Reprotoxic Impact of Environment, Diet, and Behavior
Abstract
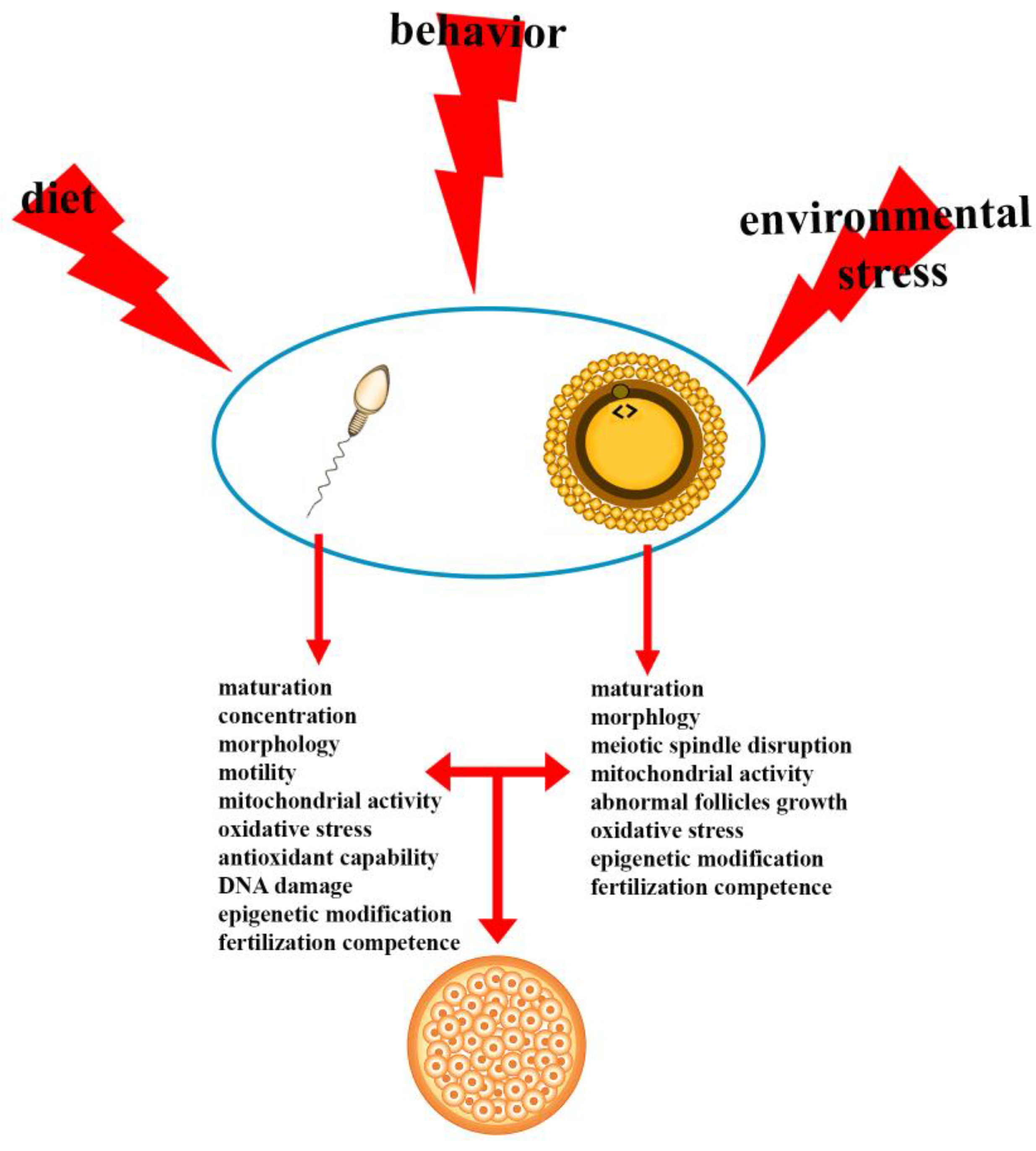
1. Introduction
2. Environmental Chemical Stressors
3. Diet and Behavior Impact on Male Reproductive Health
4. Diet and Behavior Impact on Female Reproductive Health
5. Epigenetic Regulation of Lifestyle-Affected Human Fertility
6. Resilience of Life Style Impact on Reproduction
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Chaminda, G.T.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Gallo, A.; Boni, R.; Buia, M.C.; Monfrecola, V.; Esposito, M.C.; Tosti, E. Ocean acidification impact on ascidian Ciona robusta spermatozoa: New evidence for stress resilience. Sci. Total Environ. 2019, 697, 134100. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Esposito, M.C.; Cuccaro, A.; Buia, M.C.; Tarallo, A.; Monfrecola, V.; Tosti, E.; Boni, R. Adult exposure to acidified seawater influences sperm physiology in Mytilus galloprovincialis: Laboratory and in situ transplant experiments. Environ. Pollut. 2020, 265, 115063. [Google Scholar] [CrossRef] [PubMed]
- Boni, R.; Gallo, A.; Montanino, M.; Macina, A.; Tosti, E. Dynamic changes in the sperm quality of Mytilus galloprovincialis under continuous thermal stress. Mol. Reprod. Dev. 2016, 83, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Boni, R.; Tosti, E. Gamete quality in a multistressor environment. Environ. Int. 2020, 138, 105627. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.; Wilding, M.; Coppola, G.; Tosti, E. How do spermatozoa activate oocytes? Reprod. Biomed. Online 2010, 21, 1–3. [Google Scholar] [CrossRef][Green Version]
- Tosti, E.; Ménézo, Y. Gamete activation: Basic knowledge and clinical applications. Hum. Reprod. Update 2016, 22, 420–439. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, D.; Yu, H.; Zheng, L.; Xu, Y.; Wen, H.; Dai, X.; Wang, S. Oxidative stress-related mitochondrial dysfunction as a possible reason for obese male infertility. Nutr. Clin. Métab. 2021, 35, 123–128. [Google Scholar] [CrossRef]
- Alvarez, S. Do some addictions interfere with fertility? Fertil. Steril. 2015, 103, 22–26. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Schlaff, W.D. Introduction. Fertil. Steril. 2018, 110, 557–559. [Google Scholar] [CrossRef]
- Nazni, P. Association of western diet & lifestyle with decreased fertility. Indian J. Med. Res. 2014, 140, S78. [Google Scholar] [PubMed]
- Pourmasumi, S.; Sabeti, P.; Rahiminia, T.; Mangoli, E.; Tabibnejad, N.; Talebi, A.R. The etiologies of DNA abnormalities in male infertility: An assessment and review. Int. J. Reprod. BioMed. 2017, 15, 331. [Google Scholar] [CrossRef]
- Bellver, J.; Mariani, G. Impact of parental over- and underweight on the health of offspring. Fertil. Steril. 2019, 111, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Aoun, A.; Khoury, V.E.; Malakieh, R. Can Nutrition Help in the Treatment of Infertility? Prev. Nutr. Food Sci. 2021, 26, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Wurl, O.; Obbard, J.P. A review of pollutants in the sea-surface microlayer (SML): A unique habitat for marine organisms. Mar. Pollut. Bull. 2004, 48, 1016–1030. [Google Scholar] [CrossRef] [PubMed]
- Ritter, L.; Solomon, K.; Sibley, P.; Hall, K.; Keen, P.; Mattu, G.; Linton, B. Sources, pathways, and relative risks of contaminants in surface water and groundwater: A perspective prepared for the walkerton inquiry. J. Toxicol. Environ. Health Part A 2002, 65, 1–142. [Google Scholar]
- Carnevali, O.; Santangeli, S.; Forner-Piquer, I.; Basili, D.; Maradonna, F. Endocrine-disrupting chemicals in aquatic environment: What are the risks for fish gametes? Fish Physiol. Biochem. 2018, 44, 1561–1576. [Google Scholar] [CrossRef] [PubMed]
- Falchuk, K.H.; Montorzi, M. Zinc physiology and biochemistry in oocytes and embryos. BioMetals 2001, 14, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Jamalan, M.; Ghaffari, M.A.; Hoseinzadeh, P.; Hashemitabar, M.; Zeinali, M. Human sperm quality and metal toxicants: Protective effects of some flavonoids on male reproductive function. Int. J. Fertil. Steril. 2016, 10, 215. [Google Scholar]
- Wang, E.; Huang, Y.; Du, Q.; Sun, Y. Silver nanoparticle induced toxicity to human sperm by increasing ROS(reactive oxygen species) production and DNA damage. Environ. Toxicol. Pharmacol. 2017, 52, 193–199. [Google Scholar] [CrossRef]
- Li, N.; Hou, Y.-H.; Jing, W.-X.; Dahms, H.-U.; Wang, L. Quality decline and oxidative damage in sperm of freshwater crab Sinopotamon henanense exposed to lead. Ecotoxicol. Environ. Saf. 2016, 130, 193–198. [Google Scholar] [CrossRef]
- Li, C.; Zhao, K.; Zhang, H.; Liu, L.; Xiong, F.; Wang, K.; Chen, B. Lead exposure reduces sperm quality and DNA integrity in mice. Environ. Toxicol. 2018, 33, 594–602. [Google Scholar] [CrossRef]
- Adamkovicova, M.; Toman, R.; Martiniakova, M.; Omelka, R.; Babosova, R.; Krajcovicova, V.; Grosskopf, B.; Massanyi, P. Sperm motility and morphology changes in rats exposed to cadmium and diazinon. Reprod. Biol. Endocrinol. 2016, 14, 42. [Google Scholar] [CrossRef]
- Marettová, E.; Maretta, M.; Legáth, J. Toxic effects of cadmium on testis of birds and mammals: A review. Anim. Reprod. Sci. 2015, 155, 1–10. [Google Scholar] [CrossRef]
- Oliveira, H.; Spanò, M.; Santos, C.; de Lourdes Pereira, M. Adverse effects of cadmium exposure on mouse sperm. Reprod. Toxicol. 2009, 28, 550–555. [Google Scholar] [CrossRef]
- Zhao, L.-L.; Ru, Y.-F.; Liu, M.; Tang, J.-N.; Zheng, J.-F.; Wu, B.; Gu, Y.-H.; Shi, H.-J. Reproductive effects of cadmium on sperm function and early embryonic development in vitro. PLoS ONE 2017, 12, e0186727. [Google Scholar] [CrossRef]
