, raisins, sunflower seeds, pumpkin seeds, corn, carrots, spices, soy sauce, pineapple, garlic | Mayonnaise sauce, natural yoghurt |
| S10—Italian lunch | Cabbage, lettuce, radish, cucumber, olives, toast, carrots, oil, spices | Cheese |
| S11—Pollo penne | Pasta, lettuce, cucumber, pepper, onion, spinach, capers, red cabbage | Roast chicken, sauce |
| S12—Gyros lunch | Lettuce, corn, cucumber, red cabbage, pepper, carrots, radish, onion | Chicken |
| S13—Indian lunch | Lettuce, lentils, tomato, dried tomato, celery, onion, sprouts and sunflower seeds, radish, sauce | - |
| S14—Fit | Cabbage, carrots, corn, peppers, fresh cucumber | Cheese, ham, Caesar sauce |
| S15—Tuna | Cabbage, sweet corn, pepper, canned peas | Mayonnaise, tuna paste |
| S16—Potato | Potatoes, cucumber, pickled peppers, onion, leek, spices | Mayonnaise |
| S17—Golden | Corn, peach, pineapple | Canned ham, mayonnaise |
| S18—Home | Carrots, potatoes, pickled peas, pickled cucumber, spices | Boiled egg, mayonnaise |
| S19—Athenian | Chinese cabbage, olives, canned peas, canned peppers, corn, vinaigrette | Feta cheese |
| S20—Classic | Chinese cabbage, canned peas, canned peppers, corn | Ham, cheese, mayonnaise |
| S21—Gyros | Chinese cabbage, pickled peas, pickled peppers, pineapple, corn | Chicken, gyros sauce |
| S22—Classic tuna | Chinese cabbage, canned peas, canned peppers, corn, horseradish sauce | Tuna |
| S23—Greek | Iceberg lettuce, cucumber, pepper, olives, onion, vinaigrette dressing | Feta cheese |
| S24—Balkan | Chinese cabbage, cucumber, pepper, carrots, onion | Feta, 1000 islands sauce |
| S25—Greek lunch | Iceberg lettuce, tomato, olives, arugula, cucumber, tomato, onion, sauce | Feta |
| S26—Fit oriental lunch | Iceberg lettuce, Chinese cabbage, Italian cabbage, lettuce, onion, pepper, carrots, pineapple, sunflower sprouts, rucola, sesame, vinaigrette sauce | Crab sticks |
| S27—Fit Italian lunch | Chinese cabbage, iceberg lettuce, cabbage, lettuce, onion, pepper, carrots, cucumber, corn, white bean, cherry tomato | Mozzarella cheese, yoghurt sauce |
| S28—Fit Greek lunch | Chinese cabbage, iceberg lettuce, tomatoes, white cabbage, onion, pepper, cucumber, corn, carrots, olives, red beans, spices, vinaigrette | Feta cheese |
| S29—Mexican | White cabbage, Chinese cabbage, carrots, corn, green peas, beans, spices | Mayonnaise |
| S30—Delecta | White cabbage, apples, leeks, carrots, spices | Mayonnaise |
| Salad Symbol | Nutritional Value in 100 g of Product | Shelf Life [day] | Storage [°C] | Modified Atmosphere [+/−] | Preservatives [+/−] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Energy [kJ/kcal] | Fat [g] | Saturates [g] | Sugars [g] | Protein [g] | Salt [g] | |||||
| S1 | 551/133 | 12.0 | 4.2 | 1.9 | 3.9 | 0.8 | 3 | 1–7 | + | − |
| S2 | 419/100 | 5.0 | 1.1 | 6.1 | 7.2 | 1.2 | 5 | 1–7 | + | + |
| S3 | 662/160 | 12.0 | 4.2 | 5.3 | 5.9 | 0.3 | 5 | 1–7 | + | − |
| S4 | 397/96 | 6.6 | 0.6 | 2.8 | 5.5 | 0.7 | 1 | 1–6 | + | + |
