Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice
Abstract
1. Introduction
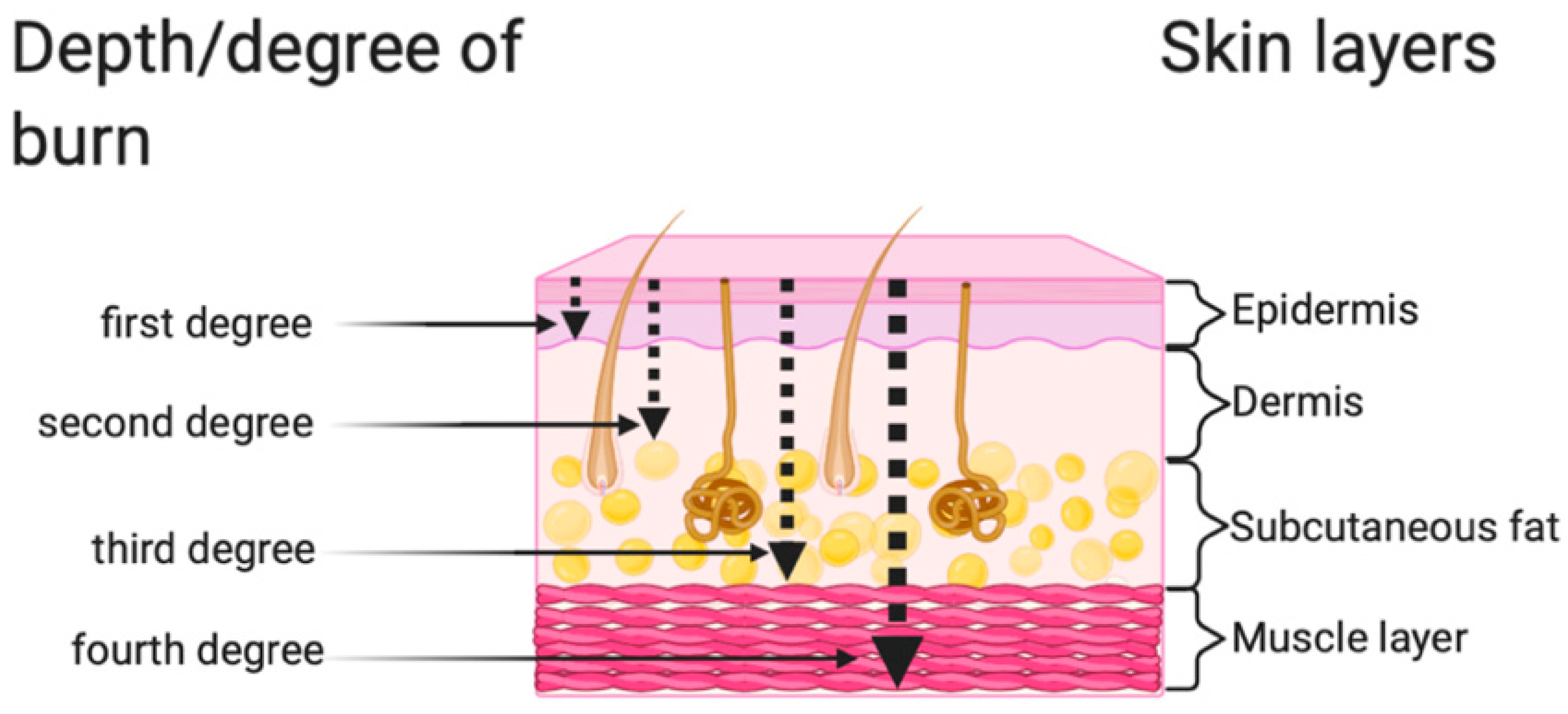
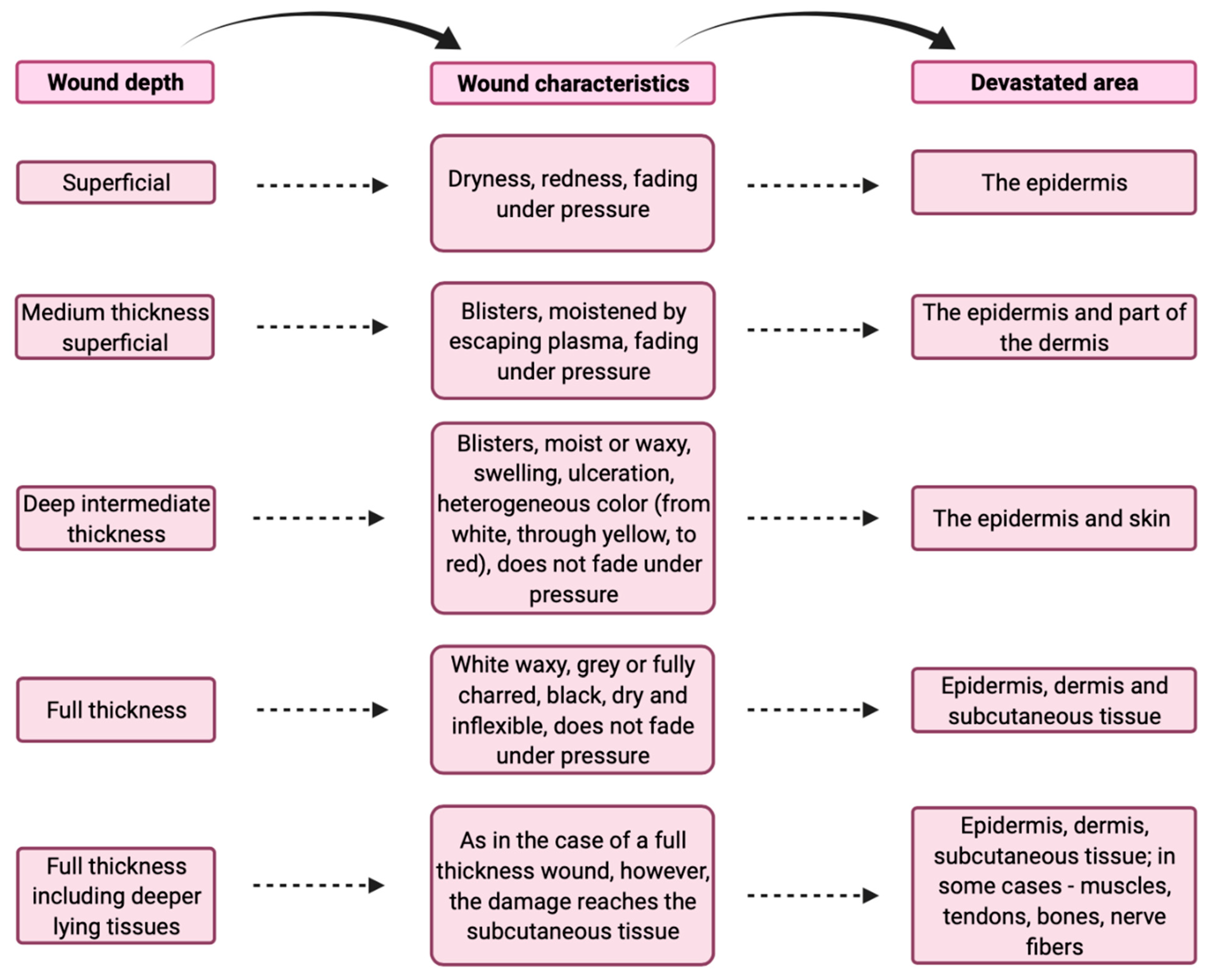
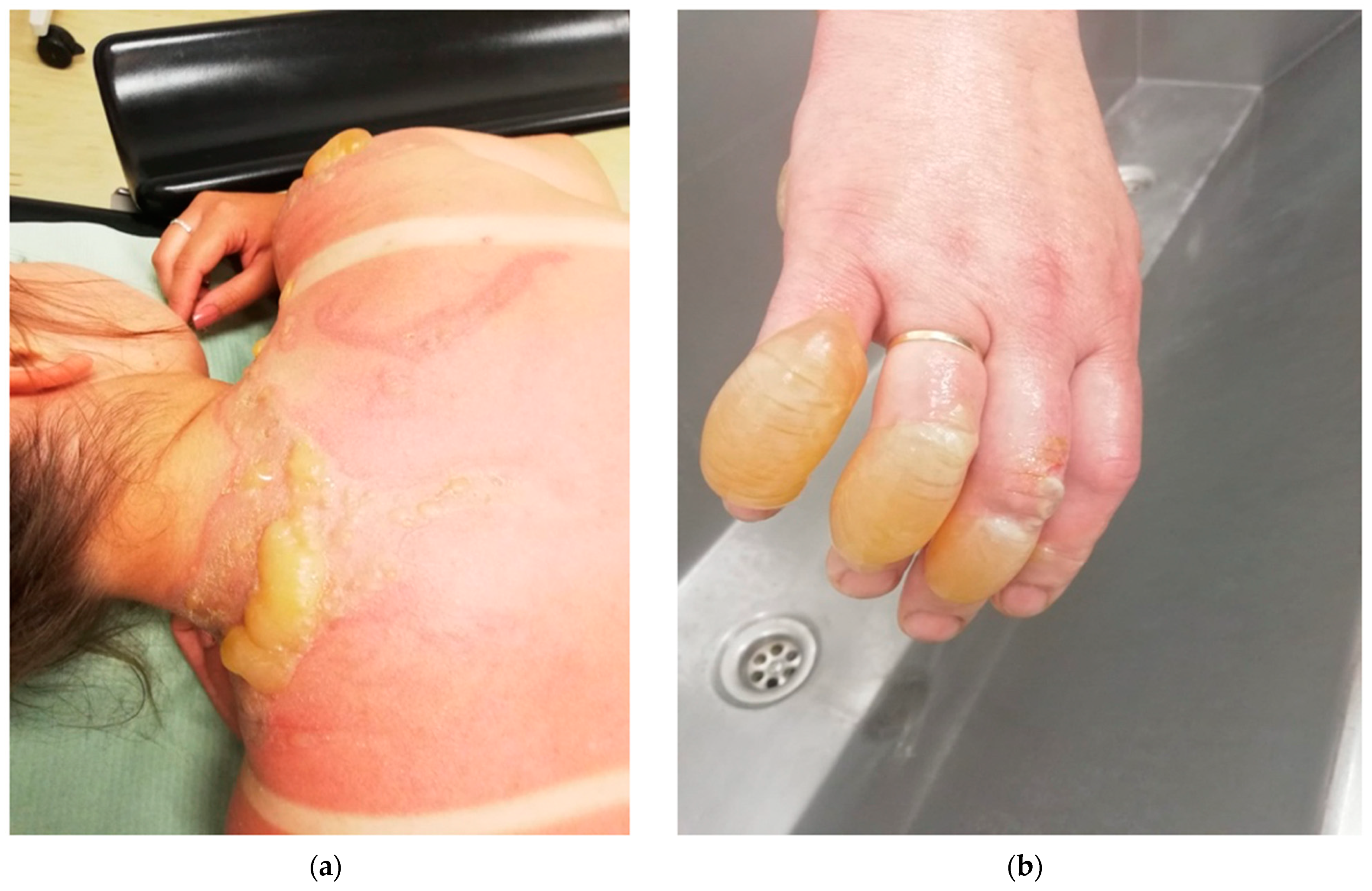
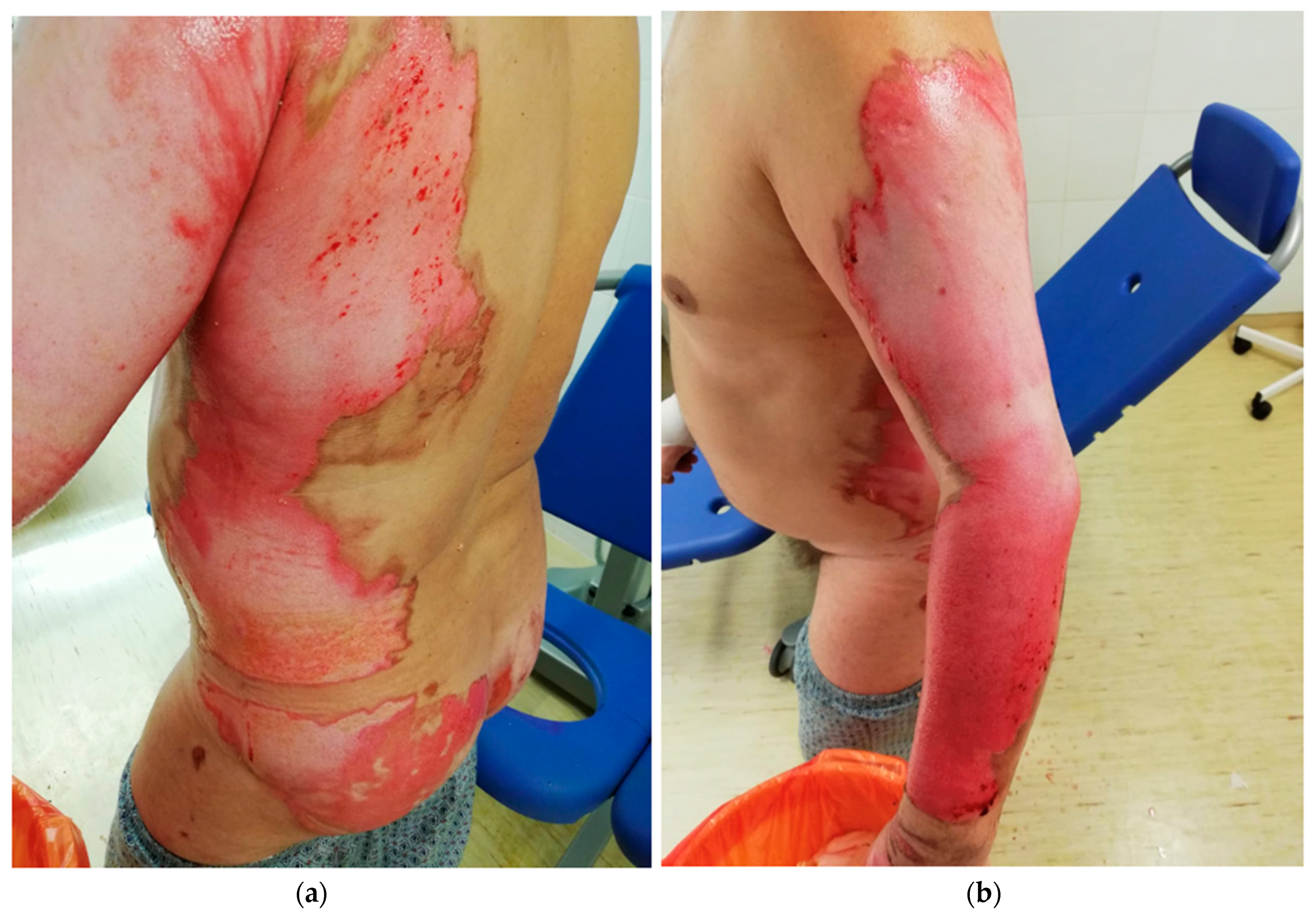
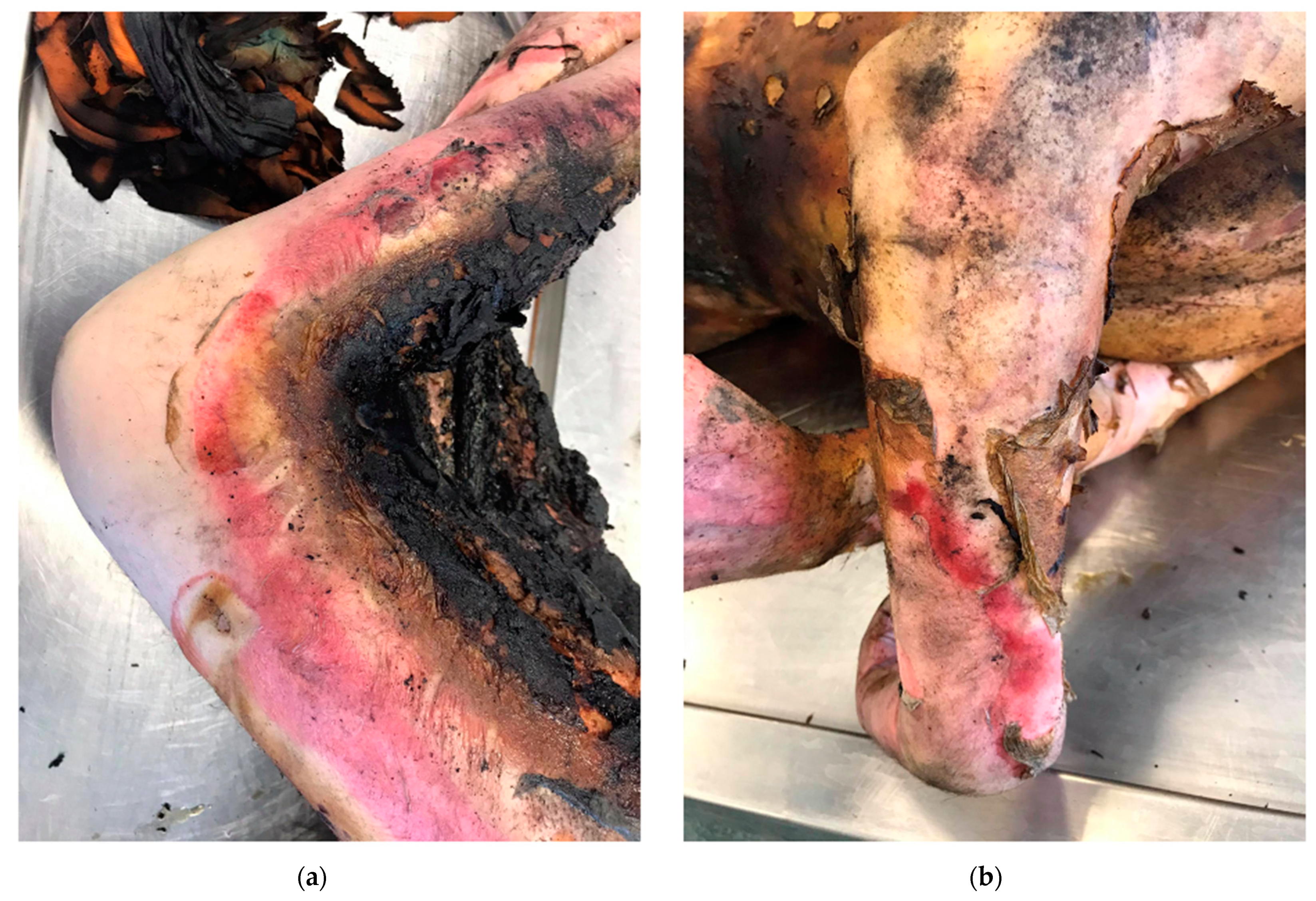
2. Classification of Burns According to the Depth of the Wound
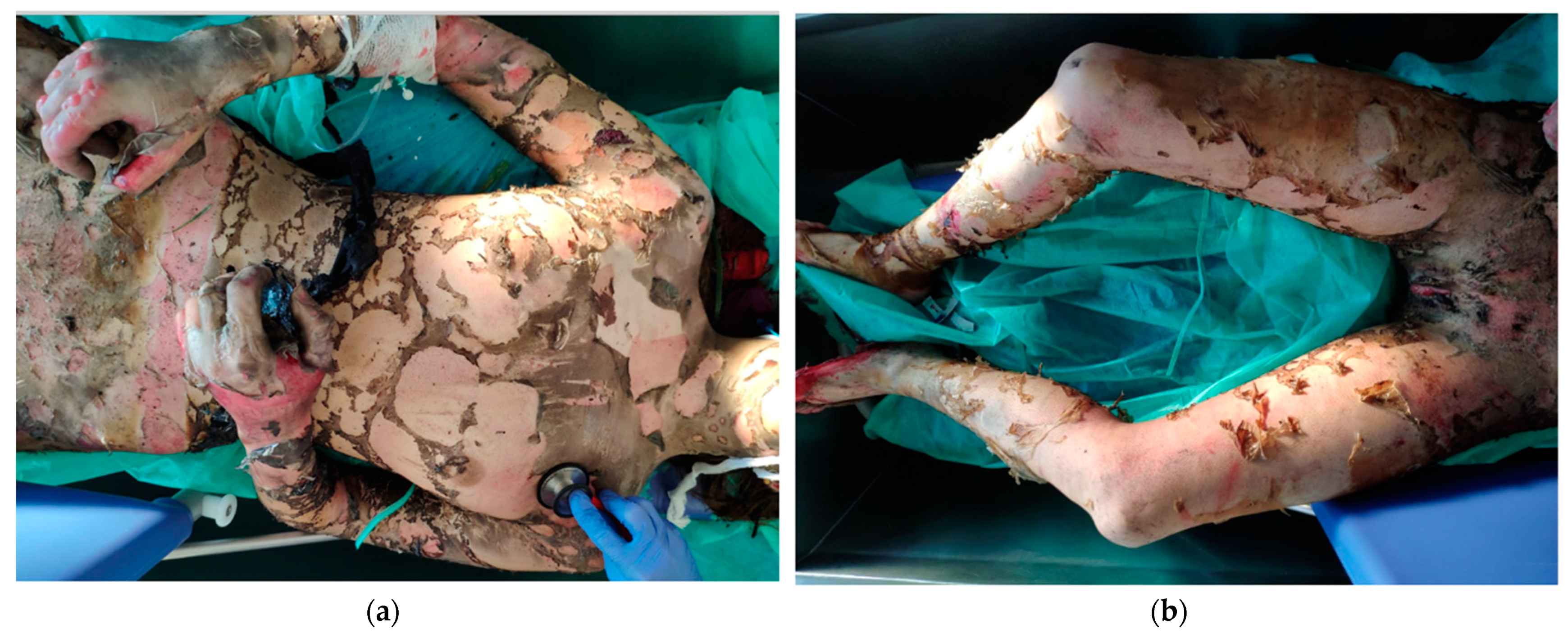
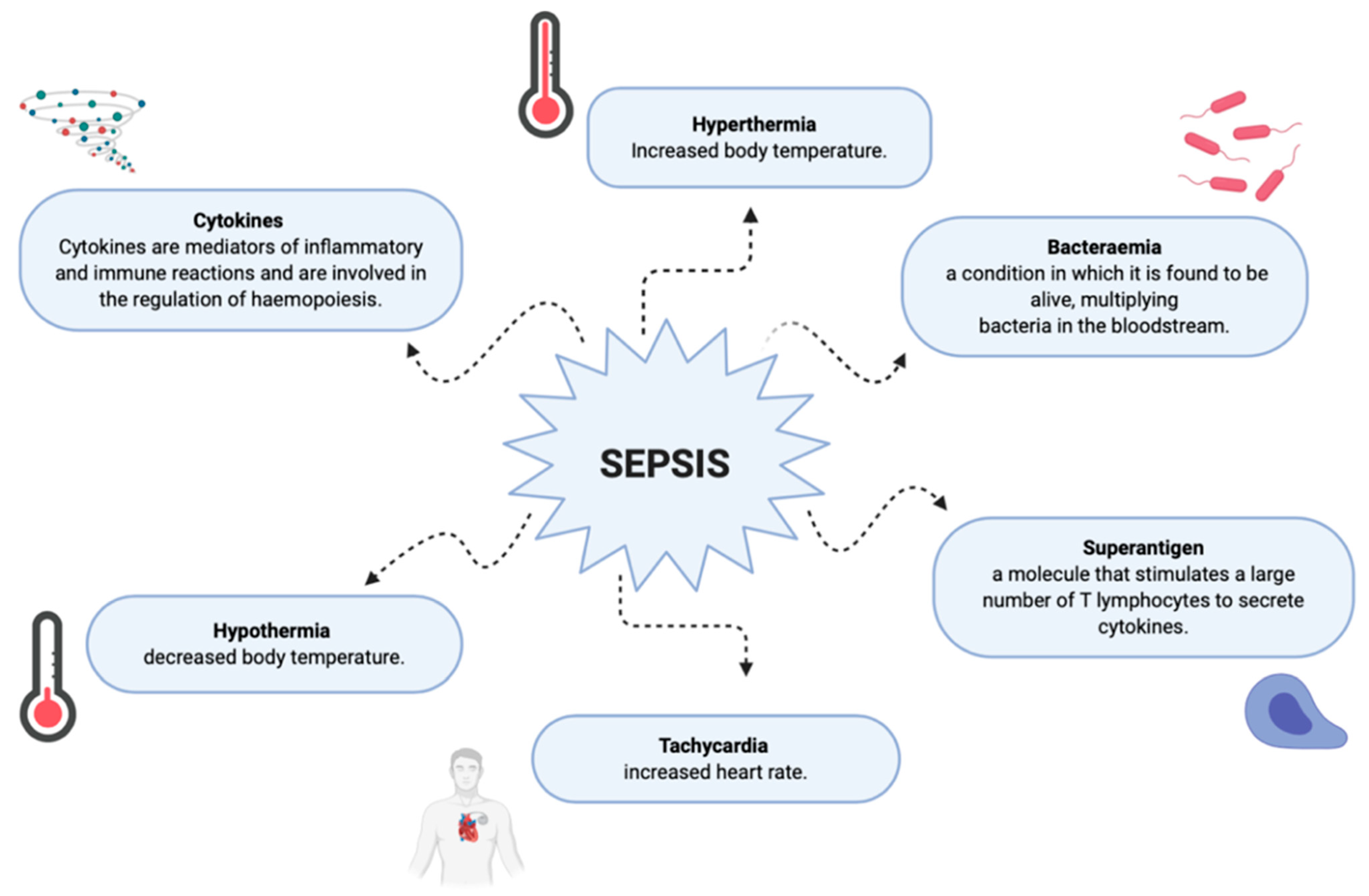
3. Complications in Burn Patients during the Healing Process
4. Oxidative Stress in Burned Patients
5. Care of a Patient with Burns
6. Pharmacological Treatment
7. Types of Dressings
7.1. Active Hydrogels for Treatment of Burn Wound Dressings
7.2. Honey-Based Dressings
7.3. Chitin-Based Dressings
8. Vacuum Therapy
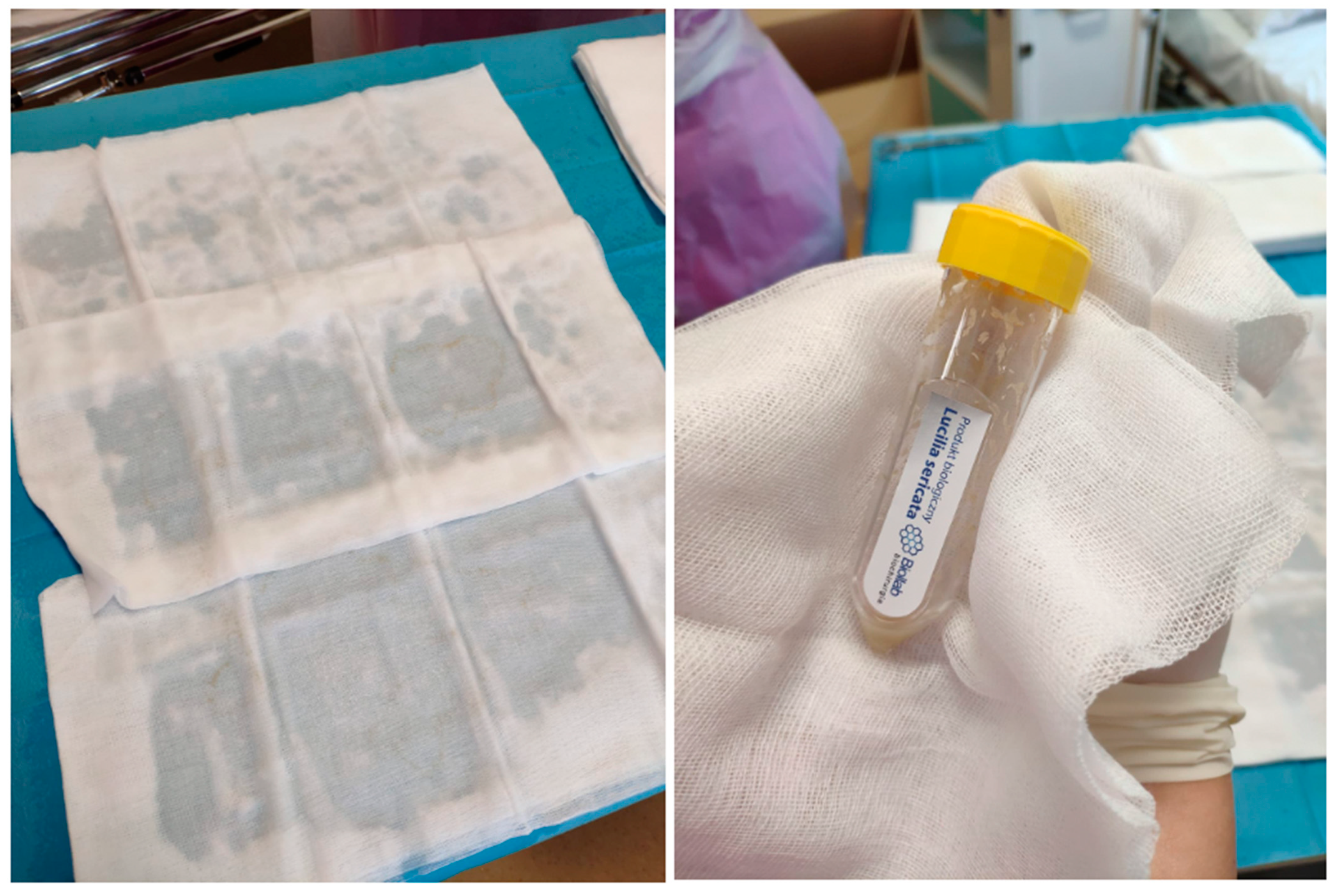
9. Use of Lucilia sericata Larvae
10. Use of Fish Skin
11. Regenerative Medicine and Burn Wounds
12. Artificial Skin—Is It a Future Treatment?
13. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Greenhalgh, D.G. Management of Burns. N. Eng. J. Med. 2019, 380, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- Nessler, M.; Chrapusta, A. Możliwości zastosowania ksenogenicznych substytutów skóry w leczeniu oparzeń–przegląd piśmiennictwa. Leczenie Ran 2013, 10, 47–52. [Google Scholar] [CrossRef]
- Esteban-Vives, R.; Choi, M.T.; Young, M.T.; Over, P.; Ziembicki, J.; Corcos, A.; Gerlach, H.C. Second-degree burns with six etiologies treated with autologous noncultured cell-spray grafting. Burns 2016, 42, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Szymański, K.; Waś, J. Potencjalne możliwości leczenia i regeneracji skóry w rozległych ranach oparzeniowych przy zastosowaniu substytutów regenerujących skórę. Leczenie Ran 2014, 11, 11–20. [Google Scholar] [CrossRef]
- Bazaliński, D.; Karnas, M.; Wołkowicz, M.; Kózka, M.; Więch, P. Zastosowanie larw Lucilla sericata w oczyszczaniu ran przewlekłych–opis trzech przypadków. Leczenie Ran 2018, 15, 153–159. [Google Scholar] [CrossRef]
- Zoriah, A.; Rasool, H.B.A. The effects of honey sulfadiazine for the treatment of burns: A systematic review of randomized controlled trials. Burns 2017, 43, 50–57. [Google Scholar] [CrossRef]
- Johnson, C. Management of burns. Surgery 2018, 36, 435–440. [Google Scholar] [CrossRef]
- Johnson, M.R.; Reg, R. Partial-Thickness burns: Identification and management. Adv. Ski. Wound Care 2003, 16, 178–187. [Google Scholar] [CrossRef]
- Chrapusta, A.; Pabiańczyk, R.; Nessler, M.; Nessler, K.; Cieślik, K. Aquacel Ag w leczeniu oparzeń IIb i IIb/III stopnia ręki u dorosłych–doświadczenia własne. Forum Zakażeń 2012, 3, 71–76. [Google Scholar]
- Paleczny, J.; Junka, A.; Bartoszewicz, M. Postępowanie przeciwbakteryjne (antyseptyka) u pacjentów oparzonych. Chir. Plast. I Oparzenia 2019, 7, 91–100. [Google Scholar] [CrossRef]
- Oryan, A.; Alezadeh, E.; Moshiri, A. Burn wound healing: Present concepts, treatment strategies, and future directions. J. Wound Care 2017, 26, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.J.; Nocolai, M.; Wiggins, R. Modern treatment of burn wound. In Total Burn Care; Herndon, D.N., Ed.; WB Saunders Company Ltd.: London, UK; Philadelphia, PA, USA; Toronto, ON, Canada; Sydney, Australia; Tokyo, Japan, 1996; pp. 136–147. [Google Scholar]
- Dantzer, E.I.; Queruel, P.; Salinier, L.; Palmier, B.; Quinot, J.F. Integra, a new surgical alternative for the treatment of massive burns. Clinical evaluation of acute and reconstructive surgery:39 cases. Ann. Chir. Plast. Esthet. 2001, 46, 173–189. [Google Scholar] [CrossRef]
- Wang, X.Q.; Mill, J.; Kravchuk, J.; Kimble, R.M. Ultrasound assessed thickness of burns scars in association with laser Doppler imaging determined depth of burns in pediatric patients. Burns 2010, 36, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, H.K.; Jay, J.; Finnerty, C.C. Pathophysiology of the burn scar. Total Burn. Care 2018, 466–475.e463. [Google Scholar] [CrossRef]
- Alexander, H.; Miller, D.L. Determining skin thickness with pulsed ultra sound. J. Investig. Dermatol. 1979, 72, 17–19. [Google Scholar] [CrossRef]
- Kreckhove, E.D.; Staes, F.; Flour, F.; Stappaerts, K.; Boeckx, W. Reproducibility of repeated mesurements on post-burn scars with Dermascan C. Ski. Res. Technol. 2003, 9, 81–84. [Google Scholar] [CrossRef]
- Kreckhove, E.D.; Stappaerts, K.; Fieuws, S.; Laperre, J.; Massage, P.; Flour, M.; Boeckx, W. The assessment of erythema and thickness on burn related scars during pressure garment therapy as a preventive measure for hypertrophic scarring. Burns 2005, 31, 696–702. [Google Scholar] [CrossRef]
- Jaskille, A.D.; Ramella-Roman, J.C.; Shupp, J.W.; Jordan, M.H.; Jeng, J.C. Critical review of burn depth assessment techniques: Part II. Review of laser doppler technology. Burn. Care Res. 2010, 31, 151–157. [Google Scholar] [CrossRef]
- Zachariah, J.R.; Rao, A.L.; Prabha, R.; Gupta, A.K.; Paul, M.K.; Lamba, S. Post burn pruritus–a review of current treatment options. Burns 2012, 38, 621–629. [Google Scholar] [CrossRef]