- De Guglielmo, V.; Puoti, R.; Notariale, R.; Maresca, V.; Ausió, J.; Troisi, J.; Verrillo, M.; Basile, A.; Febbraio, F.; Piscopo, M. Alterations in the properties of sperm protamine-like II protein after exposure of Mytilus galloprovincialis (Lamarck 1819) to sub-toxic doses of cadmium. Ecotoxicol. Environ. Saf. 2019, 169, 600–606. [Google Scholar] [CrossRef]
- Piscopo, M.; Trifuoggi, M.; Notariale, R.; Labar, S.; Troisi, J.; Giarra, A.; Rabbito, D.; Puoti, R.; Brundo, M.V.; Basile, A.; et al. Protamine-like proteins analyses as emerging biotechnique for cadmium impact assessment on male mollusk Mytilus galloprovincialis (Lamarck 1819). Acta Biochim. Pol. 2018, 65, 259–267. [Google Scholar] [CrossRef]
- Maresca, V.; Lettieri, G.; Sorbo, S.; Piscopo, M.; Basile, A. Biological responses to cadmium stress in liverwort Conocephalum conicum (Marchantiales). Int. J. Mol. Sci. 2020, 21, 6485. [Google Scholar] [CrossRef]
- Bergamo, P.; Andreassi, M.G.; Lorenzetti, S. The role of human semen as an early and reliable tool of environmental impact assessment. In Spermatozoa-Facts and Perspectives; Meccariello, R., Chianese, R., Eds.; IntechOpen: London, UK, 2018; pp. 174–202. [Google Scholar]
- Gallo, A. Toxicity of marine pollutants on the ascidian oocyte physiology: An electrophysiological approach. Zygote 2018, 26, 14–23. [Google Scholar] [CrossRef]
- Gallo, A.; Russo, G.L.; Tosti, E. T-Type Ca2+ Current Activity during Oocyte Growth and Maturation in the Ascidian Styela plicata. PLoS ONE 2013, 8, e54604. [Google Scholar] [CrossRef]
- Gallo, A.; Silvestre, F.; Cuomo, A.; Papoff, F.; Tosti, E. The impact of metals on the reproductive mechanisms of the ascidian Ciona intestinalis: Metals and reproduction. Mar. Ecol. 2011, 32, 222–231. [Google Scholar] [CrossRef]
- Au, D.W.T.; Lee, C.Y.; Chan, K.L.; Wu, R.S.S. Reproductive impairment of sea urchins upon chronic exposure to cadmium. Part I: Effects on gamete quality. Environ. Pollut. 2001, 111, 1–9. [Google Scholar] [CrossRef]
- Migliaccio, O.; Castellano, I.; Cirino, P.; Romano, G.; Palumbo, A. Maternal Exposure to Cadmium and Manganese Impairs Reproduction and Progeny Fitness in the Sea Urchin Paracentrotus lividus. PLoS ONE 2015, 10, e0131815. [Google Scholar] [CrossRef]
- Pavlaki, M.D.; Araújo, M.J.; Cardoso, D.N.; Silva, A.R.R.; Cruz, A.; Mendo, S.; Soares, A.M.V.M.; Calado, R.; Loureiro, S. Ecotoxicity and genotoxicity of cadmium in different marine trophic levels. Environ. Pollut. 2016, 215, 203–212. [Google Scholar] [CrossRef]
- Martino, N.A.; Marzano, G.; Mangiacotti, M.; Miedico, O.; Sardanelli, A.M.; Gnoni, A.; Lacalandra, G.M.; Chiaravalle, A.E.; Ciani, E.; Bogliolo, L.; et al. Exposure to cadmium during in vitro maturation at environmental nanomolar levels impairs oocyte fertilization through oxidative damage: A large animal model study. Reprod. Toxicol. 2017, 69, 132–145. [Google Scholar] [CrossRef]
- Lettieri, G.; Notariale, R.; Ambrosino, A.; Di Bonito, A.; Giarra, A.; Trifuoggi, M.; Manna, C.; Piscopo, M. Spermatozoa Transcriptional Response and Alterations in PL Proteins Properties after Exposure of Mytilus galloprovincialis to Mercury. Int. J. Mol. Sci. 2021, 22, 1618. [Google Scholar] [CrossRef]
- Lettieri, G.; Notariale, R.; Carusone, N.; Giarra, A.; Trifuoggi, M.; Manna, C.; Piscopo, M. New Insights into Alterations in PL Proteins Affecting Their Binding to DNA after Exposure of Mytilus galloprovincialis to Mercury—A Possible Risk to Sperm Chromatin Structure? Int. J. Mol. Sci. 2021, 22, 5893. [Google Scholar] [CrossRef]
- Swan, S.H. Semen quality in fertile US men in relation to geographical area and pesticide exposure. Int. J. Androl. 2006, 29, 62–68. [Google Scholar] [CrossRef]
- Silveyra, G.R.; Silveyra, P.; Vatnick, I.; Medesani, D.A.; Rodríguez, E.M. Effects of atrazine on vitellogenesis, steroid levels and lipid peroxidation, in female red swamp crayfish Procambarus clarkii. Aquat. Toxicol. 2018, 197, 136–142. [Google Scholar] [CrossRef]
- McBirney, M.; King, S.E.; Pappalardo, M.; Houser, E.; Unkefer, M.; Nilsson, E.; Sadler-Riggleman, I.; Beck, D.; Winchester, P.; Skinner, M.K. Atrazine induced epigenetic transgenerational inheritance of disease, lean phenotype and sperm epimutation pathology biomarkers. PLoS ONE 2017, 12, e0184306. [Google Scholar] [CrossRef] [PubMed]
- Lugowska, K. The effects of Roundup on gametes and early development of common carp (Cyprinus carpio L.). Fish Physiol. Biochem. 2018, 44, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Amara, I.; Miled, W.; Slama, R.B.; Ladhari, N. Antifouling processes and toxicity effects of antifouling paints on marine environment. A review. Environ. Toxicol. Pharmacol. 2018, 57, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.S. Tributyltin compounds induce male characteristics on female mud snailsNassarius obsoletus = Ilyanassa obsoleta. J. Appl. Toxicol. 1981, 1, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, E.; McClellan-Green, P. Mechanisms of imposex induction in the mud snail, Ilyanassa obsoleta: TBT as a neurotoxin and aromatase inhibitor. Mar. Environ. Res. 2002, 54, 715–718. [Google Scholar] [CrossRef]
- Franchet, C.; Goudeau, M.; Goudeau, H. Tributyltin impedes early sperm-egg interactions at the egg coat level in the ascidian Phallusia mammillata but does not prevent sperm-egg fusion in naked eggs. Aquat. Toxicol. 1998, 44, 213–228. [Google Scholar] [CrossRef]
