Geographic and Ethnic Variations in Serum Concentrations of Legacy Persistent Organic Pollutants among Men in the Nenets Autonomous Okrug, Arctic Russia
Abstract
1. Introduction
2. Materials and Methods
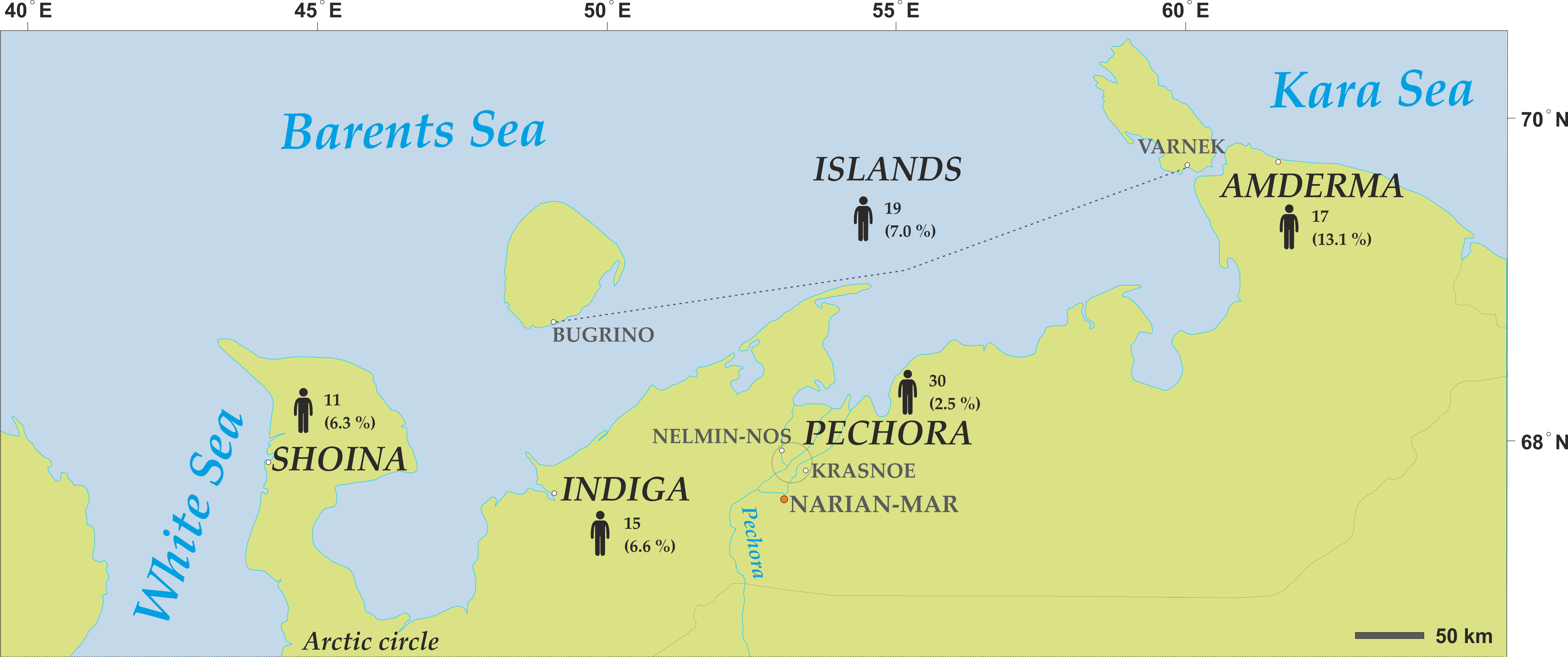
2.1. Study Design
2.2. Serum Samples
2.3. Determination of Contaminants and Total Lipids
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Serum concentrations of Lipophilic POPs
3.3. Geographic Variations in Serum Concentration of POPs
3.3.1. Crude Analysis
3.3.2. Adjusted Analysis
3.4. Ethnic Variations in Serum Concentration of POPs
3.4.1. Crude Analysis
3.4.2. Adjusted Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vested, A.; Giwercman, A.; Bonde, J.P.; Toft, G. Persistent organic pollutants and male reproductive health. Asian J. Androl. 2014, 16, 71–80. [Google Scholar] [CrossRef]
- Wania, F.; MacKay, D. Peer Reviewed: Tracking the Distribution of Persistent Organic Pollutants. Environ. Sci. Technol. 1996, 30, 390A–396A. [Google Scholar] [CrossRef] [PubMed]
- AMAP. AMAP Assessment 2009: Human Health in the Arctic; Arctic Monitoring and Assessment Programme (AMAP): Oslo, Norway, 2009; p. xiv + 256. [Google Scholar]
- Dudarev, A.A. Persistent polychlorinated hydrocarbons and heavy metals in the Arctic biosphere: The main patterns of exposure and reproductive health of indigenous people. Biosphere 2009, 1, 186–202. [Google Scholar]
- Tsygankov, V.Y.; Boyarova, M.D.; Donets, M.M.; Gumovskaya, Y.P.; Gumovskiy, A.N.; Koval, I.P.; Khristoforova, N.K.; Kiku, P.F.; Usov, V.V. Persistent organic pollutants (POPs) in the body of residents of the Russian Far East coastal regions. In Persistent Organic Pollutants (POPs) in the Far Eastern Region: Seas. Organisms. Human: Monograph; Tsygankov, V.Y., Donets, M.M., Khristoforova, N.K., Gumovskaya, Y.P., Polevshchikov, A.V., Gumovsky, A.N., Boyarova, M.D., Chernyaev, A.P., Lyagusha, M.S., Busarova, O., Eds.; Publishing House of the Far Eastern Federal University: Vladivostok, Russia, 2020; pp. 317–338. [Google Scholar]
- AMAP. Biological Effects of Contaminants on Arctic Wildlife and Fish; Arctic Monitoring and Assessment Programme (AMAP): Tromsø, Norway, 2018. [Google Scholar]
- Bravo, N.; Grimalt, J.O.; Chashchin, M.; Chashchin, V.P.; Odland, J.Ø. Drivers of maternal accumulation of organohalogen pollutants in Arctic areas (Chukotka, Russia) and 4,4′-DDT effects on the newborns. Environ. Int. 2019, 124, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Varakina, Y.; Lahmanov, D.; Aksenov, A.; Trofimova, A.; Korobitsyna, R.; Belova, N.; Sobolev, N.; Kotsur, D.; Sorokina, T.; Grjibovski, A.M.; et al. Concentrations of Persistent Organic Pollutants in Women’s Serum in the European Arctic Russia. Toxics 2021, 9, 6. [Google Scholar] [CrossRef]
- Stockholm Convention on Persistent Organic Pollutants Was Adopted on 22 May 2001 in Stockholm. Sweden. Available online: http://chm.pops.int/Portals/0/Repository/convention_text/UNEP-POPS-COP-CONVTEXT-FULL.English.PDF (accessed on 19 May 2021).
- Laird, B.D.; Goncharov, A.B.; Chan, H.M. Body burden of metals and persistent organic pollutants among Inuit in the Canadian Arctic. Environ. Int. 2013, 59, 33–40. [Google Scholar] [CrossRef]