- Numata, Y.; Terui, T.; Okuyama, R.; Hirasawa, N.; Sugiura, Y.; Miyoshi, I.; Watanabe, T.; Kuramasu, A.; Tagami, H.; Ohtsu, H. The accelerating effect of histaminę on the cutaneus wound-healing process through the action of basic fibroblast growth factor. J. Investig. Dermatol. 2006, 126, 1403–1409. [Google Scholar] [CrossRef][Green Version]
- Chung, B.Y.; Kim, H.B.; Jung, M.J.; Kang, S.Y.; Kwak, I.S.; Park, C.W.; Kim, H.O. Post-burn pruritus. Int. J. Mol. Sci. 2020, 21, 3880. [Google Scholar] [CrossRef] [PubMed]
- Willebrand, M.; Low, A.; Dyster-Aas, J.; Kildal, M.; Andersson, G.; Ekselius, L.; Gerdin, B. Pruritus, personality traits and coping in long-term follow-up of burn-injured patients. Acta Derm. Venerol. 2004, 84, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.C.; Harris, N.L.; Shami, A.E.; Sheridan, R.L.; Schulz, J.T.; Bilodeau, M.L.; Rayan, C.M. A descriptive review of neuropathic-like pain arfer burn injury. Burn. Care Res. 2006, 27, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.L.; Gabriel, V. Evidence based review for the treatment of post-burn pruritus. Burn. Care Res. 2009, 30, 55–61. [Google Scholar] [CrossRef]
- Pucułek, M.; Baj, J.; Portincasa, P.; Sitarz, M.; Grochowski, C.; Radzikowska, E. The morphology and application of stem cells in digestive system surgery. Folia Morphol. 2020, 80, 13–19. [Google Scholar] [CrossRef]
- Kocik, J. Udział cytokin i innych mediatorów w procesie gojenia rany. Postępy Biol. Komórki 1996, 23, 63–92. [Google Scholar]
- Pikuła, M.; Langa, P.; Kosikowska, P.; Trzonkowski, P. Komórki macierzyste i czynniki wzrostu w gojeniu ran. Postępy Hig. Med. Dosw. 2015, 69, 874–885. [Google Scholar] [CrossRef]
- Auger, C.; Samadi, O.; Jeschke, M.G. Biochemical alterations underlying post-burn hypermetabolism. Biochem. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2633–2644. [Google Scholar] [CrossRef]
- Okonkwo, U.A.; DiPietro, L.A. Diabetes and wound angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Durham, J.T.; Herman, I.M. Wound healing angiogenesis: Innovations and challenges in acute and chronic wound healing. Adv. Wound Care 2012, 1, 17–22. [Google Scholar] [CrossRef]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Dliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Heng, J.S.; Clancy, O.; Atkins, J.; Leon-Villapalos, J.; Williams, A.J.; Keays, R.; Hayes, M.; Jones, I.; Vizcaychipi, M.P. Revised Baux Score and updated Charlson comorbidity index are independently associated with mortality in burns intensive care patients. Burns 2016, 41, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Ogura, H.; Asahara, T.; Nomoto, K.; Matsushima, A.; Hayakawa, K.; Ikegawa, H.; Tasaki, O.; Kuwagata, Y.; Shimazu, T. Gut microbiota and environment in patients with major burns–a preliminary report. Burns 2015, 41, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Bahemia, I.A.; Muganza, A.; Moore, R.; Sahid, F.; Menzes, C.N. Microbiology and antibiotic resistance in severe burns patients: A 5-year review in an adult burn’s unit. Burns 2015, 41, 1536–1542. [Google Scholar] [CrossRef]
- Ward, J.; Philips, G.; Radotra, I.; Smailes, S.; Dziwulski, P.; Zhang, J.; Martin, N. Frailty: An independent predictor of burns mortality following in patient admission. Burns 2018, 44, 1895–1902. [Google Scholar] [CrossRef]
- Ramirez-Blanco, C.E.; Ramirez-Rivero, C.E.; Diaz-Martinez, L.A.; Sosa-Avila, L.M. Infection in burn patients in a referral center in Columbia. Burns 2017, 43, 642–653. [Google Scholar] [CrossRef]
- Lachiewicz, A.M.; Hauck, C.G.; Weber, D.J.; Cairns, B.A.; van Duin, D. Bacterial infections after burn injuries: Impact of multidrug resistance. Clin. Infect. Dis. 2017, 65, 2130–2136. [Google Scholar] [CrossRef]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for wound healing and infection control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef]
- Junaidi, K.; Mustafa, A.U.; Arshadm, S.; Al Farraj, A.D.; Younas, S.; Ejaz, H. Burn wound infections: A serious threat of multidrug-resistant Staphylococcus aureus. Pak. J. Med. Health Sci. 2019, 13, 804. [Google Scholar]
- Kozioł, M.M.; Sikora, A.; Targońska, S.; Sikora, A. Nasal carriage of Staphylococcus aureus as a risk of nosocomial infections. Forum Zakażeń 2014, 5, 205–209. [Google Scholar] [CrossRef]
- Hałgas, M.; Kuś, R.; Kozioł, M.; Zuzak, T.; Olender, A. Evaluation of drug-sensitivity of Staphylococcus aureus strains isolated from upper respiratory tract and antibiotherapy of infections. Nauk. Przyr. 2018, 3, 24–31. [Google Scholar]
- Bayram, Y.; Parlak, M.; Aypak, C.; Bayram, I. Three-year review of bacteriological profile and anibiogram of burn wound izolates in Van Turkey. Int. J. Med. Sci. 2013, 10, 19–23. [Google Scholar] [CrossRef]
- Azimi, L.; Moteballian, A.; Namvar, E.A.; Asghari, B.; Lari, A.R. Nosocomial infections in burned patients in Mothari Hospital, Teheran, Iran. Derm. Res. Pract. 2011, 2011, 436952. [Google Scholar] [CrossRef]
- Alebachew, T.; Yismaw, G.; Derabe, A.; Sisay, Z. Staphylococcus aureus burn wound infection among patients attending Yekatit 12 hospital burn unit, Addis Ababa, Ethiopia. Ethiop. J. Health Sci. 2012, 22, 209–213. [Google Scholar] [PubMed]