- Gallo, A.; Tosti, E. Adverse Effect of Antifouling Compounds on the Reproductive Mechanisms of the Ascidian Ciona intestinalis. Mar. Drugs 2013, 11, 3554–3568. [Google Scholar] [CrossRef]
- Gallo, A.; Tosti, E. Reprotoxicity of the Antifoulant Chlorothalonil in Ascidians: An Ecological Risk Assessment. PLoS ONE 2015, 10, e0123074. [Google Scholar] [CrossRef]
- Akcha, F.; Spagnol, C.; Rouxel, J. Genotoxicity of diuron and glyphosate in oyster spermatozoa and embryos. Aquat. Toxicol. 2012, 106–107, 104–113. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Xu, X.-L.; Deng, S.-L.; Lian, Z.-X.; Yu, K. Oestrogenic endocrine disruptors in the placenta and the fetus. Int. J. Mol. Sci. 2020, 21, 1519. [Google Scholar] [CrossRef]
- Duty, S.M.; Silva, M.J.; Barr, D.B.; Brock, J.W.; Ryan, L.; Chen, Z.; Herrick, R.F.; Christiani, D.C.; Hauser, R. Phthalate Exposure and Human Semen Parameters. Epidemiology 2003, 14, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R. The Environment and Male Fertility: Recent Research on Emerging Chemicals and Semen Quality. Semin. Reprod. Med. 2006, 24, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Hanke, W.; Radwan, M.; Bonde, J. Environmental factors and semen quality. Int. J. Occup. Med. Environ. Health 2009, 22, 305–329. [Google Scholar] [CrossRef] [PubMed]
- Wirth, J.J.; Rossano, M.G.; Potter, R.; Puscheck, E.; Daly, D.C.; Paneth, N.; Krawetz, S.A.; Protas, B.M.; Diamond, M.P. A Pilot Study Associating Urinary Concentrations of Phthalate Metabolites and Semen Quality. Syst. Biol. Reprod. Med. 2008, 54, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, I.; Tagliaferri, S.; Sommella, E.; Salviati, E.; Porri, D.; Raspini, B.; Cena, H.; Campiglia, P.; La Rocca, C.; Cerbo, R.M.; et al. Lifestyle Habits and Exposure to BPA and Phthalates in Women of Childbearing Age from Northern Italy: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 9710. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Mínguez-Alarcón, L.; Hauser, R.; Gaskins, A.J. Effects of bisphenol A on male and couple reproductive health: A review. Fertil. Steril. 2016, 106, 864–870. [Google Scholar] [CrossRef]
- Chianese, R.; Troisi, J.; Richards, S.; Scafuro, M.; Fasano, S.; Guida, M.; Pierantoni, R.; Meccariello, R. Bisphenol A in reproduction: Epigenetic effects. Curr. Med. Chem. 2018, 25, 748–770. [Google Scholar] [CrossRef]
- Ziv-Gal, A.; Flaws, J.A. Evidence for bisphenol A-induced female infertility: A review (2007–2016). Fertil. Steril. 2016, 106, 827–856. [Google Scholar] [CrossRef]
- Pivonello, C.; Muscogiuri, G.; Nardone, A.; Garifalos, F.; Provvisiero, D.P.; Verde, N.; de Angelis, C.; Conforti, A.; Piscopo, M.; Auriemma, R.S.; et al. Bisphenol A: An emerging threat to female fertility. Reprod. Biol. Endocrinol. 2020, 18, 22. [Google Scholar] [CrossRef]
- Raimondo, S.; Gentile, M.; Esposito, G.; Gentile, T.; Ferrara, I.; Crescenzo, C.; Palmieri, M.; Cuomo, F.; De Filippo, S.; Lettieri, G. Could Kallikrein-Related Serine Peptidase 3 Be an Early Biomarker of Environmental Exposure in Young Women? Int. J. Environ. Res. Public Health 2021, 18, 8833. [Google Scholar] [CrossRef]
- Rubes, J.; Selevan, S.G.; Evenson, D.P.; Zudova, D.; Vozdova, M.; Zudova, Z.; Robbins, W.A.; Perreault, S.D. Episodic air pollution is associated with increased DNA fragmentation in human sperm without other changes in semen quality. Hum. Reprod. 2005, 20, 2776–2783. [Google Scholar] [CrossRef]
- Somers, C.M. Ambient air pollution exposure and damage to male gametes: Human studies and in situ ‘sentinel’ animal experiments. Syst. Biol. Reprod. Med. 2011, 57, 63–71. [Google Scholar] [CrossRef]
- Montano, L.; Donato, F.; Bianco, P.M.; Lettieri, G.; Guglielmino, A.; Motta, O.; Bonapace, I.M.; Piscopo, M. Semen quality as a potential susceptibility indicator to SARS-CoV-2 insults in polluted areas. Environ. Sci. Pollut. Res. 2021, 28, 37031–37040. [Google Scholar] [CrossRef]
- Montano, L.; Donato, F.; Bianco, P.M.; Lettieri, G.; Guglielmino, A.; Motta, O.; Bonapace, I.M.; Piscopo, M. Air pollution and COVID-19: A possible dangerous synergy for male fertility. Int. J. Environ. Res. Public Health 2021, 18, 6846. [Google Scholar] [CrossRef]
- Vahedian-Azimi, A.; Karimi, L.; Makvandi, S.; Jamialahmadi, T.; Sahebkar, A. Does SARS-CoV-2 Threaten Male Fertility? Clin. Biol. Mol. Asp. COVID-19 2021, 19, 139–146. [Google Scholar]
- Maresca, V.; Fusaro, L.; Sorbo, S.; Siciliano, A.; Loppi, S.; Paoli, L.; Monaci, F.; Karam, E.A.; Piscopo, M.; Guida, M.; et al. Functional and structural biomarkers to monitor heavy metal pollution of one of the most contaminated freshwater sites in Southern Europe. Ecotoxicol. Environ. Saf. 2018, 163, 665–673. [Google Scholar] [CrossRef]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the involvement in DNA oxidative damage of human sperm nuclear basic proteins of healthy young men living in polluted areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef]
- Lettieri, G.; Marra, F.; Moriello, C.; Prisco, M.; Notari, T.; Trifuoggi, M.; Giarra, A.; Bosco, L.; Montano, L.; Piscopo, M. Molecular alterations in spermatozoa of a family case living in the land of fires. A first look at possible transgenerational effects of pollutants. Int. J. Mol. Sci. 2020, 21, 6710. [Google Scholar] [CrossRef]
- Montano, L.; Ceretti, E.; Donato, F.; Bergamo, P.; Zani, C.; Viola, G.C.V.; Notari, T.; Pappalardo, S.; Zani, D.; Ubaldi, S.; et al. Effects of a Lifestyle Change Intervention on Semen Quality in Healthy Young Men Living in Highly Polluted Areas in Italy: The FASt Randomized Controlled Trial. Eur. Urol. Focus 2021. [Google Scholar] [CrossRef]