| S5 | 733/176 | 11.5 | 8.0 | 8.4 | 8.8 | 0.3 | 1 | 1–6 | + | + |
| S6 | 334/80 | 3.4 | 1.4 | 5.9 | 5.4 | 1.8 | 1 | 1–6 | + | − |
| S7 | 665/154 | 9.5 | 4.5 | 15.0 | 2.0 | 0.6 | 6 | 1–8 | − | − |
| S8 | 253/61 | 3.0 | 0.7 | 4.4 | 2.5 | 0.6 | 2 | 1–6 | − | − |
| S9 | 665/160 | 10.3 | 1.4 | 11.1 | 4.5 | 0.4 | 2 | 1–6 | − | − |
| S10 | 470/113 | 8.3 | 1.9 | 5.0 | 3.4 | 0.8 | 2 | 1–6 | − | − |
| S11 | 896/214 | 14.8 | 8.5 | 18.0 | 2.2 | 1.8 | 7 | 1–8 | − | − |
| S12 | 439/105 | 6.3 | 0.8 | 4.5 | 7.2 | 0.3 | 2 | 1–8 | − | − |
| S13 | 452/108 | 6.0 | 1.6 | 9.0 | 3.0 | 0.8 | 6 | 0–7 | − | − |
| S14 | 419/100 | 4.5 | 3.5 | 8.4 | 6.5 | 1.5 | 6 | 2–7 | − | + |
| S15 | 406/97 | 5.4 | 4.4 | 3.0 | 9.2 | 1.7 | 1 | 2–7 | − | − |
| S16 | 389/93 | 3.5 | 0.4 | 14.5 | 1.0 | 1.2 | 1 | 0–7 | − | − |
| S17 | 574/137 | 6.6 | 1.3 | 16.0 | 3.5 | 1.8 | 6 | 0–10 | − | − |
| S18 | 389/93 | 4.6 | 0.3 | 11.8 | 1.2 | 1.2 | 3 | 0–4 | − | − |
| S19 | 447/108 | 9.0 | 1.4 | 10.8 | 2.7 | 1.0 | 3 | 0–8 | − | − |
| S20 | 461/111 | 8.4 | 1.9 | 5.9 | 4.6 | 0.7 | 2 | 1–8 | − | − |
| S21 | 395/95 | 6.9 | 0.7 | 7.1 | 3.1 | 0.4 | 2 | 1–8 | − | − |
| S22 | 345/83 | 5.5 | 0.5 | 12.2 | 4.5 | 0.5 | 2 | 1–8 | − | − |
| S23 | 440/105 | 9.5 | 3.5 | 3.2 | 3.0 | 1.7 | 3 | 2–4 | − | − |
| S24 | 276/66 | 5.1 | 0.8 | 4.5 | 2.0 | 1.2 | 3 | 2–4 | − | − |
| S25 | 553/134 | 12.0 | 3.3 | 2.9 | 3.2 | 0.6 | 3 | 2–4 | − | − |
| S26 | 528/126 | 9.1 | 4.6 | 3.3 | 7.6 | 0.9 | 3 | 0–8 | − | − |
| S27 | 391/93 | 2.6 | 0.4 | 9.8 | 7.3 | 0.8 | 3 | 0–8 | − | − |
| S28 | 645/154 | 13.5 | 4.1 | 4.9 | 1.6 | 1.1 | 3 | 0–8 | − | − |
| S29 | 620/148 | 9.1 | 3.6 | 13.0 | 2.0 | 1.6 | 1 | 1–4 | − | − |
| S30 | 590/141 | 9.6 | 1.1 | 11.1 | 1.8 | 0.3 | 1 | 1–4 | − | − |
| Salad Symbol | Count of Microorganisms [log CFU g−1] | Presence [+/−] | ||||||
|---|---|---|---|---|---|---|---|---|
| AC | STA | EB | EC | LAB | YM | SAL | LM | |
| S1 | 5.00 ± 0.11 | <1.00 | 3.91 ± 0.23 | <1.00 | 8.43 ± 0.17 | 3.60 ± 0.00 | + | − |
| S2 | 5.00 ± 0.00 | <1.00 | 3.83 ± 0.01 | <1.00 | <1.00 | 4.32 ± 0.20 | − | − |
| S3 | 6.98 ± 0.12 | <1.00 | 4.04 ± 0.06 | 2.11 ± 0.01 | 7.10 ± 0.01 | 5.00 ± 0.00 | + | − |
| S4 | 2.36 ± 0.06 | <1.00 | 2.77 ± 0.13 | <1.00 | 2.45 ± 0.10 | 2.00 ± 0.00 | + | − |
| S5 | 3.89 ± 0.07 | <1.00 | 2.70 ± 0.00 | 2.69 ± 0.02 | 3.26 ± 0.00 | 6.00 ± 0.00 | − | − |
| S6 | 5.00 ± 0.00 | <1.00 | 4.57 ± 0.02 | 5.00 ± 0.00 | 3.22 ± 0.00 | 2.42 ± 0.01 | + | − |
| S7 | 3.26 ± 0.00 | 2.00 ± 0.00 | 6.32 ± 0.00 | <1.00 | 4.48 ± 0.00 | 6.00 ± 0.00 | − | + |
| S8 | 8.23 ± 0.16 | <1.00 | 3.89 ± 0.00 | 4.08 ± 0.02 | 2.48 ± 0.00 | 6.00 ± 0.00 | + | − |
| S9 | 7.91 ± 0.01 | <1.00 | 6.83 ± 0.00 | <1.00 | 4.69 ± 0.13 | 3.96 ± 0.08 | − | + |
| S10 | 7.18 ± 0.00 | <1.00 | 7.48 ± 0.12 | <1.00 | 3.67 ± 0.27 | 6.00 ± 0.05 | − | + |
| S11 | 7.76 ± 0.04 | <1.00 | 5.00 ± 0.00 | 2.48 ± 0.12 | 1.86 ± 0.00 | 2.91 ± 0.19 | − | − |
| S12 | 6.32 ± 0.00 | <1.00 | 5.00 ± 0.00 | 2.99 ± 0.05 | 1.00 ± 0.00 | 5.00 ± 0.00 | − | + |