- Chen, K.; Lin, S.; Li, P.; Song, Q.; Liu, T.; Zeng, L.; Zhang, W. Characterization of Staphylococcus aureus isolated from patients with burns in a regional burn center, Southeastern China. BMC Infec. Dis. 2018, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, E.A.; Azzopardi, E.; Camilleri, L.; Villapalos, J.; Boyce, D.E.; Dziewulski, P.; Dickson, W.A.; Whitaker, I.S. Gram negative wound infection in hospitalized burn patients-systematic review and metanalysis. PLoS ONE 2014, 9, e95042. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, C.Z.; Ahmad, K.; Farzaneg, F.; Hosein, A.; Mahmoudi, A.A. Biofilm formation and virulence factors among Pseudomonas aeruginosa isolated from burn patients. Jundishaour J. Microbiol. 2015, 8, e22345. [Google Scholar] [CrossRef]
- Bhat, P.; Rathi, K.R.; Hazra, S.; Sharma, A.; Shete, V. Prevalence of multidrug resistant Pseudomonas aeruginosa infection in burn patients at a tertiary care center. Indian J. Burn. 2015, 23, 56–59. [Google Scholar] [CrossRef]
- Dou, Y.; Huan, J.; Guo, F.; Zhou, Z.; Shi, Y. Pseudomonas aeruginosa prevalence, antibiotic resistance and antimicrobial use in Chinese burn wards from 2007 to 2014. J. Int. Med. Res. 2017, 45, 1124–1137. [Google Scholar] [CrossRef]
- Brandenburg, K.S.; Weaver, A.J., Jr.; Karna, S.L.R.; You, T.; Chen, P.; Stryk, S.V.; Qian, L.; Pineda, U.; Abercrombie, J.J.; Leung, K.P. Formation of Pseudomonas aeruginosa biofilms in full-thickness scald burn wounds in rats. Sci. Rep. 2019, 9, 13627. [Google Scholar] [CrossRef]
- Ramirez, T.; Shrestha, A.; Kishen, A. Inflammatory potential of monospecies biofilm matrix components. Int. Endod. J. 2019, 52, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Borkowska, U. Sepsa. Pytania bez odpowiedzi. Anestezjol. I Ratow. 2019, 13, 358–364. [Google Scholar]
- Markowska, M.; Wach, R.; Chrapusta, A. Sepsa u pacjenta oparzonego–trudności i wyzwania. Chir. Plast. I Oparzenia 2019, 7, 181–189. [Google Scholar] [CrossRef]
- Nunez Lopez, O.; Cambiaso-Daniel, J.; Branski, L.K.; Norbury, W.B.; Herdon, D.N. Predicting and managing sepsis in burn patients: Current perspectives. Ther. Clin. Risk Manag. 2017, 13, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Hill, W.F.; Rehou, S.; Pinto, R.; Shahrokhi, S.; Jeschke, M.G. Sepsis criteria versus clinical diagnosis of sepsis in burn patients: A validation of current sepsis scores. Surgery 2018, 164, 1241–1245. [Google Scholar] [CrossRef]
- Cabral, L.; Afreixo, V.; Santos, F.; Almeida, L.; Paiva, J.A. Procalcitonin for the early diagnosis of sepsis in burn patients: A retrospective study. Burns 2017, 43, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Manning, J. Sepsis in the burn patient. Crit. Care Nurs. Clin. N. Am. 2018, 30, 423–430. [Google Scholar] [CrossRef]
- Wardhana, A.; Djan, R.; Halim, Z. Bacterial and antimicrobial susceptibility profile, and the prevalence of sepsis among burn patients at the burn unit of Cipto Mangunkusumo Hospital. Ann. Burn. Fire Disasters 2017, 30, 107–115. [Google Scholar]
- Englert, N.C.; Ross, C. The older adult experiencing sepsis. Crit. Care Nurs. Q. 2015, 38, 175–181. [Google Scholar] [CrossRef]
- Mann-Salinas, E.A.; Baun, M.M.; Meininger, J.C.; Murray, C.K.; Aden, J.K.; Wolf, S.E.; Wade, C.E. Novel predictors of sepsis outperform the American Burn Association. Sepsis criteria in the burn intensive care patient. J. Burn Care Res. 2013, 34, 31–43. [Google Scholar] [CrossRef]
- Holavanahalli, R.K.; Helm, P.A.; Kowalske, K.J. Long-term outcomes in patients surviving large burns: The musculoskeletal system. J. Burn Care Res. 2016, 37, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Blears, E.; Ross, E.; Ogunbileje, J.O.; Porter, C.; Murton, A.J. The impact of catecholamines on skeletal muscle following massive burns: Friend or foe. Burns 2021, 47, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Bonucci, J.; Gragnani, A.; Tricado, M.M.; Vincentin, V.; Aparecida, A.C.S.; Ferreira, L.M. The role of vitamin C in the gene expression of oxidative stress markers in fibroblasts from burn patients. Acta Cir. Bras. 2018, 33, 703–712. [Google Scholar] [CrossRef]
- Nwosu, A.D.G.; Ossai, E.N.; Onwuasoigwe, O.; Ahaotu, F.N.; Aneze, J.K.; Umeji, E.I.; Okonedo, B.; Ogboji, O. Evaluation of ascorbic acid therapy and oxidative stress parameters in burns patients. JAMMR 2020, 32, 296–306. [Google Scholar] [CrossRef]
- Tian, K.Y.; Liu, X.J.; Xu, J.D.; Deng, L.J.; Wang, G. Propofol inhibits burn injury-induced hyperpermeability though an apoptotic signal pathway in microvascular endothelial cells. Braz. J. Med. Biol. Res. 2015, 48, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Foldi, V.; Csontos, C.; Bogar, L.; Roth, E.; Lantos, J. Effects of fluid resuscitation methods on burn trauma-induced oxidative stress. J. Burn Care Res. 2009, 30, 957–966. [Google Scholar] [CrossRef]
- Qin, F.J.; Hu, X.H.; Chen, Z.; Chen, X.; Shen, Y.M. Protective effects of tiopronin against oxidative stress in severely burned patients. Drug Des. Devel. Ther. 2019, 13, 2827–2832. [Google Scholar] [CrossRef]
- Valachova, K.; Svik, K.; Biro, C.; Soltes, L. Skin wound healing with composite biomembranes loaded by troponin or captopril. J. Biotechnol. 2020, 310, 49–53. [Google Scholar] [CrossRef]