- Caron, C.; Govin, J.; Rousseaux, S.; Khochbin, S. How to Pack the Genome for a Safe Trip. In Epigenetics and Chromatin; Jeanteur, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 38, pp. 65–89. [Google Scholar]
- Boissonneault, G. Chromatin remodeling during spermiogenesis: A possible role for the transition proteins in DNA strand break repair. FEBS Lett. 2002, 514, 111–114. [Google Scholar] [CrossRef]
- Ménézo, Y.; Dale, B.; Cohen, M. DNA damage and repair in human oocytes and embryos: A review. Zygote 2010, 18, 357–365. [Google Scholar] [CrossRef]
- Setti, A.S.; de Almeida Ferreira Braga, D.P.; Provenza, R.R.; Iaconelli, A., Jr.; Borges, E., Jr. Oocyte ability to repair sperm DNA fragmentation: The impact of maternal age on intracytoplasmic sperm injection outcomes. Fertil. Steril. 2021, 116, 123–129. [Google Scholar] [CrossRef]
- Bungum, M.; Humaidan, P.; Axmon, A.; Spano, M.; Bungum, L.; Erenpreiss, J.; Giwercman, A. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum. Reprod. 2007, 22, 174–179. [Google Scholar] [CrossRef]
- Galotto, C.; Cambiasso, M.Y.; Julianelli, V.L.; Valzacchi, G.J.R.; Rolando, R.N.; Rodriguez, M.L.; Calvo, L.; Calvo, J.C.; Romanato, M. Human sperm decondensation in vitro is related to cleavage rate and embryo quality in IVF. J. Assist. Reprod. Genet. 2019, 36, 2345–2355. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sikka, S. The role of free radicals and antioxidants in reproduction. Curr. Opin. Obstet. Gynecol. 2006, 18, 325–332. [Google Scholar] [CrossRef]
- Bisht, S.; Dada, R. Oxidative stress: Major executioner in disease pathology, role in sperm DNA damage and preventive strategies. Front. Biosci. 2017, 9, 420–447. [Google Scholar]
- Tirpák, F.; Greifová, H.; Lukáč, N.; Stawarz, R.; Massányi, P. Exogenous Factors Affecting the Functional Integrity of Male Reproduction. Life 2021, 11, 213. [Google Scholar] [CrossRef]
- Daumler, D.; Chan, P.; Lo, K.; Takefman, J.; Zelkowitz, P. Men’s knowledge of their own fertility: A population-based survey examining the awareness of factors that are associated with male infertility. Hum. Reprod. 2016, 31, 2781–2790. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Tusimin, M.B.; İrez, T.; Krajewska-Kulak, E. Sperm counts in Asian men: Reviewing the trend of past 50 years. Asian Pac. J. Reprod. 2018, 7, 87–92. [Google Scholar] [CrossRef]
- Sengupta, P.; Nwagha, U.; Dutta, S.; Krajewska-Kulak, E.; Izuka, E. Evidence for decreasing sperm count in African population from 1965 to 2015. Afr. Health Sci. 2017, 17, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Biedenharn, K.R.; Fedor, J.M.; Agarwal, A. Lifestyle factors and reproductive health: Taking control of your fertility. Reprod. Biol. Endocrinol. 2013, 11, 66. [Google Scholar] [CrossRef]
- Chambers, T.; Anderson, R. The impact of obesity on male fertility. Hormones 2015, 14, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, Z. Obesity, a serious etiologic factor for male subfertility in modern society. Reproduction 2017, 154, R123–R131. [Google Scholar] [CrossRef] [PubMed]
- Bellastella, G.; Menafra, D.; Puliani, G.; Colao, A.; Savastano, S. How much does obesity affect the male reproductive function? Int. J. Obes. Suppl. 2019, 9, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Agbaje, I.M.; Rogers, D.A.; McVicar, C.M.; McClure, N.; Atkinson, A.B.; Mallidis, C.; Lewis, S.E.M. Insulin dependant diabetes mellitus: Implications for male reproductive function. Hum. Reprod. 2007, 22, 1871–1877. [Google Scholar] [CrossRef]
- Abdullah, F.; Nor-Ashikin, M.N.K.; Agarwal, R.; Kamsani, Y.S.; Abd Malek, M.; Bakar, N.S.; Kamal, A.-A.M.; Sarbandi, M.-S.; Rahman, N.-S.A.; Musa, N.H. Glutathione (GSH) improves sperm quality and testicular morphology in streptozotocin-induced diabetic mice. Asian J. Androl. 2021, 23, 281. [Google Scholar]
- Mulholland, J.; Mallidis, C.; Agbaje, I.; McClure, N. Male diabetes mellitus and assisted reproduction treatment outcome. Reprod. Biomed. Online 2011, 22, 215–219. [Google Scholar] [CrossRef][Green Version]
- Hackney, A.C.; Lane, A.R. Exercise and the Regulation of Endocrine Hormones. In Progress in Molecular Biology and Translational Science; Academic Press Inc.: Amsterdam, The Netherlands, 2015; Volume 135, pp. 293–311. [Google Scholar]
- Jung, A.; Strauss, P.; Lindner, H.; Schuppe, H. Influence of moderate cycling on scrotal temperature. Int. J. Androl. 2008, 31, 403–407. [Google Scholar] [CrossRef]
- Sansone, A.; Sansone, M.; Vaamonde, D.; Sgrò, P.; Salzano, C.; Romanelli, F.; Lenzi, A.; Di Luigi, L. Sport, doping and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 114. [Google Scholar] [CrossRef]
- Zampieri, N.; Dall’Agnola, A. Subclinical varicocele and sports: A longitudinal study. Urology 2011, 77, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Ilacqua, A.; Izzo, G.; Emerenziani, G.P.; Baldari, C.; Aversa, A. Lifestyle and fertility: The influence of stress and quality of life on male fertility. Reprod. Biol. Endocrinol. 2018, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Cramer, D.W.; Hornstein, M.D.; Ashby, R.K.; Missmer, S.A. Physical activity and semen quality among men attending an infertility clinic. Fertil. Steril. 2011, 95, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh Maleki, B.; Tartibian, B. Moderate aerobic exercise training for improving reproductive function in infertile patients: A randomized controlled trial. Cytokine 2017, 92, 55–67. [Google Scholar] [CrossRef]