- Health Canada. Results of the Canadian Health Measures Survey Cycle 1 (2007–2009); Canadian Health Measures Survey: Ottawa, Canada, 2010; ISBN 78-1-100-15618-7. [Google Scholar]
- Deutch, B. Recent dietary studies in the Arctic AMAP 2003; ch 7: 75–87. Available online: https://www.amap.no/documents/download/181/inline (accessed on 1 December 2021).
- Glynn, A.W.; Wolk, A.; Aune, M.; Atuma, S.; Zettermark, S.; Mæhle-Schmid, M.; Darnerud, P.O.; Becker, W.; Vessby, B.; Adami, H.-O. Serum concentrations of organochlorines in men: A search for markers of exposure. Sci. Total Environ. 2000, 263, 197–208. [Google Scholar] [CrossRef]
- Zani, C.; Magoni, M.; Speziani, F.; Leonardi, L.; Orizio, G.; Scarcella, C.; Gaia, A.; Donato, F. Polychlorinated biphenyl serum levels, thyroid hormones and endocrine and metabolic diseases in people living in a highly polluted area in North Italy: A population-based study. Heliyon 2019, 5, e01870. [Google Scholar] [CrossRef]
- Sala, M.; Sunyer, J.; Otero, R.; Santiago-Silva, M.; Camps, C.; Grimalt, J. Organochlorine in the serum of inhabitants living near an electrochemical factory. Occup. Environ. Med. 1999, 56, 152–158. [Google Scholar] [CrossRef]
- Popova, E.K.; Arkhipova, N.S.; Popov, I.O. Risk predictors of coronary heart disease in men of older age groups living in the conditions of far north. Hum. Ecol. 2020, 2, 4–11. [Google Scholar] [CrossRef][Green Version]
- Svetlichnaya, T.G.; Vorobyeva, N.A. Lifestyle and Self-Percieved health of the Nenets population living on the arctic island of vaigach. Hum. Ecol. 2019, 12, 20–25. [Google Scholar] [CrossRef]
- Gumovskaya, Y.P.; Gumovskiy, A.N.; Tsygankov, V.; Polevshchikov, A.V. Persistent organic pollutants (POPs) in the human body: The experience of Russia and the ex-USSR republics. In Persistent Organic Pollutants (POPs) in the Far Eastern Region: Seas. Organisms. Human: Monograph; Tsygankov, V.Y., Donets, M.M., Khristoforova, N.K., Gumovskaya, Y.P., Polevshchikov, A.V., Gumovsky, A.N., Boyarova, M.D., Chernyaev, A.P., Lyagusha, M.S., Busarova, O.Y., et al., Eds.; Publishing House of the Far Eastern Federal University: Vladivostok, Russia, 2020; pp. 283–316. [Google Scholar]
- Moshansky, V.F.; Kagan, S.A.; Tektinsky, O.L. Differential diagnosis of two forms of necrospermia. Urol. Nephrol. 1987, 4, 57–59. [Google Scholar]
- Gromenko, D.S.; Galimov, S.N.; Shemagonov, D.V.; Farkhutdinov, R.R. The role of reactive oxygen species in the formation of male infertility. Kazan Med. J. 2007, 88, 23–24. [Google Scholar]
- Murugesan, P.; Senthilkumar, J.; Balasubramanian, K.; Aruldhas, M.M.; Arunakaran, J. Impact of polychlorinated biphenyl Aroclor 1254 on testicular antioxidant system in adult rats. Hum. Exp. Toxicol. 2005, 24, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Khurtsilava, O.G.; Chashchin, V.P.; Meltser, A.V.; Dardynskaia, I.V.; Erastova, N.V.; Chashchin, M.V.; Dardynskiy, O.A.; Bazilevskaya, E.M.; Belikova, T.M.; Kovshov, A.A.; et al. Pollution of the environment with persistent toxic substances and prevention of their harmful impact on the health of the indigenous population residing in the arctic zone of the Russian federation. Hyg. Sanit. 2017, 96, 409–414. [Google Scholar] [CrossRef]