- Głowacka, A.; Baczyk, G.; Ultanowska, A. Prevention of infection effective control and treatment of infected burn wounds–the role of a nurse in an interdisciplinary team. Pielęgniarstwo Pol. 2017, 5, 633–639. [Google Scholar] [CrossRef][Green Version]
- Bayuo, J. Nurses’ experiences of caring for severely burned patients. Collegian 2018, 25, 27–32. [Google Scholar] [CrossRef]
- Clark, A.; Imran, J.; Madni, T.; Wolf, S.E. Nutrition and metabolism in burn patients. Burn. Trauma 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Czerwińska-Rogowska, M.; Krajewski, A.; Rudnicki, B.; Nogal, T.; Pacek, D.; Kuras, S.; Domin, M.; Stachura, A.; Markowska, M.; Polakowska, Z.; et al. Żywienie immunomodulujące w kontekście leczenia oparzeń. Chir. Plast. I Oparzenia 2018, 6, 67–70. [Google Scholar] [CrossRef]
- Porter, C.; Tompkins, R.G.; Finnerty, C.C.; Sidossis, L.S.; Suman, O.E.; Herdon, D.N. The metabolic stress response to burn trauma: Current understanding and therapies. Lancet 2016, 388, 1417–1426. [Google Scholar] [CrossRef]
- Mochizuki, H.; Trocki, O.; Dominioni, L.; Backett, K.A.; Joffe, S.N.; Alexander, J.W. Mechanism of prevention of postburn hypermetabolism and catabolism by early enteral feeding. Ann. Surg. 1984, 200, 287–310. [Google Scholar] [CrossRef]
- Rooi, A.; Ndobe, E.; Muganza, A.; Mannell, A.; Nel, M. Enteral nutrition in the unstable burn’s patients. J. Clin. Med. 2020, 2, 163–166. [Google Scholar] [CrossRef]
- Turza, K.C.; Krenisthy, J.; Sawyer, R.G. Enteral feeding, and vasoactive agents: Suggested guidelines for clinicians. Pract. Gastro. 2009, 78, 11–22. [Google Scholar]
- Bittner, E.A.; Shank, E.; Woodson, L.; Martyn, J.A. Acute and perioperative care of the burn-injured patient. Anesthesiology 2015, 122, 448–464. [Google Scholar] [CrossRef]
- Williams, P.I.; Sarginson, R.E.; Ratcliffe, J.M. Use of methadone in the morphine-tolerant burned pediatric patient. Br. J. Anaesth. 1998, 80, 92–95. [Google Scholar] [CrossRef]
- Kariya, N.; Shindoh, M.; Nishi, S.; Yukioka, H.; Asada, A. Oral clonidine for sedation and analgesia in a burn patient. J. Clin. Anaesth. 1998, 10, 514–517. [Google Scholar] [CrossRef]
- Gurfinkel, R.; Czeiger, D.; Douvdevani, A.; Shapira, Y.; Artu, A.A.; Sufaro, Y.; Mazar, J.; Shaked, G. Ketamine improves survival in burn injury followed by sepsis in rats. Anesth. Analg. 2006, 103, 396–402. [Google Scholar] [CrossRef]
- Ambrose, C.; Sale, S.; Howells, R.; Bevan, C.; Jenkins, I.; Weir, P.; Wolf, A. Intravenous clonidine infusion in critically ill children: Dose-dependent sedative effects and cardiovascular stability. Br. J. Anaesth. 2000, 84, 794–796. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, M.; Sakalli, S.; Gunes, Y.; Kesiktas, E.; Ozcengiz, D.; Isik, G. Comparsion of effects of ketamine, ketamine-dexmedetomidine and ketamine-midazolam on dressing changes of burn patients. J. Anaesthesiol. Clin. Pharmacol. 2011, 27, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Marret, E.; Kurdi, O.; Zufferey, P.; Bonnet, F. Effects of nonsteroidal antiimflammatory drugs on patient-controlled analgesia morphine side effects: Meta-analysis of randomized controlled trials. Anesthesiology 2005, 102, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Demling, R.H. Burns: What are the pharmacological treatment options? Expert Opin. Pharm. 2008, 9, 1895–1908. [Google Scholar] [CrossRef]
- Patterson, D.R.; Hofland, H.W.; Espey, K.; Sharar, S. Nursing committee of the international society for burn injuries, Pain Management. Burns 2004, 30, A-10-15. [Google Scholar] [CrossRef]
- Griggs, C.; Goverman, J.; Bittner, E.A.; Levi, B. Sedation, and pain management in burn patients. Clin. Plast. Surg. 2017, 44, 535–540. [Google Scholar] [CrossRef]
- Gray, P.; Williams, B.; Cramond, T. Successful use of gabapentin in acute pain management following burn injury: A case series. Pain Med. 2008, 9, 371–376. [Google Scholar] [CrossRef]
- Gatti, A.; Sabato, A.F.; Occhioni, R.; Collini Baldeschi, G.; Reale, C. Controlled-relase oxycodone and pregabalin in the treatment of neuropathic pain: Results of multicenter Italian study. Eur. Neurol. 2009, 61, 129–137. [Google Scholar] [CrossRef]
- Duhmke, R.M.; Cornblath, D.D.; Hollingshead, J.R. Tramadol for neuropathic pain. Cochrane Database Syst. Rev. 2004, 2, CD003726. [Google Scholar] [CrossRef]
- Wasiak, J.; Spinks, A.; Costello, V.; Ferrano, F.; Paul, E.; Konstantatos, A.; Cleland, H. Adjuvant use of intravenous lidocaine for procedural burn pain relief: A randomized double-blind, placebo-controlled, cross-over trial. Burns 2011, 37, 951–957. [Google Scholar] [CrossRef]
- Witkowski, W. Wykorzystanie opatrunków hydrożelowych w leczeniu wojennych ran oparzeniowych. Chir. Plast. I Oparzenia 2019, 7, 37–41. [Google Scholar] [CrossRef]
- Elbadawy, A.K.; El-Refaie, S.K.; Xin, C. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Goodwin, N.S.; Spinks, A.; Wasiak, J. The efficacy of hydrogel dressings as a first aid measure for burn wound management in the pre-hospital setting: A systematic review of the literature. Int. Wound J. 2015, 13, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Navare, K.J.; Eggeermont, L.; Rogers, Z.J.; Mohammed, H.S.; Colombani, T.; Bencherif, S. Antimicrobial hydrogels: Key considerations and engineering strategies for biomedical applications. Racing Surf. 2020, 511–542. [Google Scholar] [CrossRef]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and modern uses of natural honey in human diseases: A review, Iran. J. Basic Med. Sci. 2013, 16, 731–742. [Google Scholar]