- Sharpe, R.M. Environment, lifestyle and male infertility. Best Pract. Res. Clin. Endocrinol. Metab. 2000, 14, 489–503. [Google Scholar] [CrossRef]
- Sharpe, R.M.; Franks, S. Environment, lifestyle and infertility—An inter-generational issue. Nat. Cell Biol. 2002, 4, S33–S40. [Google Scholar] [CrossRef]
- Asare-Anane, H.; Bannison, S.B.; Ofori, E.K.; Ateko, R.O.; Bawah, A.T.; Amanquah, S.D.; Oppong, S.Y.; Gandau, B.B.N.; Ziem, J.B. Tobacco smoking is associated with decreased semen quality. Reprod. Health 2016, 13, 90. [Google Scholar] [CrossRef]
- Bundhun, P.K.; Janoo, G.; Bhurtu, A.; Teeluck, A.R.; Soogund, M.Z.S.; Pursun, M.; Huang, F. Tobacco smoking and semen quality in infertile males: A systematic review and meta-analysis. BMC Public Health 2019, 19, 36. [Google Scholar] [CrossRef]
- Dechanet, C.; Anahory, T.; Mathieu Daude, J.C.; Quantin, X.; Reyftmann, L.; Hamamah, S.; Hedon, B.; Dechaud, H. Effects of cigarette smoking on reproduction. Hum. Reprod. Update 2011, 17, 76–95. [Google Scholar] [CrossRef]
- Talbot, P.; Lin, S. The effect of cigarette smoke on fertilization and pre-implantation development: Assessment using animal models, clinical data, and stem cells. Biol. Res. 2011, 44, 189–194. [Google Scholar] [CrossRef]
- Nassan, F.L.; Arvizu, M.; Mínguez-Alarcón, L.; Williams, P.L.; Attaman, J.; Petrozza, J.; Hauser, R.; Chavarro, J.; EARTH Study Team. Marijuana smoking and markers of testicular function among men from a fertility centre. Hum. Reprod. 2019, 34, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Francou, M.M.; Girela, J.L.; De Juan, A.; Ten, J.; Bernabeu, R.; De Juan, J. Human sperm motility, capacitation and acrosome reaction are impaired by 2-arachidonoylglycerol endocannabinoid. Histol. Histopathol. 2017, 32, 1351–1358. [Google Scholar] [PubMed]
- Drobnis, E.Z.; Nangia, A.K.; Drobnis, E.Z.; Nangia, A.K. Pain Medications and Male Reproduction. In Impacts of Medications on Male Fertility; Springer International Publishing: Cham, Switzerland, 2017; Volume 1034, pp. 39–57. [Google Scholar]
- Safarinejad, M.R.; Asgari, S.A.; Farshi, A.; Ghaedi, G.; Kolahi, A.A.; Iravani, S.; Khoshdel, A.R. The effects of opiate consumption on serum reproductive hormone levels, sperm parameters, seminal plasma antioxidant capacity and sperm DNA integrity. Reprod. Toxicol. 2013, 36, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Nazmara, Z.; Najafi, M.; Rezaei-Mojaz, S.; Movahedin, M.; Zandiyeh, Z.; Shirinbayan, P.; Roshanpajouh, M.; Asgari, H.R.; Lavasani, L.H.J.; Koruji, M. The Effect of Heroin Addiction on Human Sperm Parameters, Histone-To-Protamine Transition, and Serum Sexual Hormone Levels. Urol. J. 2018, 16, 289–294. [Google Scholar]
- González, B.; Pantoja, C.R.G.; Sosa, M.H.; Vitullo, A.D.; Bisagno, V.; González, C.R. Cocaine alters the mouse testicular epigenome with direct impact on histone acetylation and DNA methylation marks. Reprod. BioMed. Online 2018, 37, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Witkiewitz, K.; Litten, R.Z.; Leggio, L. Advances in the science and treatment of alcohol use disorder. Sci. Adv. 2019, 5, eaax4043. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A.; Esteves, S.C.; Ko, E.; Ramasamy, R.; Zini, A. Clinical utility of sperm DNA fragmentation testing: Practice recommendations based on clinical scenarios. Transl. Androl. Urol. 2016, 5, 935–950. [Google Scholar] [CrossRef]
- Borges, E.; de Almeida Ferreira Braga, D.P.; Provenza, R.R.; de Cassia Savio Figueira, R.; Iaconelli, A.; Setti, A.S. Paternal lifestyle factors in relation to semen quality and in vitro reproductive outcomes. Andrologia 2018, 50, e13090. [Google Scholar] [CrossRef]
- Muthusami, K.; Chinnaswamy, P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil. Steril. 2005, 84, 919–924. [Google Scholar] [CrossRef]
- Ricci, E.; Al Beitawi, S.; Cipriani, S.; Candiani, M.; Chiaffarino, F.; Viganò, P.; Noli, S.; Parazzini, F. Semen quality and alcohol intake: A systematic review and meta-analysis. Reprod. Biomed. Online 2017, 34, 38–47. [Google Scholar] [CrossRef]
- Talebi, A.R.; Sarcheshmeh, A.A.; Khalili, M.A.; Tabibnejad, N. Effects of ethanol consumption on chromatin condensation and DNA integrity of epididymal spermatozoa in rat. Alcohol 2011, 45, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Boeri, L.; Capogrosso, P.; Ventimiglia, E.; Pederzoli, F.; Cazzaniga, W.; Chierigo, F.; Dehò, F.; Montanari, E.; Montorsi, F.; Salonia, A. Heavy cigarette smoking and alcohol consumption are associated with impaired sperm parameters in primary infertile men. Asian J. Androl. 2019, 21, 478. [Google Scholar] [PubMed]
- Kazimoglu, H.; Topdagi, Y.E.; Solakhan, M.; Guzel, A.I. May Smoking and Alcohol Consumption Worsen the Spermiogram Results? Gynecol. Obstet. Reprod. Med. 2020, 27, 44–48. [Google Scholar] [CrossRef]
- Evans, E.P.P.; Scholten, J.T.M.; Mzyk, A.; Reyes-San-Martin, C.; Llumbet, A.E.; Hamoh, T.; Arts, E.G.J.M.; Schirhagl, R.; Cantineau, A.E.P. Male subfertility and oxidative stress. Redox Biol. 2021, 46, 102071. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Kumar, S.; Nirala, J.; Siddiqui, M.H.; Behari, J. Biophysical Evaluation of Radiofrequency Electromagnetic Field Effects on Male Reproductive Pattern. Cell Biochem. Biophys. 2013, 65, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.A.; Galloway, T.S.; Mondal, D.; Esteves, S.C.; Mathews, F. Effect of mobile telephones on sperm quality: A systematic review and meta-analysis. Environ. Int. 2014, 70, 106–112. [Google Scholar] [CrossRef]