- Sorokina, T.Y. A national system of biological monitoring in the Russian Arctic as a tool for the implementation of the Stockholm Convention. Int. Environ. Agreem. Politi. Law Econ. 2019, 19, 341–355. [Google Scholar] [CrossRef]
- Lakhmanov, D.; Varakina, Y.; Aksenov, A.; Sorokina, T.; Sobolev, N.; Kotsur, D.; Plakhina, E.; Chashchin, V.; Thomassen, Y. Persistent Organic Pollutants (POPs) in Fish Consumed by the Indigenous Peoples from Nenets Autonomous Okrug. Environments 2019, 7, 3. [Google Scholar] [CrossRef]
- Sobolev, N.; Ellingsen, D.G.; Belova, N.; Aksenov, A.; Sorokina, T.; Trofimova, A.; Varakina, Y.; Kotsur, D.; Grjibovski, A.M.; Chashchin, V.; et al. Essential and non-essential elements in biological samples of inhabitants residing in Nenets Autonomous Okrug of the Russian Arctic. Environ. Int. 2021, 152, 106510. [Google Scholar] [CrossRef]
- Sorokina, T.; Sobolev, N.; Ellingsen, D.G.; Aksenov, A.; Trofimova, A.; Varakina, Y.; Kotsur, D.; Grjibovski, A.M.; Chashchin, V.; Thomassen, Y. Essential and non-essential elements in whole blood and their association with diet and iron status: Results from an indigenous population study in Nenets Autonomous Okrug of the Russian Arctic. Environ. Int. 2021, submitted. [Google Scholar]
- Bernert, J.T.; Turner, W.E.; Patterson, D.G., Jr.; Needham, L.L. Calculation of serum “total lipid” concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere 2007, 68, 824–831. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.; Kim, H.-J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Moon, H.-B.; Kim, S.; et al. Association between Several Persistent Organic Pollutants and Thyroid Hormone Levels in Cord Blood Serum and Bloodspot of the Newborn Infants of Korea. PLoS ONE 2015, 10, e0125213. [Google Scholar] [CrossRef][Green Version]
- Dudarev, A.A.; Odland, J.O. Human health in connection with arctic pollution-results and perspectives of international studies under the aegis of AMAP. Hum. Ecol. 2017, 9, 3–14. [Google Scholar] [CrossRef]
- Odland, J.; Deutch, B.; Hansen, J.C.; Burkow, I.C. The importance of diet on exposure to and effects of persistent organic pollutants on human health in the Arctic. Acta Paediatr. 2007, 92, 1255–1266. [Google Scholar] [CrossRef]
- Kang, J.H.; Chang, Y.-S. Organochlorine Pesticides in Human Serum. In Pesticides—Strategies for Pesticides Analysis; Stoytcheva, M., Ed.; IntechOpen Publishing: Rijeka, Croatia, 2011; pp. 215–240. [Google Scholar]
- Rylander, C.; Sandanger, T.M.; Petrenya, N.; Konoplev, A.; Bojko, E.; Odland, J.Ø. Indications of decreasing human PTS concentrations in North West Russia. Glob. Health Action 2011, 4, 8427. [Google Scholar] [CrossRef][Green Version]
- Long, M.; Wielsøe, M.; Bonefeld-Jørgensen, E. Time Trend of Persistent Organic Pollutants and Metals in Greenlandic Inuit during 1994–2015. Int. J. Environ. Res. Public Health 2021, 18, 2774. [Google Scholar] [CrossRef]
- Porta, M.; Pumarega, J.; Henríquez-Hernández, L.A.; Gasull, M.; Bartoll, X.; Arrebola, J.P.; Morales, E.; Ibarluzea, J.; Alguacil, J.; Bilal, U.; et al. Reductions in blood concentrations of persistent organic pollutants in the general population of Barcelona from 2006 to 2016. Sci. Total. Environ. 2021, 777, 146013. [Google Scholar] [CrossRef]