- Mirzaei, B.; Etemadian, S.; Goli, H.R.; Bahonar, S.; Gohlami, S.A.; Karami, P.; Farhadi, M.; Tavakoli, R. Construction and analysis of alginate-based honey hydrogel as an ointment to heal of rat burn wound related infections. Int. J. Burn. Trauma 2018, 8, 88–97. [Google Scholar]
- El-Kased, R.F.; Amer, R.; Attia, D.; Elmazar, M.M. Honey-based hydrogel: In vitro and comparative evaluation for burn wound gealing. Sci. Rep. 2017, 7, 9692. [Google Scholar] [CrossRef]
- Wilemska-Kucharzewska, K.; Klimek, M.; Rojczyk, E.; Kucharzewski, M.; Sopata, M. Rola miodu w procesie gojenia ran. Leczenie Ran 2017, 14, 151–158. [Google Scholar] [CrossRef]
- Febriyenti, F.; Lucida, H.; Almahdy, A.; Alfikriyah, I.; Hanif, M. Wound-healing effect of honey gel and film. J. Pharm. Bioallied. Sci. 2019, 11, 176–180. [Google Scholar] [CrossRef]
- Huang, J.; Frauenlob, M.; Shibata, Y.; Wang, L.; Nakajima, T.; Nonoyama, T.; Tsuda, M.; Tanaka, S.; Kurkova, T.; Ping, G.J. Chitin-based double network hydrogel as potential superficial soft tissue repairing material. Biomacromolecules 2020, 21, 4220–4230. [Google Scholar] [CrossRef]
- Singh, R.; Shitiz, K.; Singh, A. Chitin, and chitosan: Biopolymers for wound management. Int. Wound J. 2017, 14, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- Mezzana, P. Clinical efficacy of new chitin nanofibrils-based gel in wound healing. Acta Chir. Plast. 2008, 50, 81–84. [Google Scholar] [PubMed]
- Latańska, I.; Kolesińska, B.; Draczyński, Z.; Sujka, W. The use of chitin and chitosan in manufacturing dressing materials. Prog. Chem. Appl. Chitin Its Deriv. 2020, 25, 16–36. [Google Scholar] [CrossRef]
- Kozłowska, E.; Banaszkiewicz, Z.; Cierzniakowska, K.; Łabuńska, A.; Szewczyk, M.T.; Popow, A. Zastosowanie terapii podciśnieniowej w leczeniu rozległej rany oparzeniowej–opis przypadku. Leczenie Ran 2018, 15, 141–145. [Google Scholar] [CrossRef]
- Pawlica, P.; Całka, M. Zastosowanie terapii podciśnieniowej w leczeniu ran. Piel. Zdr. Publ. 2020, 10, 127–132. [Google Scholar] [CrossRef]
- Białomyzy, A.; Banasiewicz, T.; Niewęgłowski, T.; Kortych, D.; Tosik, P. Nowe drogi w leczeniu ran przewlekłych–jednoczesne zastosowanie terapii podciśnieniowej i ozonoterapii z wykorzystaniem uniwersalnego portu–opis przypadków. Forum Leczenia Ran 2020, 1, 87–94. [Google Scholar] [CrossRef]
- Polat, E.; Kutlubay, Z.; Sirekbasan, S.; Gokalp, H.; Akarirmak, U. Treatment of pressure ulcers with larvae Lucilla sericata. Turk. J. Phys. Med. Rehabil. 2017, 63, 307–312. [Google Scholar] [CrossRef]
- Mościcka, P.; Szewczyk, M.; Cwajda-Białasik, J. Komplikacje w procesie gojenia rany po aplikacji przeszczepu skórnego–opis przypadków. Leczenie Ran 2017, 14, 159–163. [Google Scholar] [CrossRef]
- Cazander, G.; Pritchard, D.I.; Nigam, Y.; Jung, W.; Nibbering, P.H. Multiple actions of Lucilia sericata larvae in hard to heal wounds. BioEssays 2013, 35, 1083–1092. [Google Scholar] [CrossRef]
- Baer, W.S. The treatment of chronic osteomyelitis with the maggot (larvae of the blow fly). JBJS 1931, 13, 438–475. [Google Scholar]
- Bugaj, M.; Strużyna, J.; Mądry, R.J.; Korzeniowski, T.; Antonov, S. Zastosowanie larw Lucilia sericata w leczeniu oparzeń. Chir. Plast. 2014, 2, 91–96. [Google Scholar] [CrossRef]
- Bazaliński, D.; Kózka, M.; Karnas, M.; Więch, P. Effectiveness of chronic wound debridement with the use of larvae of Lucilia sericata. J. Clin. Med. 2019, 8, 1845. [Google Scholar] [CrossRef] [PubMed]
- Limsopatham, K.; Khamnoi, P.; Sukontason, K.L.; Boonyawan, D.; Chaiwong, T.; Sukontason, K. Sterilization of blow fly eggs, Chrysomya megacephala and Lucilia cuprina, (Diptera: Calliphoridae) for marggot debridement therapy application. Parasitol. Res. 2017, 116, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, A.; Steven, S.L.A. Acellular fish skin grafts for management of split thickness donor sites and partial thickness burns: A case series. Mil. Med. 2019, 184, 16–20. [Google Scholar] [CrossRef]
- Stone, R.; Saathoff, E.C.; Larson, D.A.; Wall, J.T.; Wienandt, N.A.; Skuli, M.; Kjartasson, H.; Natesan, S.; Christy, R.J. Accelerated wound closure of deep partial thickness burns with acellular fish skin graft. Int. J. Mol. Sci. 2021, 22, 1590. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, S.; Kjartansson, H.; Baldursson, B.T.; Astradsdottir, K.; Ågren, M.S.; Hilmarsson, H.; Sigurjonsson, G.F. Acellular Fish Skin Grafts and Pig Urinary Bladder Matrix Assessed in the Collagen-Induced Arthritis Mouse Model. Int. J. Low Extrem. Wounds 2018, 17, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Fiakos, G.; Kuang, Z.; Lo, E. Improved skin regeneration with acellular fish skin grafts. Eng. Regen. 2020, 1, 95–101. [Google Scholar] [CrossRef]
- Afifah, A.; Suparno, O.; Haditjaroko, L.; Tarman, K. Utilization of fish skin waste as a collagen wound dressing on burn injuries: A mini review. Earth Env. Sci. 2019, 335, 012031. [Google Scholar] [CrossRef]