| S13 | 6.52 ± 0.00 | <1.00 | 6.32 ± 0.12 | 2.74 ± 0.24 | 1.00 ± 0.00 | 3.11 ± 0.00 | − | + |
| S14 | 6.52 ± 0.00 | <1.00 | 6.00 ± 0.00 | 4.08 ± 0.00 | 2.51 ± 0.19 | 2.00 ± 0.00 | − | − |
| S15 | 6.66 ± 0.10 | <1.00 | 6.36 ± 0.14 | 2.90 ± 0.00 | 2.33 ± 0.00 | 2.00 ± 0.02 | − | + |
| S16 | 8.64 ± 0.06 | <1.00 | <1.00 | <1.00 | 5.0 ± 0.00 | 7.00 ± 0.67 | + | + |
| S17 | 4.95 ± 0.01 | 3.54 ± 0.00 | 2.95 ± 0.10 | <1.00 | <1.00 | 2.00 ± 0.00 | + | + |
| S18 | 4.04 ± 0.00 | 2.91 ± 0.03 | <1.00 | <1.00 | <1.00 | 1.00 ± 0.00 | − | − |
| S19 | 8.12 ± 0.58 | <1.00 | 2.08 ± 0.00 | 1.80 ± 0.00 | 6.60 ± 0.02 | 2.30 ± 0.00 | − | − |
| S20 | 6.54 ± 0.00 | <1.00 | 1.30 ± 0.05 | 1.00 ± 0.00 | 1.30 ± 0.05 | <1.00 | − | − |
| S21 | 4.45 ± 0.05 | <1.00 | <1.00 | <1.00 | <1.00 | 4.30 ± 0.02 | − | − |
| S22 | 6.30 ± 0.00 | <1.00 | <1.00 | <1.00 | 1.55 ± 0.00 | 3.14 ± 0.08 | − | + |
| S23 | 9.30 ± 0.20 | <1.00 | 6.84 ± 0.50 | 5.55 ± 0.05 | 7.80 ± 0.50 | 6.60 ± 0.00 | − | − |
| S24 | 5.12 ± 0.43 | <1.00 | 3.12 ± 0.03 | 2.30 ± 0.01 | 4.40 ± 0.03 | 3.01 ± 0.06 | + | − |
| S25 | 7.90 ± 0.00 | <1.00 | 2.34 ± 0.00 | 1.22 ± 0.08 | 5.90 ± 0.04 | 5.80 ± 0.00 | − | − |
| S26 | 6.86 ± 0.00 | <1.00 | 5.00 ± 0.12 | <1.00 | 3.70 ± 0.10 | 2.12 ± 0.04 | − | − |
| S27 | 5.30 ± 0.10 | <1.00 | 4.14 ± 0.02 | <1.00 | 2.60 ± 0.00 | <1.00 | − | − |
| S28 | 6.99 ± 0.01 | <1.00 | <1.00 | <1.00 | 5.63 ± 0.07 | 4.38 ± 0.14 | − | + |
| S29 | 7.24 ± 0.00 | <1.00 | <1.00 | <1.00 | 2.45 ± 0.30 | 4.16 ± 0.02 | − | − |
| S30 | 6.00 ± 0.00 | <1.00 | 4.38 ± 0.01 | 2.90 ± 0.12 | 1.90 ± 0.00 | 1.18 ± 0.03 | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łepecka, A.; Zielińska, D.; Szymański, P.; Buras, I.; Kołożyn-Krajewska, D. Assessment of the Microbiological Quality of Ready-to-Eat Salads—Are There Any Reasons for Concern about Public Health? Int. J. Environ. Res. Public Health 2022, 19, 1582. https://doi.org/10.3390/ijerph19031582
Łepecka A, Zielińska D, Szymański P, Buras I, Kołożyn-Krajewska D. Assessment of the Microbiological Quality of Ready-to-Eat Salads—Are There Any Reasons for Concern about Public Health? International Journal of Environmental Research and Public Health. 2022; 19(3):1582. https://doi.org/10.3390/ijerph19031582
Chicago/Turabian StyleŁepecka, Anna, Dorota Zielińska, Piotr Szymański, Izabela Buras, and Danuta Kołożyn-Krajewska. 2022. "Assessment of the Microbiological Quality of Ready-to-Eat Salads—Are There Any Reasons for Concern about Public Health?" International Journal of Environmental Research and Public Health 19, no. 3: 1582. https://doi.org/10.3390/ijerph19031582
APA StyleŁepecka, A., Zielińska, D., Szymański, P., Buras, I., & Kołożyn-Krajewska, D. (2022). Assessment of the Microbiological Quality of Ready-to-Eat Salads—Are There Any Reasons for Concern about Public Health? International Journal of Environmental Research and Public Health, 19(3), 1582. https://doi.org/10.3390/ijerph19031582