- Singh, K.; Chan, H.M. Association of blood polychlorinated biphenyls and cholesterol levels among Canadian Inuit. Environ. Res. 2018, 160, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Pavuk, M.; Olson, J.; Sjödin, A.; Wolff, P.; Turner, W.; Shelton, C.; Dutton, N.; Bartell, S. Serum concentrations of polychlorinated biphenyls (PCBs) in participants of the Anniston Community Health Survey. Sci. Total Environ. 2014, 473-474, 286–297. [Google Scholar] [CrossRef]
- Thomas, G.O.; Wilkinson, M.; Hodson, S.; Jones, K.C. Organohalogen chemicals in human blood from the United Kingdom. Environ. Pollut. 2006, 141, 30–41. [Google Scholar] [CrossRef]
- Zapevalov, M.A.; Samsonov, D.P.; Kochetkov, A.I.; Pasynkova, E.M.; Bogacheva, E.G. Global Atmospheric Transport of Persistent Organic Pollutants to the Russian Arctic. Russ. Meteorol. Hydrol. 2020, 45, 658–668. [Google Scholar] [CrossRef]
- Novikov, M.A. Persistent organic pollutants in bottom sediments of the Barents Sea. Water Resour. 2021, 48, 334–343. [Google Scholar] [CrossRef]
- Long, M.; Knudsen, A.-K.S.; Pedersen, H.S.; Bonefeld-Jørgensen, E.C. Food intake and serum persistent organic pollutants in the Greenlandic pregnant women: The ACCEPT sub-study. Sci. Total Environ. 2015, 529, 198–212. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, C.; Torres, J.P.M.; Malm, O. DDT (dicloro difenil tricloroetano): Toxicidade e contaminação ambiental-uma revisão. Química Nova 2002, 25, 995–1002. [Google Scholar] [CrossRef]
- Muir, D.; Savinova, T.; Savinov, V.; Alexeeva, L.; Potelov, V.; Svetochev, V. Bioaccumulation of PCBs and chlorinated pesticides in seals, fishes and invertebrates from the White Sea, Russia. Sci. Total Environ. 2003, 306, 111–131. [Google Scholar] [CrossRef]
- Hop, H.; Borgå, K.; Gabrielsen, G.W.; Kleivane, L.; Skaare, J.U. Food Web Magnification of Persistent Organic Pollutants in Poikilotherms and Homeotherms from the Barents Sea. Environ. Sci. Technol. 2002, 36, 2589–2597. [Google Scholar] [CrossRef]
- Polder, A.; Savinova, T.; Tkachev, A.; Løken, K.; Odland, J.Ø.; Skaare, J. Levels and patterns of Persistent Organic Pollutants (POPS) in selected food items from Northwest Russia (1998–2002) and implications for dietary exposure. Sci. Total Environ. 2010, 408, 5352–5361. [Google Scholar] [CrossRef] [PubMed]
- Davydov, A.N. Climate change and living conditions in the Arctic as perceived by the Nenets of Vaygach Island. Ekologiya cheloveka Hum. Ecol. 2013, 2, 29–34. [Google Scholar] [CrossRef]
- AMAP. Human Health in the Arctic; Arctic Monitoring and Assessment Programme (AMAP): Oslo, Norway, 2015; Available online: https://www.amap.no/documents/doc/amap-assessment-2015-human-health-in-the-arctic/1346 (accessed on 7 June 2021).
- Bjermo, H.; Darnerud, P.O.; Lignell, S.; Pearson, M.; Rantakokko, P.; Nälsén, C.; Barbieri, H.E.; Kiviranta, H.; Lindroos, A.K.; Glynn, A. Fish intake and breastfeeding time are associated with serum concentrations of organochlorines in a Swedish population. Environ. Int. 2013, 51, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Törnkvist, A.; Glynn, A.; Aune, M.; Darnerud, P.O.; Ankarberg, E.H. PCDD/F, PCB, PBDE, HBCD and chlorinated pesticides in a Swedish market basket from 2005–Levels and dietary intake estimations. Chemosphere 2011, 83, 193–199. [Google Scholar] [CrossRef]