- Chen, H.; Yin, B.; Hu, B.; Zhang, B.; Liu, J.; Jing, Y.; Fan, Z.; Tian, Y.; Wei, X.; Zhang, W. Acellular fish skin enhances wound healing by promoting angiogenesis and collagen deposition. Biomed. Mater. 2021, 16, 045011. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Isolation and characterization of collagen from the cartilages of brownbanded bamboo shark (Chiloscyllium punctatum) and blacktip shark (Carcharhinus limbatus). LWT-Food Sci. Technol. 2009, 43, 792–800. [Google Scholar] [CrossRef]
- Singh, O.; Benjakul, S.; Maqsood, S.; Kishimura, H. Isolation and characterization of collagen extracted from the skin of striped catfish (Pangasianodon hypothalamus). Food Chem. 2011, 124, 97–105. [Google Scholar] [CrossRef]
- Sitje, T.S.; Grøndahl, E.C.; Sørensen, J.A. Clinical innovation fish-derived wound product for cutaneous wounds. Wounds Int. 2018, 9, 44–50. [Google Scholar]
- Coalson, E.; Bishop, E.; Liu, W.; Feng, Y.; Spezia, M.; Liu, B.; Shen, Y.; Wu, D.; Du, S.; Li, A.F.; et al. Stem cell therapy chronic skin wounds in the era of personalized medicine: From bench to bedside. Genes Dis. 2019, 6, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. A concise review on tissue engineered artificial skin grafts for chronic wound treatment: Can we reconstruct functional skin tissue in vitro? Cells 2020, 9, 1622. [Google Scholar] [CrossRef] [PubMed]
- Fathke, C. Contribution of bone marrow-derived cells to skin: Collagen deposition and wound repair. Stem Cells 2004, 22, 813–822. [Google Scholar] [CrossRef]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune regulation of skin wound healing: Mechanisms and novel therapeutic targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Frykberg, R.G.C.; Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Benko, A.; Nocun, M.; Przekora, A. Novel chitosan/agarose/hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: Comprehensive of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells. Int. J. Nanomed. 2019, 14, 6615–6630. [Google Scholar] [CrossRef]
- Atalay, S.; Coruh, A.; Deniz, K. Stromal vascular fraction improves deep thickness burn wound healing. Burns 2014, 40, 1375–1383. [Google Scholar] [CrossRef]
- Zhu, M.; Xue, J.; Lu, S.; Yuan, Y.; Liao, Y.; Qui, J.; Liu, C.; Liao, Q. Anti-inflammatory effect of stromal vascular fraction cells in fat transplantation. Exp. Ther. Med. 2019, 17, 1435–1439. [Google Scholar] [CrossRef]
- Bowles, A.C.; Wise, R.M.; Gerstein, B.Y.; Thomas, R.C.; Ogelman, R.; Febbo, I.; Bunnell, B.A. Immunomodulatory effects of adipose stromal vascular fraction cells promote alternative activation macrophages to repair tissue damage. Stem Cells 2017, 35, 2198–2207. [Google Scholar] [CrossRef] [PubMed]
- Karina, K.; Rosadi, I.; Sobariah, S.; Afini, I.; Widyastuti, T.; Rosalina, I. Comparable effect of adipose-derived stromal vascular fraction and mesenchymal stem cells for wound healing: An in vitro study. Biomed. Res. Ther. 2019, 6, 3412–3421. [Google Scholar] [CrossRef]
- Sun, M.; He, Y.; Zhou, T.; Zhang, P.; Gao, J.; Lu, F. Adipose extracellular matrix/stromal vascular fraction gel secretes angiogenic factors and enhances skin wound healing in a murine model. Biomed. Res. Int. 2017, 2017, 3105780. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Wang, L.; Feng, J.; Lu, F. Treatment of human chronic wounds with autologous extracellular matrix/stromal vascular fraction gel: A strobe-compliant study. Medicine 2018, 97, e11667. [Google Scholar] [CrossRef] [PubMed]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef]
- Jaller, J.A.; Herskovits, I.; Borda, L.J.; Mervis, J.; Darwin, E.; Hirt, P.A.; Lev-Tov, H.; Kirsner, R.S. Evaluation of donor site pain after fractional autologous full-thickness skin grafting. Adv. Wound Care 2018, 7, 309–314. [Google Scholar] [CrossRef]
- Patterson, C.W.; Stark, M.; Sharma, S.; Mundinger, G.S. Regeneration and expansion of autologous full-thickness kin through a self-propagating autologous skin graft technology. Clin. Case Rep. 2019, 7, 2449–2455. [Google Scholar] [CrossRef]
- Gupta, D.K. Thin and ultra-thin split thickness skin grafts (STSG-UT, STSG-T). In Mikroskin Grafting for Vitiligo; Springer: London, UK, 2009; pp. 15–18. [Google Scholar] [CrossRef]
- Dixit, S.; Baganizi, D.R.; Sahu, R.; Dosunmu, E.; Chaudhari, A.; Vig, K.; Pillai, S.R.; Dennis, V.A. Immunological challenges associated with artificial skin grafts: Available solutions and stem cells in future design of synthetic skin. J. Biol. Eng. 2017, 11, 1–23. [Google Scholar] [CrossRef]
- Varkey, M.; Ding, J.; Tredget, E. Advances in skin substitutes–potential of tissue engineered skin for facilitating antifibrotic healing. J. Funct. Biomater. 2015, 6, 547–563. [Google Scholar] [CrossRef]
- Nathoo, R.; Howe, N.; Cohen, G. Skin substitutes: An overview of the key players in wound management. J. Clin. Aesthet. Dermatol. 2014, 7, 44–48. [Google Scholar]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Michniak-Kohn, B.B. Tissue engineered human skin equivalents. Pharmaceutics 2012, 4, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced smart biometerials and constructs for hard tissue engineering and regeneration. Bone Res. 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Frisman, I.; Seliktar, D.; Bianco-Peled, H. Nanostructuring of PEG-fibrinogen polymeric scaffolds. Acta Biomater. 2010, 6, 2518–2524. [Google Scholar] [CrossRef]