- Agarwal, A.; Desai, N.R.; Makker, K.; Varghese, A.; Mouradi, R.; Sabanegh, E.; Sharma, R. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: An in vitro pilot study. Fertil. Steril. 2009, 92, 1318–1325. [Google Scholar] [CrossRef]
- De Iuliis, G.N.; Newey, R.J.; King, B.V.; Aitken, R.J. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS ONE 2009, 4, e6446. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.; Vicari, E.; D’Agata, R.; Calogero, A.E. Diabetes mellitus and sperm parameters. J. Androl. 2012, 33, 145–153. [Google Scholar] [CrossRef]
- Tirpak, F.; Slanina, T.; Tomka, M.; Zidek, R.; Halo Jr, M.; Ivanic, P.; Gren, A.; Formicki, G.; Stachanczyk, K.; Lukac, N. Exposure to non-ionizing electromagnetic radiation of public risk prevention instruments threatens the quality of spermatozoids. Reprod. Domest. Anim. 2019, 54, 150–159. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Simionescu, G.; Doroftei, B.; Maftei, R.; Obreja, B.-E.; Anton, E.; Grab, D.; Ilea, C.; Anton, C. The complex relationship between infertility and psychological distress. Exp. Ther. Med. 2021, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Fenster, L.; Katz, D.F.; Wyrobek, A.J.; Pieper, C.; Rempel, D.M.; Oman, D.; Swan, S.H. Effects of Psychological Stress on Human Semen Quality. J. Urol. 1998, 18, 194–202. [Google Scholar]
- Anderson, K.; Nisenblat, V.; Norman, R. Lifestyle factors in people seeking infertility treatment—A review. Aust. N. Z. J. Obstet. Gynaecol. 2010, 50, 8–20. [Google Scholar] [CrossRef]
- Pook, M.; Tuschen-Caffier, B.; Kubek, J.; Schill, W.B.; Krause, W. Personality, coping and sperm count. Andrologia 2005, 37, 29–35. [Google Scholar] [CrossRef]
- Chiang, C.; Mahalingam, S.; Flaws, J. Environmental Contaminants Affecting Fertility and Somatic Health. Semin. Reprod. Med. 2017, 35, 241–249. [Google Scholar] [CrossRef]
- Garolla, A.; Torino, M.; Sartini, B.; Cosci, I.; Patassini, C.; Carraro, U.; Foresta, C. Seminal and molecular evidence that sauna exposure affects human spermatogenesis. Hum. Reprod. 2013, 28, 877–885. [Google Scholar] [CrossRef]
- Avendaño, C.; Mata, A.; Sarmiento, C.A.S.; Doncel, G.F. Use of laptop computers connected to internet through Wi-Fi decreases human sperm motility and increases sperm DNA fragmentation. Fertil. Steril. 2012, 97, 39–45.e32. [Google Scholar] [CrossRef]
- Hamerezaee, M.; Dehghan, S.F.; Golbabaei, F.; Fathi, A.; Barzegar, L.; Heidarnejad, N. Assessment of Semen Quality among Workers Exposed to Heat Stress: A Cross-Sectional Study in a Steel Industry. Saf. Health Work. 2018, 9, 232–235. [Google Scholar] [CrossRef]
- Song, Y.; Li, R. Effects of Environment and Lifestyle Factors on Anovulatory Disorder. In Environment and Female Reproductive Health; Springer: Singapore, 2021; pp. 113–136. [Google Scholar]
- Meldrum, D.R. Introduction: Obesity and reproduction. Fertil. Steril. 2017, 107, 831–832. [Google Scholar] [CrossRef]
- Grieger, J.A. Preconception diet, fertility, and later health in pregnancy. Curr. Opin. Obstet. Gynecol. 2020, 32, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Best, D.; Bhattacharya, S. Obesity and fertility. Horm. Mol. Biol. Clin. Investig. 2015, 24, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Fabozzi, G.; Cimadomo, D.; Allori, M.; Vaiarelli, A.; Colamaria, S.; Argento, C.; Amendola, M.G.; Innocenti, F.; Soscia, D.; Maggiulli, R.; et al. Maternal body mass index associates with blastocyst euploidy and live birth rates: The tip of an iceberg? Reprod. BioMed. Online 2021, 43, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Broughton, D.E.; Moley, K.H. Obesity and female infertility: Potential mediators of obesity’s impact. Fertil. Steril. 2017, 107, 840–847. [Google Scholar] [CrossRef]
- Luke, B. Adverse effects of female obesity and interaction with race on reproductive potential. Fertil. Steril. 2017, 107, 868–877. [Google Scholar] [CrossRef]
- Warren, M.P.; Perlroth, N. Hormones and sport-the effects of intense exercise on the female reproductive system. J. Endocrinol. 2001, 170, 3–12. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Rich-Edwards, J.W.; Missmer, S.A.; Rosner, B.; Chavarro, J.E. Association of fecundity with changes in adult female weight. Obstet. Gynecol. 2015, 126, 850. [Google Scholar] [CrossRef]
- Budani, M.C.; Tiboni, G.M. Ovotoxicity of cigarette smoke: A systematic review of the literature. Reprod. Toxicol. 2017, 72, 164–181. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, B.; Chen, Y.; Hu, Y.; Zhu, R.; Ye, D.; Mao, Y.; Sun, X. Genetically Predicted Cigarette Smoking in Relation to Risk of Polycystic Ovary Syndrome. Clin. Epidemiol. 2021, 13, 527. [Google Scholar] [CrossRef]
- Bourdon, M.; Ferreux, L.; Maignien, C.; Patrat, C.; Marcellin, L.; Pocate-Cheriet, K.; Chapron, C.; Santulli, P. Tobacco consumption is associated with slow-growing day-6 blastocysts. FS Rep. 2020, 1, 30–36. [Google Scholar] [CrossRef]
- Weizman, N.F.; Wyse, B.A.; Szaraz, P.; Defer, M.; Jahangiri, S.; Librach, C.L. Cannabis alters epigenetic integrity and endocannabinoid signalling in the human follicular niche. Hum. Reprod. 2021, 36, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- de Angelis, C.; Nardone, A.; Garifalos, F.; Pivonello, C.; Sansone, A.; Conforti, A.; Di Dato, C.; Sirico, F.; Alviggi, C.; Isidori, A.; et al. Smoke, alcohol and drug addiction and female fertility. Reprod. Biol. Endocrinol. 2020, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.B.; Decherney, A.H. Fertility and Endometriosis. Clin. Obstet. Gynecol. 2017, 60, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Celik, O.; Hascalik, S. Effect of electromagnetic field emitted by cellular phones on fetal heart rate patterns. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 112, 55–56. [Google Scholar] [CrossRef]