- Sobolev, N.; Nieboer, E.; Aksenov, A.; Sorokina, T.; Chashchin, V.; Ellingsen, D.G.; Varakina, Y.; Plakhina, E.; Kotsur, D.; Kosheleva, A.; et al. Concentration dataset for 4 essential and 5 non-essential elements in fish collected in Arctic and sub-Arctic territories of the Nenets Autonomous and Arkhangelsk regions of Russia. Data Brief 2019, 27, 104631. [Google Scholar] [CrossRef]
- Savinov, V.; Muir, D.; Svetochev, V.; Svetocheva, O.; Belikov, S.; Boltunov, A.; Alekseeva, L.; Reiersen, L.-O.; Savinova, T. Persistent organic pollutants in ringed seals from the Russian Arctic. Sci. Total Environ. 2011, 409, 2734–2745. [Google Scholar] [CrossRef] [PubMed]
- Abass, K.; Emelyanova, A.; Rautio, A. Temporal trends of contaminants in Arctic human populations. Environ. Sci. Pollut. Res. 2018, 25, 28834–28850. [Google Scholar] [CrossRef] [PubMed]
| Analyte | % > LOD a | LOD | LOQ | ||
|---|---|---|---|---|---|
| Range (ng/g Lipid) | Range (ng/g Lipid) | ||||
| PCB28 | - b | 0.40 | 1.14 | 1.80 | 5.07 |
| PCB52 | - | 2.92 | 8.24 | 9.74 | 27.5 |
| PCB101 | - | 4.11 | 11.6 | 13.7 | 38.6 |
| PCB105 | - | 5.92 | 16.7 | 19.7 | 55.6 |
| PCB118 | 18.5 | 8.20 | 19.3 | 23.9 | 67.4 |
| PCB123 | - | 4.43 | 12.5 | 14.8 | 41.6 |
| PCB128 | - | 4.22 | 11.9 | 14.1 | 39.7 |
| PCB138 | 42.4 | 9.50 | 22.4 | 27.8 | 78.4 |
| PCB153 | 83.7 | 2.52 | 5.95 | 7.38 | 20.8 |
| PCB180 | 58.7 | 1.23 | 2.90 | 3.59 | 10.1 |
| PCB183 | 5.43 | 5.49 | 12.9 | 16.1 | 45.3 |
| o, p′-DDE | 82.6 | 2.06 | 4.86 | 6.03 | 17.0 |
| p, p′-DDE | 90.2 | 10.9 | 25.7 | 31.9 | 89.9 |
| o, p′-DDD | 6.52 | 0.84 | 1.98 | 2.46 | 6.94 |
| p, p′-DDD | 34.8 | 4.78 | 11.3 | 14.0 | 39.5 |
| HCB | 98.9 | 3.50 | 8.25 | 10.2 | 28.9 |
| α-HCH | - | 5.59 | 15.8 | 18.6 | 52.5 |
| β-HCH | 96.7 | 2.00 | 4.71 | 5.84 | 16.5 |
| γ-HCH | - | 3.62 | 10.2 | 12.1 | 34.0 |
| cis-nonachlor | - | 3.51 | 9.91 | 11.7 | 33.0 |
| trans-nonachlor | 8.70 | 8.85 | 20.9 | 25.9 | 73.0 |
| cis-chlordane | - | 3.51 | 9.91 | 11.7 | 33.0 |
| trans-chlordane | - | 8.56 | 24.1 | 28.5 | 80.4 |
| Heptachlor | - | 7.68 | 21.7 | 25.6 | 72.2 |
| Aldrin | 6.52 | 9.53 | 22.5 | 21.7 | 61.2 |
| Mirex | 2.17 | 6.10 | 14.4 | 17.9 | 50.3 |
| 1,2,3,5-tetrachlorobenzene | 22.8 | 1.09 | 2.58 | 3.20 | 9.02 |
| 1,2,4,5-tetrachlorobenzene | - | 0.96 | 2.71 | 3.20 | 9.02 |
| Characteristic | Measures | Islands | Shoina | Amderma | Indiga | Pechora | p-Value a | Nenets | Non-Nenets | p-Value b |
|---|---|---|---|---|---|---|---|---|---|---|
| N | 19 | 11 | 17 | 15 | 30 | 51 | 41 | |||
| Age, years | GM | 37.5 | 44.1 | 45.0 | 36.9 | 40.9 | 0.502 | 40.2 | 41.06 | 0.801 |
| Min | 18.0 | 28.0 | 28.0 | 19.0 | 20.0 | 20.0 | 18 | |||
| Max | 78.0 | 63.0 | 72.0 | 59.0 | 70.0 | 78.0 | 72 | |||
| Total lipid, mg/dL | GM | 578 | 599 | 627 | 560 | 595 | 0.615 | 588 | 597 | |
| Min | 395 | 459 | 468 | 414 | 417 | 395 | 414 | 0.485 | ||
| Max | 1110 | 859 | 977 | 821 | 874 | 1110 | 977 | |||
| BMI, (kg/m2) | GM | 25.0 | 27.4 | 28.3 | 27.7 | 25.2 | 0.018 | 26.1 | 26.7 | |