- Lee, C.H.; Chang, S.H.; Chen, W.J.; Hung, K.C.; Lin, Y.; Liu, S.; Hsieh, M.J.; Pang, J.H.S.; Juang, J.H. Augemtation of diabetic wound healing and enhancement of collagen content using nanofibrous Glucophage-loaded collagen/PLGA scaffold membranes. J. Colloid Interface Sci. 2015, 439, 88–98. [Google Scholar] [CrossRef]
- Kaur, A.; Midha, S.; Giri, S.; Mohanty, S. Functional skin grafts: Where biomaterials meet stem cells. Stem Cell Int. 2019, 2019, 1286054. [Google Scholar] [CrossRef]
- Savoji, H.; Godau, B.; Hassani, M.S.; Akbari, M. Skin tissue substitutes and biomaterial risk assacment and testing. Front. Bioeng. Biotechnol. 2018, 6, 86. [Google Scholar] [CrossRef]
- Paggiaro, A.O.; Mathor, M.B.; Teodoro, W.R.; Isaac, C.; Capelozzi, V.L.; Gemperli, R. Evaluation of radiosterilized glyercerolated amniotic membranes as a substrate for cultured human epithelial cells. Organogenesis 2020, 16, 27–41. [Google Scholar] [CrossRef]
- Kumar, R.J.; Kimble, R.M.; Boots, R.; Pegg, S.P. Treatment of partial-thickness burns: A prospective, randomized trial using transcyte. ANZ J. Surg. 2004, 74, 622–626. [Google Scholar] [CrossRef]
- Myers, S.; Partha, V.; Soranzo, C.; Price, R.; Navsaria, H. Hyalomatrix: A temporary epidermal barrier, hyaluronan delivery and neodermis induction system for keratinocyte stem cell therapy. Tissue Eng. 2007, 13, 2733–2741. [Google Scholar] [CrossRef]
- Cubo, N.; Garcia, M.; Del Canizo, J.; Velasco, D.; Jarcano, J. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2016, 9, 015006. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, T.; Merola, J.; Catarino, C.; Xie, C.; Kirkiles-Smith, N.; Lee, V.; Hotta, S.; Dai, G.; Xu, X.; Ferreira, F.; et al. Three dimensional bioprinting of a vascularized and perfusable skin graft using human keratinocytes, fibroblasts, pericytes and endothelial cells. Tissue Eng. Part A 2019, 26, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.R.; Ju, H.W.; Lee, J.M.; Kim, D.K.; Lee, O.J.; Moon, B.M.; Park, H.J.; Jeong, J.Y.; Yeon, Y.K.; Park, C.H. Three-dimensional electrospun silk-fibroin nanofiber for skin tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced materials from fungal mycelium: Fabrication and tuning of physical properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Chan, E.; To, E.; Lau, C.; Yen, S.; King, W. Development, and evaluation of a new composite Laserskin graft. J. Trauma 1999, 47, 918–922. [Google Scholar] [CrossRef]
- Soejima, K.; Kashimura, T.; Kazama, T.; Matsumoto, T.; Nakazawa, H. Effect of mature adipocyte-derived dedifferentiated fat cells on formation of basement membrane after cultured epithelial autograft on artificial dermis. Plast. Reconstr. Surg. 2019, 143, 983e–992e. [Google Scholar] [CrossRef]
- Hayashi, M.; Yoshitake, K.; Tokunaka, R.; Yoshida, Y.; Oshima, M.; Tatsuta, S.; Hamada, T.; Kamitomo, A.; Hamajima, A. Combination of meshed dermis graft and cultured epithelial autograft for massive burns. Three case reports. Medicine 2018, 97, 48. [Google Scholar] [CrossRef]
- Hayashi, M.; Muramatsu, H.; Nakani, M.; Yamamoto, N.; Tokunaka, R.; Umezwa, K.; Hamajima, A.; Araki, N.; Yoshumoto, S. Changes in the dorsal structure during cultured epidermal autograft engrafment process. Plast. Reconstr. Surg. 2016, 4, e870. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Paggiaro, A.O.; Isaac, C.; Teixeira Neto, N.; Santos, G.B. dos Subitutos cutaneous: Conceitos atuais e proposta de classificaco. Rev. Bras. Cir. Plastica 2011, 26, 696–702. [Google Scholar] [CrossRef]
- Vana, L.P.M.; Battlehner, C.N.; Ferreira, M.A.; Caldini, E.G.; Gemperli, R.; Alonso, N. Comparative long-term study between two dermal regeneration templates for the reconstruction of burn scar contractures in humans: Clinical and histological results. Burns 2019, 46, 596–608. [Google Scholar] [CrossRef]
- Kang, S.W.; Park, J.K.; Shon, H.C.; Choi, E.S.; Kim, D.S.; Min, K.T. Skin grafts using MartiDerm® for plantar defects after excision of skin cancer. Cancer Manag. Res. 2019, 11, 2947–2950. [Google Scholar] [CrossRef]
- Cristofari, S.; Guenane, Y.; Atlan, M.; Hallier, A.; Revol, M.; Stivala, A. Coverage of radial forearm flap donor site with full thickness skin frat and Martiderm®: An alternative reliable solution? Ann. Chir. Plast. Esthet. 2020, 65, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Lucas, D.; Di Rocco, D.; Muller, C.T.; Jurjus, A.R.; Raffoul, W.; Di Summa, P.G.; Watfa, W. Application of dermal skin substitutes for hand and finger palmar soft tissue loss. Plast. Reconstr. Surg. 2019, 7, e2551. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.; Cho, Y.S.; Joo, S.Y.; Seo, C.H.; Cho, Y.S. A clinical trial with a novel collagen dermal substitute for wound healing in burn patients. Biomater. Sci. 2020, 8, 823–829. [Google Scholar] [CrossRef]