- Rezk, A.Y.; Abdulqawi, K.; Mustafa, R.M.; El-Azm, T.A.; Al-Inany, H. Fetal and neonatal responses following maternal exposure to mobile phones. Saudi Med. J. 2008, 29, 218. [Google Scholar]
- Merhi, Z.O. Challenging cell phone impact on reproduction: A review. J. Assist. Reprod. Genet. 2012, 29, 293–297. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef]
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 2016, 23, 36. [Google Scholar] [CrossRef]
- Negris, O.; Lawson, A.; Brown, D.; Warren, C.; Galic, I.; Bozen, A.; Swanson, A.; Jain, T. Emotional stress and reproduction: What do fertility patients believe? J. Assist. Reprod. Genet. 2021, 38, 877–887. [Google Scholar] [CrossRef]
- Lakatos, E.; Szabó, G.; Szigeti, J.F.; Balog, P. Relationships between psychological well-being, lifestyle factors and fertility. Orv. Hetil. 2015, 156, 483–492. [Google Scholar] [CrossRef]
- Palomba, S.; Daolio, J.; Romeo, S.; Battaglia, F.A.; Marci, R.; La Sala, G.B. Lifestyle and fertility: The influence of stress and quality of life on female fertility. Reprod. Biol. Endocrinol. 2018, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Barzilai-Pesach, V.; Sheiner, E.K.; Sheiner, E.; Potashnik, G.; Shoham-Vardi, I. The Effect of Women’s Occupational Psychologic Stress on Outcome of Fertility Treatments. J. Occup. Environ. Med. 2006, 48, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Kimmins, S.; Sassone-Corsi, P. Chromatin remodelling and epigenetic features of germ cells. Nature 2005, 434, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Schatten, H.; Sun, Q.-Y. Environmental epigenetic inheritance through gametes and implications for human reproduction. Hum. Reprod. Update 2015, 21, 194–208. [Google Scholar] [CrossRef]
- Vecoli, C.; Montano, L.; Andreassi, M.G. Environmental pollutants: Genetic damage and epigenetic changes in male germ cells. Environ. Sci. Pollut. Res. 2016, 23, 23339–23348. [Google Scholar] [CrossRef] [PubMed]
- Fullston, T.; McPherson, N.O.; Zander-Fox, D.; Lane, M. The most common vices of men can damage fertility and the health of the next generation. J. Endocrinol. 2017, 234, F1–F6. [Google Scholar] [CrossRef]
- Keyhan, S.; Burke, E.; Schrott, R.; Huang, Z.; Grenier, C.; Price, T.; Raburn, D.; Corcoran, D.L.; Soubry, A.; Hoyo, C.; et al. Male obesity impacts DNA methylation reprogramming in sperm. Clin. Epigenetics 2021, 13, 17. [Google Scholar] [CrossRef]
- Ding, G.-L.; Liu, Y.; Liu, M.-E.; Pan, J.-X.; Guo, M.-X.; Sheng, J.-Z.; Huang, H.-F. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J. Androl. 2015, 17, 948. [Google Scholar]
- Agarwal, P.; Morriseau, T.S.; Kereliuk, S.M.; Doucette, C.A.; Wicklow, B.A.; Dolinsky, V.W. Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit. Rev. Clin. Lab. Sci. 2018, 55, 71–101. [Google Scholar] [CrossRef]
- Soubry, A.; Guo, L.; Huang, Z.; Hoyo, C.; Romanus, S.; Price, T.; Murphy, S.K. Obesity-related DNA methylation at imprinted genes in human sperm: Results from the TIEGER study. Clin. Epigenetics 2016, 8, 51. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, W.; Duan, E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat. Rev. Genet. 2016, 17, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Rando, O.J. Intergenerational transfer of epigenetic information in sperm. Cold Spring Harb. Perspect. Med. 2016, 6, a022988. [Google Scholar] [CrossRef] [PubMed]
- Hieronimus, B.; Ensenauer, R. Influence of maternal and paternal pre-conception overweight/obesity on offspring outcomes and strategies for prevention. Eur. J. Clin. Nutr. 2021, 75, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Schagdarsurengin, U.; Steger, K. Epigenetics in male reproduction: Effect of paternal diet on sperm quality and offspring health. Nat. Rev. Urol. 2016, 13, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Zeid, D.; Gould, T.J. Impact of nicotine, alcohol, and cocaine exposure on germline integrity and epigenome. Neuropharmacology 2020, 173, 108127. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Kaufman, F.; Sandy, M.S.; Cardenas, A. Cannabis exposure during critical windows of development: Epigenetic and molecular pathways implicated in neuropsychiatric disease. Curr. Environ. Health Rep. 2020, 7, 325–342. [Google Scholar] [CrossRef]
- Cariati, F.; Carbone, L.; Conforti, A.; Bagnulo, F.; Peluso, S.R.; Carotenuto, C.; Buonfantino, C.; Alviggi, E.; Alviggi, C.; Strina, I. Bisphenol A-induced epigenetic changes and its effects on the male reproductive system. Front. Endocrinol. 2020, 11, 453. [Google Scholar] [CrossRef]
- Gallo, A.; Tosti, E. Effects of ecosystem stress on reproduction and development. Mol. Reprod. Dev. 2019, 86, 1269–1272. [Google Scholar] [CrossRef]
- Caldwell, G.S. The influence of bioactive oxylipins from marine diatoms on invertebrate reproduction and development. Mar. Drugs 2009, 7, 367–400. [Google Scholar] [CrossRef]
- Vasconcelos, V.; Azevedo, J.; Silva, M.; Ramos, V. Effects of marine toxins on the reproduction and early stages development of aquatic organisms. Mar. Drugs 2010, 8, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Rolton, A.; Soudant, P.; Vignier, J.; Pierce, R.; Henry, M.; Shumway, S.E.; Bricelj, V.M.; Volety, A.K. Susceptibility of gametes and embryos of the eastern oyster, Crassostrea virginica, to Karenia brevis and its toxins. Toxicon 2015, 99, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Ianora, A.; Miralto, A. Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: A review. Ecotoxicology 2010, 19, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Tosti, E.; Gallo, A. Ion currents involved in gamete physiology. Int. J. Dev. Biol. 2015, 59, 261–270. [Google Scholar]