| Min | 19.8 | 20.5 | 19.7 | 19.4 | 20.8 | 19.8 | 19.4 | 0.719 | ||
| Max | 34.5 | 37.1 | 37.1 | 36.0 | 40.0 | 37.1 | 40.0 | |||
| BMI > 30, n (%) | 2 (10.5) | 2 (18.2) | 8 (47.1) | 6 (40) | 4 (13.3) | 10 (20) | 12 (29) | |||
| Proportion of Nenets, n (%) | 17 (89.5) | 1 (9.10) | 5 (29.4) | 6 (40.0) | 22 (73.3) |
| Analyte | Measures | Islands | Shoina | Amderma | Indiga | Pechora | p-Value 3 | Total | Nenets | Non-Nenets | p-Value 4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 19 | 11 | 17 | 15 | 30 | 92 | 51 | 41 | |||
| PCBs, ng/g lipid | |||||||||||
| PCB 118 | GM | 8.24 | <LOD | 14.7 | 12.3 | <LOD | 9.08 | <LOD | 10.5 | ||
| Min | <LOD 5 | <LOD | <LOD | <LOD | <LOD | <0.001 | <LOD | <LOD | <LOD | 0.049 | |
| Max | 45.0 | 24.0 | 42.1 | 89.0 | <LOD | 89.0 | 45.0 | 89.0 | |||
| PCB 138 | GM | 37.7 | 30.7 | 19.1 | 12.8 | <LOD | 16.9 | 16.3 | 17.7 | ||
| Min | <LOD | <LOD | <LOD | <LOD | <LOD | <0.001 | <LOD | <LOD | <LOD | 0.489 | |
| Max | 515 | 96.8 | 60.0 | 163 | 54.6 | 515 | 515 | 163 | |||
| PCB 153 | GM | 90.4 | 41.6 | 31.5 | 12.7 | 8.34 | 22.7 | 27.5 | 17.8 | ||
| Min | <LOD | 8.84 | <LOD | <LOD | <LOD | <0.001 | <LOD | <LOD | <LOD | 0.156 | |
| Max | 2640 | 142 | 83.3 | 292 | 77.2 | 2640 | 2640 | 292 | |||
| PCB 180 | GM | 52.9 | 11.9 | 15.7 | 3.32 | <LOD | 5.82 | 5.84 | 5.80 | ||
| Min | 6.55 | 2.22 | <LOD | <LOD | <LOD | <0.001 | <LOD | <LOD | <LOD | 0.102 | |
| Max | 1420 | 37.4 | 52.4 | 118 | <LOD | 1420 | 1420 | 118 | |||
| PCB 183 | GM | 6.84 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | ||
| Min | <LOD | <LOD | <LOD | <LOD | <LOD | - | <LOD | <LOD | <LOD | 0.064 | |
| Max | 45.6 | <LOD | <LOD | <LOD | <LOD | 45.6 | 45.6 | 6.03 | |||
| ∑ PCB5 1 | GM | 222 | 110 | 92.2 | 52.5 | 33.6 | 74.1 | 80.2 | 67.2 | ||
| Min | 28.8 | 35.1 | 20.6 | 18.9 | 15.0 | <0.001 | 15.0 | 19.3 | 15.0 | 0.165 | |
| Max | 4600 | 257 | 217 | 666 | 137 | 4600 | 4600 | 666 | |||
| OCPs ng/g lipid | |||||||||||
| o, p-DDE | GM | 21.7 | 23.0 | 6.57 | 3.81 | 18.6 | 12.6 | 13.0 | 12.0 | ||
| Min | 2.70 | 11.8 | <LOD | <LOD | <LOD | <0.001 | <LOD | <LOD | <LOD | 0.407 | |
| Max | 61.8 | 38.5 | 23.7 | 24.0 | 75.2 | 75.2 | 65.6 | 75.2 | |||
| p, p′-DDE | GM | 121 | 152 | 123 | 41.8 | 32.5 | 68.3 | 48.7 | 104 | ||
| Min | 30.6 | 43.3 | 22.7 | <LOD | <LOD | <0.001 | <LOD | <LOD | 16.0 | 0.009 | |
| Max | 467 | 549 | 1320 | 336 | 278 | 1320 | 467 | 1320 | |||
| o, p-DDD | GM | <LOD | <LOD | <LOD | <LOD | 0.99 | 0.92 | 0.86 | 1.00 | ||
| Min | <LOD | <LOD | <LOD | <LOD | <LOD | - | <LOD | <LOD | <LOD | 0.262 | |
| Max | <LOD | <LOD | <LOD | <LOD | 129 | 129 | 1.73 | 129 | |||
| p, p′-DDD | GM | <LOD | <LOD | <LOD | <LOD | 47.5 | 9.62 | 12.1 | 7.22 | ||
| Min | <LOD | <LOD | <LOD | <LOD | 17.3 | <LOD | <LOD | <LOD | 0.165 | ||
| Max | <LOD | <LOD | 8.41 | 6.03 | 183 | 183 | 183 | 109 | |||
| ∑ DDT 2 | GM | 161 | 193 | 146 | 55.0 | 124 | 125 | 109 | 146 | ||
| Min | 61.3 | 72.4 | 41.1 | 15.3 | 36.2 | 0.002 | 15.3 | 15 | 21.4 | 0.019 | |
| Max | 506 | 570 | 1321 | 342 | 534 | 1320 | 506 | 1321 | |||
| HCB | GM | 83.1 | 26.3 | 34.2 | 133 | 65.1 | 61.2 | 81.7 | 42.8 | ||
| Min | <LOD | 10.85 | 9.4 | 22.2 | 12.5 | <0.001 | <LOD | <LOD | 9.38 | 0.014 | |
| Max | 358 | 109 | 250 | 469 | 713 | 713 | 713 | 370 | |||