- Ribeiro, L.M.; Serras, R.; Guimares, D.; Viela, M.; Mouzinho, M.M. Bilateral third-degree burn of the legs: Lower limb salvage with dermal regenerative matrix. Ann. Burn. Fire Disasters 2018, 31, 228–232. [Google Scholar]
- Yiğitbaş, H.; Yavuz, E.; Beken Özdemir, E.; Önen, Ö.; Pençe, H.; Meriç, S. Our experience with dermal substitute Nevelia® in the treatment of severity burned patients. Ulus Travma Acil Cerrahi. Derg. 2019, 25, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Uccioli, L.A. A clinical investigation on the characteristics and outcomes of treating chronic lower extremity wounds using the tissutech autograft system. Int. J. Low Extrem. Wounds 2003, 2, 140–151. [Google Scholar] [CrossRef]
- Hart, C.E.; Loewen-Rodgiguez, A.; Lessern, J. Dermgraft: Use in the treatment of chronic wounds. Adv. Wound Care 2012, 1, 138–141. [Google Scholar] [CrossRef]
- Fang, J.J.; Li, P.E.; Wu, J.J.; Zhou, H.Y.; Xie, L.P.; Lu, H. Reconstruction of massive skin avulsion of the scrota and penis by combined application of dermal regeneration template (Pelnac) and split-thickness skin graft with vacuum-sccisted closure: A case report. World J. Clin. Cases 2019, 7, 4349–4354. [Google Scholar] [CrossRef] [PubMed]
- PELNACTM–Robust, Stable, Infection Resistant and Affordable–The Ideal Dermal Substitute. Available online: https://www.eurosurgical.co.uk/wound-care-burns/pelnac/ (accessed on 19 March 2020).
- Waymack, P.; Duff, R.G.; Sabolinski, M. The effect of a tissue engineered bilayered living skin along, over meshed split-thickness autografts on the healing of excised burn wounds. Burn 2020, 26, 609–619. [Google Scholar] [CrossRef]
- Curran, M.P.; PLosker, G.I. Bilayered bioengineered skin substitute (Apligraf): A review of its use in the treatment of venous leg ulcers and diabetic foot ulcers. BioDrugs 2002, 16, 439–455. [Google Scholar] [CrossRef] [PubMed]
| Type of the Graft | Commercial Product | Polymeric Composition | Indications for Use | Ref. |
|---|---|---|---|---|
| Epidermal Cellular: keratinocytes | Laserskin® (Fidia Advanced Biopolymers Ltd., Abano Terme [PD], Italy) | Benzyl esterified hyaluronan derivative | Burn wounds | [157] |
| Epidermal Cellular: keratinocytes (Keratinocyte sheet prepared based on green method) | JACE® (Japan Tissue Engineering Co., Ltd., Aichi, Japan) | No polymer used | Extensive burn wounds | [158,159,160] |
| Dermal Acellular | Matriderm® (Medskin Solutiions Dr. Suwelack Skin & Health Care AG, Billerbeck, Germany) | Bovine type I collagen, elastin | Full-thickness burns | [161,162,163,164,165] |
| Dermal Acellular | Insuregraf® (SK-Bioland Co. Ltd., South Korea) | Porcine type I collagen | Burn wounds | [166] |
| Dermal Acellular | Integra® (Integra LifeSciences Servoces, USA) | Bovine Type I collagen, chondroitin-6- sulfate | Partial- and full-thickness burns | [162,167] |
| Dermal Acellular | Nevelia® (Symatese Aesthetics, Lyon, France) | Calf type I collagen | Burn wounds | [168] |
| Dermal Cellular: fibroblasts | Hyalograft 3D® (Anika Therapeutics, Bedford, MA, USA) | Hyaluronic acid | Deep burns | [169] |
| Dermal Cellular: human neonatal fibroblasts | Dermagraft® (Organogenesis, Canton, MA, USA) | Polyglactin | Burn wounds | [170] |
| Dermo-epidermal Acellular | Biobrane® (Smith & Nephew UK Limited, London, UK) | Porcine type I collagen, nylon, silicone | Partial- and full-thickness burns in children | [136,137,138,139,140,141] |
| Dermo-epidermal Acellular | Hyalomatrix® (Fidia Advanced Biopolymers, FAB, Italy) | Hyaluronic acid, silicone | Burn wounds | [152] |
| Dermo-epidermal Acellular | PELNAC™ (Gunze Co., Ltd., Kyoto, Japan) | Porcine atelocollagen, silicone | Large acute burns | [171,172] |
| Dermo-epidermal Cellular: fibroblasts, keratinocytes | Apligraf® (Organogenesis, Canton, MA, USA) | Bovine type I collagen | Partial- and full-thickness burns | [173,174] |
| Dermo-epidermal Cellular: human neonatal fibroblasts | TransCyte® (Advanced Tissue Sciences, La Jolla, Calif) | Porcine type I collagen, polyglactin | Partial- and full-thickness burns | [151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markiewicz-Gospodarek, A.; Kozioł, M.; Tobiasz, M.; Baj, J.; Radzikowska-Büchner, E.; Przekora, A. Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice. Int. J. Environ. Res. Public Health 2022, 19, 1338. https://doi.org/10.3390/ijerph19031338
Markiewicz-Gospodarek A, Kozioł M, Tobiasz M, Baj J, Radzikowska-Büchner E, Przekora A. Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice. International Journal of Environmental Research and Public Health. 2022; 19(3):1338. https://doi.org/10.3390/ijerph19031338
Chicago/Turabian StyleMarkiewicz-Gospodarek, Agnieszka, Małgorzata Kozioł, Maciej Tobiasz, Jacek Baj, Elżbieta Radzikowska-Büchner, and Agata Przekora. 2022. "Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice" International Journal of Environmental Research and Public Health 19, no. 3: 1338. https://doi.org/10.3390/ijerph19031338
APA StyleMarkiewicz-Gospodarek, A., Kozioł, M., Tobiasz, M., Baj, J., Radzikowska-Büchner, E., & Przekora, A. (2022). Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice. International Journal of Environmental Research and Public Health, 19(3), 1338. https://doi.org/10.3390/ijerph19031338