- Saeed, M.; Arain, M.A.; Fazlani, S.A.; Marghazani, I.B.; Umar, M.; Soomro, J.; Bhutto, Z.A.; Soomro, F.; Noreldin, A.E.; El-Hack, M.E.A.; et al. A comprehensive review on the health benefits and nutritional significance of fucoidan polysaccharide derived from brown seaweeds in human, animals and aquatic organisms. Aquac. Nutr. 2021, 27, 633–654. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Diet and Lifestyle in the Prevention of Ovulatory Disorder Infertility. Obstet. Gynecol. 2007, 110, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. A prospective study of dietary carbohydrate quantity and quality in relation to risk of ovulatory infertility. Eur. J. Clin. Nutr. 2009, 63, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.M.; Fleming, R. Obesity and reproduction: Impact and interventions. Curr. Opin. Obstet. Gynecol. 2007, 19, 384–389. [Google Scholar] [CrossRef]
- Donkin, I.; Versteyhe, S.; Ingerslev, L.R.; Qian, K.; Mechta, M.; Nordkap, L.; Mortensen, B.; Appel, E.V.R.; Jørgensen, N.; Kristiansen, V.B.; et al. Obesity and Bariatric Surgery Drive Epigenetic Variation of Spermatozoa in Humans. Cell Metab. 2016, 23, 369–378. [Google Scholar] [CrossRef]
- McPherson, N.O.; Bakos, H.W.; Owens, J.A.; Setchell, B.P.; Lane, M. Improving Metabolic Health in Obese Male Mice via Diet and Exercise Restores Embryo Development and Fetal Growth. PLoS ONE 2013, 8, e71459. [Google Scholar]
- Benito, E.; Gómez-Martin, J.M.; Vega-Piñero, B.; Priego, P.; Galindo, J.; Escobar-Morreale, H.F.; Botella-Carretero, J.I. Fertility and Pregnancy Outcomes in Women with Polycystic Ovary Syndrome Following Bariatric Surgery. J. Clin. Endocrinol. Metab. 2020, 105, e3384–e3391. [Google Scholar] [CrossRef]
- Lim, S.S.; Hutchison, S.K.; Van Ryswyk, E.; Norman, R.J.; Teede, H.J.; Moran, L.J. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.-D.; Wang, W.-W.; Xu, S.; Shang, X.-J. Impacts of electromagnetic radiation from cellphones and Wi-Fi on spermatogenesis. Zhonghua Nan Ke Xue Natl. J. Androl. 2019, 25, 451–455. [Google Scholar]
- Li, Y.; Zhang, X.; Shi, M.; Guo, S.; Wang, L. Resilience acts as a moderator in the relationship between infertility-related stress and fertility quality of life among women with infertility: A cross-sectional study. Health Qual. Life Outcomes 2019, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Sansone, A.; Di Dato, C.; de Angelis, C.; Menafra, D.; Pozza, C.; Pivonello, R.; Isidori, A.; Gianfrilli, D. Smoke, alcohol and drug addiction and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, A.; Liu, X.; Li, E. Effects of resveratrol on reducing spermatogenic dysfunction caused by high-intensity exercise. Reprod. Biol. Endocrinol. 2019, 17, 42. [Google Scholar] [CrossRef]
- Alahmar, A. Role of oxidative stress in male infertility: An updated review. J. Hum. Reprod. Sci. 2019, 12, 4. [Google Scholar] [CrossRef]
- Sheweita, S.; Tilmisany, A.; Al-Sawaf, H. Mechanisms of Male Infertility: Role of Antioxidants. Curr. Drug Metab. 2005, 6, 495–501. [Google Scholar] [CrossRef]
- Henkel, R.; Sandhu, I.S.; Agarwal, A. The excessive use of antioxidant therapy: A possible cause of male infertility? Andrologia 2019, 51, e13162. [Google Scholar] [CrossRef]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. BioMed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef]
- Majzoub, A.; Arafa, M.; Mahdi, M.; Agarwal, A.; Al Said, S.; Al-Emadi, I.; El Ansari, W.; Alattar, A.; Al Rumaihi, K.; Elbardisi, H. Oxidation–reduction potential and sperm DNA fragmentation, and their associations with sperm morphological anomalies amongst fertile and infertile men. Arab. J. Urol. 2018, 16, 87–95. [Google Scholar] [CrossRef]
- Bergh, C.; Pinborg, A.; Wennerholm, U.-B. Parental age and child outcomes. Fertil. Steril. 2019, 111, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L.; Dunleavy, J.; Gemmell, N.J.; Nakagawa, S. Consistent age-dependent declines in human semen quality: A systematic review and meta-analysis. Ageing Res. Rev. 2015, 19, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Verón, G.L.; Tissera, A.D.; Bello, R.; Beltramone, F.; Estofan, G.; Molina, R.I.; Vazquez-Levin, M.H. Impact of age, clinical conditions, and lifestyle on routine semen parameters and sperm kinematics. Fertil. Steril. 2018, 110, 68–75.e64. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.J. Physiological aspects of female fertility: Role of the environment, modern lifestyle, and genetics. Physiol. Rev. 2016, 96, 873–909. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, A. Reprotoxic Impact of Environment, Diet, and Behavior. Int. J. Environ. Res. Public Health 2022, 19, 1303. https://doi.org/10.3390/ijerph19031303
Gallo A. Reprotoxic Impact of Environment, Diet, and Behavior. International Journal of Environmental Research and Public Health. 2022; 19(3):1303. https://doi.org/10.3390/ijerph19031303
Chicago/Turabian StyleGallo, Alessandra. 2022. "Reprotoxic Impact of Environment, Diet, and Behavior" International Journal of Environmental Research and Public Health 19, no. 3: 1303. https://doi.org/10.3390/ijerph19031303
APA StyleGallo, A. (2022). Reprotoxic Impact of Environment, Diet, and Behavior. International Journal of Environmental Research and Public Health, 19(3), 1303. https://doi.org/10.3390/ijerph19031303