| β-HCH | GM | 13.2 | 26.4 | 17.1 | 19.8 | 12.7 | 15.9 | 13.2 | 19.9 | ||
| Min | 2.75 | 3.17 | 4.00 | 7.21 | 2.10 | 0.227 | 2.10 | 2.75 | 2.10 | 0.006 | |
| Max | 31.5 | 178 | 118 | 139 | 89.2 | 178 | 139 | 178 | |||
| Aldrin | GM | 18.1 | <LOD | <LOD | <LOD | <LOD | <LOD | 10.5 | <LOD | ||
| Min | <LOD | <LOD | <LOD | <LOD | <LOD | - | <LOD | <LOD | <LOD | 0.138 | |
| Max | 987 | <LOD | 24.6 | <LOD | <LOD | 987 | 987 | 24.6 | |||
| Mirex | GM | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | ||
| Min | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0.207 | ||
| Max | 36.6 | <LOD | <LOD | <LOD | <LOD | - | 36.6 | 36.6 | 7.24 | ||
| trans-nonachlor | GM | 11.9 | 9.60 | <LOD | <LOD | <LOD | <LOD | 9.05 | <LOD | ||
| Min | <LOD | <LOD | <LOD | <LOD | <LOD | - | <LOD | <LOD | <LOD | 0.207 | |
| Max | 95.7 | 34.8 | <LOD | 26.1 | <LOD | 95.7 | 95.7 | 34.8 | |||
| 1,2,3,5-TCB | GM | <LOD | <LOD | 1.73 | 64.9 | <LOD | 1.96 | 1.52 | 2.70 | ||
| Min | <LOD | <LOD | <LOD | 12.5 | <LOD | - | <LOD | <LOD | <LOD | 0.798 | |
| Max | <LOD | <LOD | 10.4 | 502 | <LOD | 502 | 502 | 250 | |||
| Variable | Crude Differences between Pechora and Other Locations | Crude Ethnic Differences | Age | BMI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Islands | Shoina | Amderma | Indiga | Nenets vs. non-Nenets | ||||||||||
| bc * | 95% CI | bc | 95% CI | bc | 95% CI | bc | 95% CI | bc | 95% CI | bc | 95% CI | B * | 95% CI | |
| p, p′-DDE | 1.31 | 0.66; 1.96 | 1.55 | 0.76; 2.32 | 1.33 | 0.66; 2.01 | 0.25 | −0.45; 0.95 | −0.76 | −1.27; −0.25 | 0 | −0.02; 0.02 | 0.07 | 0.01; 0.13 |
| ∑ DDT | 0.26 | −0.16; 0.69 | 0.44 | −0.07; 0.95 | 0.16 | −0.28; 0.60 | −0.81 | −1.27; −0.36 | −0.29 | −0.63; 0.04 | 0.01 | 0.00; 0.02 | 0.03 | −0.01; 0.06 |
| PCB 118 | 0.20 | −0.12; 0.53 | 0.13 | −0.26; 0.52 | 0.78 | 0.44; 1.12 | 0.61 | 0.26; 0.96 | −0.26 | −0.51; −0.01 | 0.01 | 0.00; 0.02 | 0.04 | 0.01; 0.08 |
| PCB 138 | 1.46 | 0.96; 1.96 | 1.26 | 0.66; 1.85 | 0.78 | 0.27; 1.30 | 0.38 | −0.16; 0.91 | −0.08 | −0.50; 0.34 | 0.02 | 0.01; 0.03 | 0.04 | −0.01; 0.07 |
| PCB 153 | 2.38 | 1.64; 3.13 | 1.61 | 0.71; 2.51 | 1.33 | 0.56; 2.10 | 0.42 | −0.38; 1.23 | 0.43 | −0.21; 1.08 | 0.03 | 0.01; 0.05 | 0.01 | −0.06; 0.09 |
| PCB 180 | 4.14 | 3.50; 4.79 | 2.65 | 1.87; 3.43 | 2.93 | 2.26; 3.60 | 1.37 | 0.68; 2.07 | 0.01 | −0.80; 0.82 | 0.02 | −0.01; 0.05 | 0.03 | −0.06; 0.12 |
| ∑ PCB | 1.89 | 1.39; 2.38 | 1.19 | 0.59; 1.78 | 1.01 | 0.50; 1.52 | 0.45 | −0.09; 0.98 | 0.18 | −0.28; 0.63 | 0.02 | 0.01; 0.03 | 0.02 | −0.03; 0.08 |
| HCB | 0.25 | −0.28; 0.77 | −0.91 | −1.53; −0.28 | −0.64 | −1.18; −0.11 | 0.71 | 0.15; 1.27 | 0.65 | 0.24; 1.05 | 0.01 | −0.01; 0.02 | 0.02 | −0.03; 0.06 |
| β-HCH | 0.04 | −0.46; 0.54 | 0.73 | 0.13; 1.33 | 0.30 | −0.22; 0.81 | 0.44 | −0.10; 0.98 | −0.41 | −0.76; −0.05 | 0.02 | 0.01; 0.03 | 0.06 | 0.02; 0.10 |
| Variable | Adjusted Differences between Pechora and Other Locations | Adjusted Ethnic Differences | Age | BMI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Islands | Shoina | Amderma | Indiga | Nenets vs. Non-Nenets | ||||||||||
| ba * | 95% CI | ba * | 95% CI | ba * | 95% CI | ba * | 95% CI | ba * | 95% CI | ba * | 95% CI | ba * | 95% CI | |
| p, p′-DDE | 1.38 | 0.75;2.02 | 0.91 | 0.08; 1.73 | 0.62 | −0.10; 1.43 | −0.30 | −1.01; 0.41 | −0.80 | −1.32; −0.27 | 0.00 | −0.02; 0.01 | 0.07 | 0.02; 0.13 |
| ∑ DDT | 0.33 | −0.08; 0.74 | 0.01 | −0.52; 0.54 | −0.32 | −0.78; 0.15 | −1.13 | −1.59; −0.68 | −0.51 | −0.85; −0.17 | 0.01 | −0.01; 0.02 | 0.04 | 0.01; 0.08 |
| PCB 118 | 0.23 | −0.11; 0.56 | 0 | −0.44; 0.43 | 0.60 | 0.22; 0.98 | 0.50 | 0.12; 0.88 | 0.13 | −0.41; 0.15 | 0.01 | 0.00; 0.02 | 0.02 | −0.01; 0.05 |
| PCB 138 | 1.53 | 1.04; 2.01 | 1.13 | 0.50; 1.76 | 0.56 | 0.01; 1.11 | 0.33 | −0.22; 0.87 | −0.07 | −0.48; 0.33 | 0.02 | 0.01; 0.03 | 0.03 | −0.01; 0.07 |
| PCB 153 | 2.33 | 1.60; 3.06 | 1.82 | 0.89; 2.78 | 1.47 | 0.64; 2.29 | 0.70 | −0.12; 1.52 | 0.54 | −0.06; 1.15 | 0.03 | 0.01; 0.04 | 0.00 | −0.06; 0.06 |
| PCB 180 | 4.24 | 3.58; 4.90 | 2.70 | 1.85; 3.56 | 2.95 | 2.21; 3.70 | 1.48 | 0.74; 2.22 | 0.17 | −0.38; 0.72 | 0.02 | 0.00; 0.04 | 0.00 | −0.06; 0.05 |
| ∑ PCB | 1.90 | 1.42; 2.38 | 1.19 | 0.57; 1.82 | 0.94 | 0.40; 1.49 | 0.52 | −0.03; 1.01 | 0.17 | −0.23; 0.57 | 0.02 | 0.01; 0.03 | 0.01 | −0.03; 0.05 |
| HCB | 0.20 | −0.32; 0.73 | −0.73 | −1.41; −0.05 | −0.66 | −1.25; −0.07 | 0.74 | 0.15; 1.33 | 0.42 | −0.02; 0.86 | 0.01 | 0.00; 0.02 | 0.02 | −0.02; 0.07 |
| β-HCH | 0.06 | −0.40; 0.52 | 0.39 | −0.21; 0.99 | −0.15 | −0.67; 0.37 | 0.25 | −0.27; 0.76 | −0.30 | −0.68; 0.09 | 0.02 | 0.01; 0.03 | 0.05 | 0.01; 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varakina, Y.; Aksenov, A.; Lakhmanov, D.; Trofimova, A.; Korobitsyna, R.; Belova, N.; Kotsur, D.; Sorokina, T.; Grjibovski, A.M.; Popova, L.; et al. Geographic and Ethnic Variations in Serum Concentrations of Legacy Persistent Organic Pollutants among Men in the Nenets Autonomous Okrug, Arctic Russia. Int. J. Environ. Res. Public Health 2022, 19, 1379. https://doi.org/10.3390/ijerph19031379
Varakina Y, Aksenov A, Lakhmanov D, Trofimova A, Korobitsyna R, Belova N, Kotsur D, Sorokina T, Grjibovski AM, Popova L, et al. Geographic and Ethnic Variations in Serum Concentrations of Legacy Persistent Organic Pollutants among Men in the Nenets Autonomous Okrug, Arctic Russia. International Journal of Environmental Research and Public Health. 2022; 19(3):1379. https://doi.org/10.3390/ijerph19031379
Chicago/Turabian StyleVarakina, Yulia, Andrey Aksenov, Dmitry Lakhmanov, Anna Trofimova, Rimma Korobitsyna, Natalia Belova, Dmitry Kotsur, Tatiana Sorokina, Andrej M. Grjibovski, Ludmila Popova, and et al. 2022. "Geographic and Ethnic Variations in Serum Concentrations of Legacy Persistent Organic Pollutants among Men in the Nenets Autonomous Okrug, Arctic Russia" International Journal of Environmental Research and Public Health 19, no. 3: 1379. https://doi.org/10.3390/ijerph19031379
APA StyleVarakina, Y., Aksenov, A., Lakhmanov, D., Trofimova, A., Korobitsyna, R., Belova, N., Kotsur, D., Sorokina, T., Grjibovski, A. M., Popova, L., Chashchin, V., Odland, J. Ø., & Thomassen, Y. (2022). Geographic and Ethnic Variations in Serum Concentrations of Legacy Persistent Organic Pollutants among Men in the Nenets Autonomous Okrug, Arctic Russia. International Journal of Environmental Research and Public Health, 19(3), 1379. https://doi.org/10.3390/ijerph19031379







