COVID-19 Pandemic Lockdown: An Excellent Opportunity to Study the Effects of Trawling Disturbance on Macrobenthic Fauna in the Shallow Waters of the Gulf of Gabès (Tunisia, Central Mediterranean Sea)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
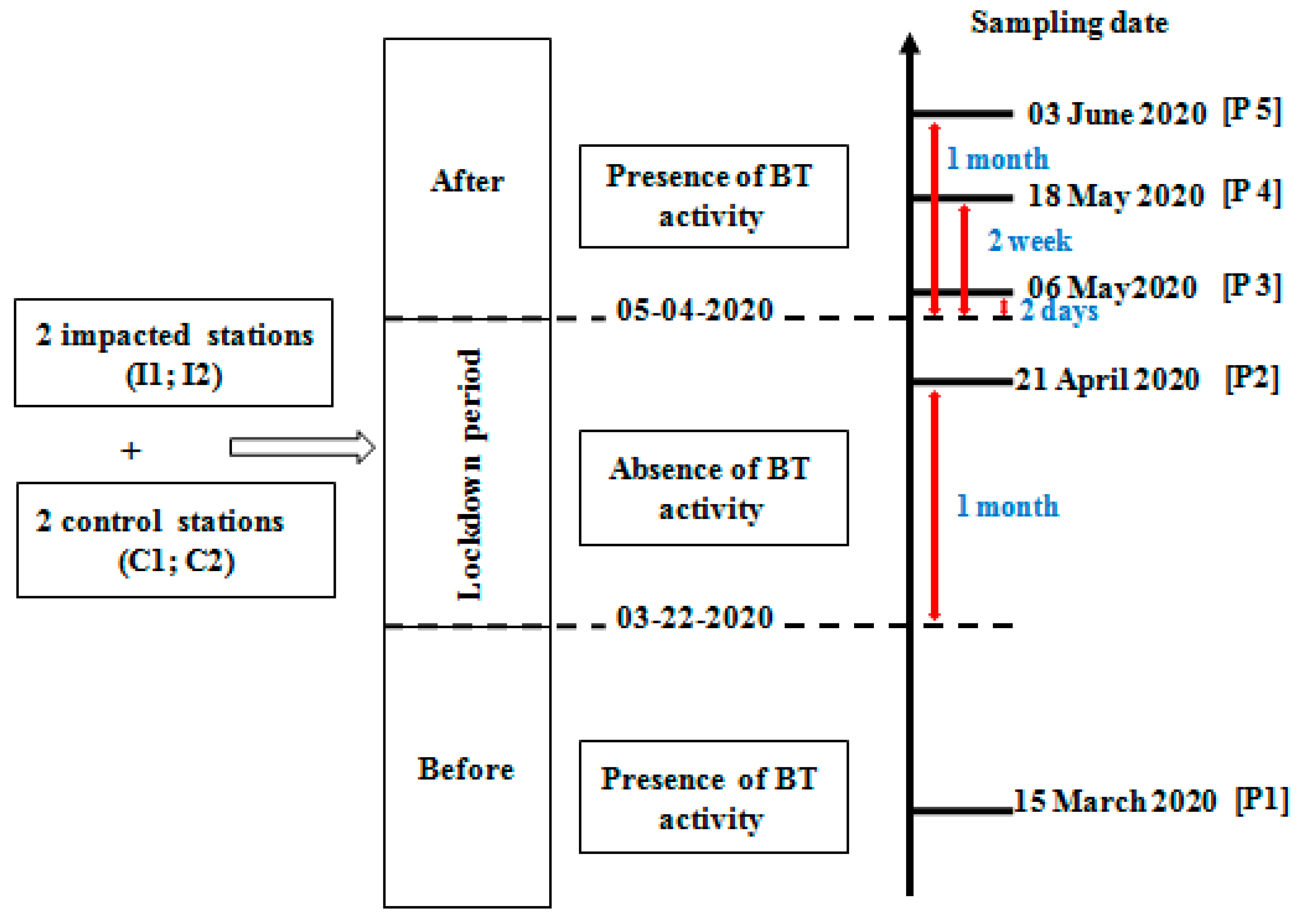
2.2. Sampling and Laboratory Procedures
2.2.1. Macrobenthic Sampling and Measurement of Physicochemical Variables
2.2.2. Sediment Grain-Size Analysis and Organic Matter Content
2.3. Statistical Analyses
3. Results
3.1. Environmental Variables
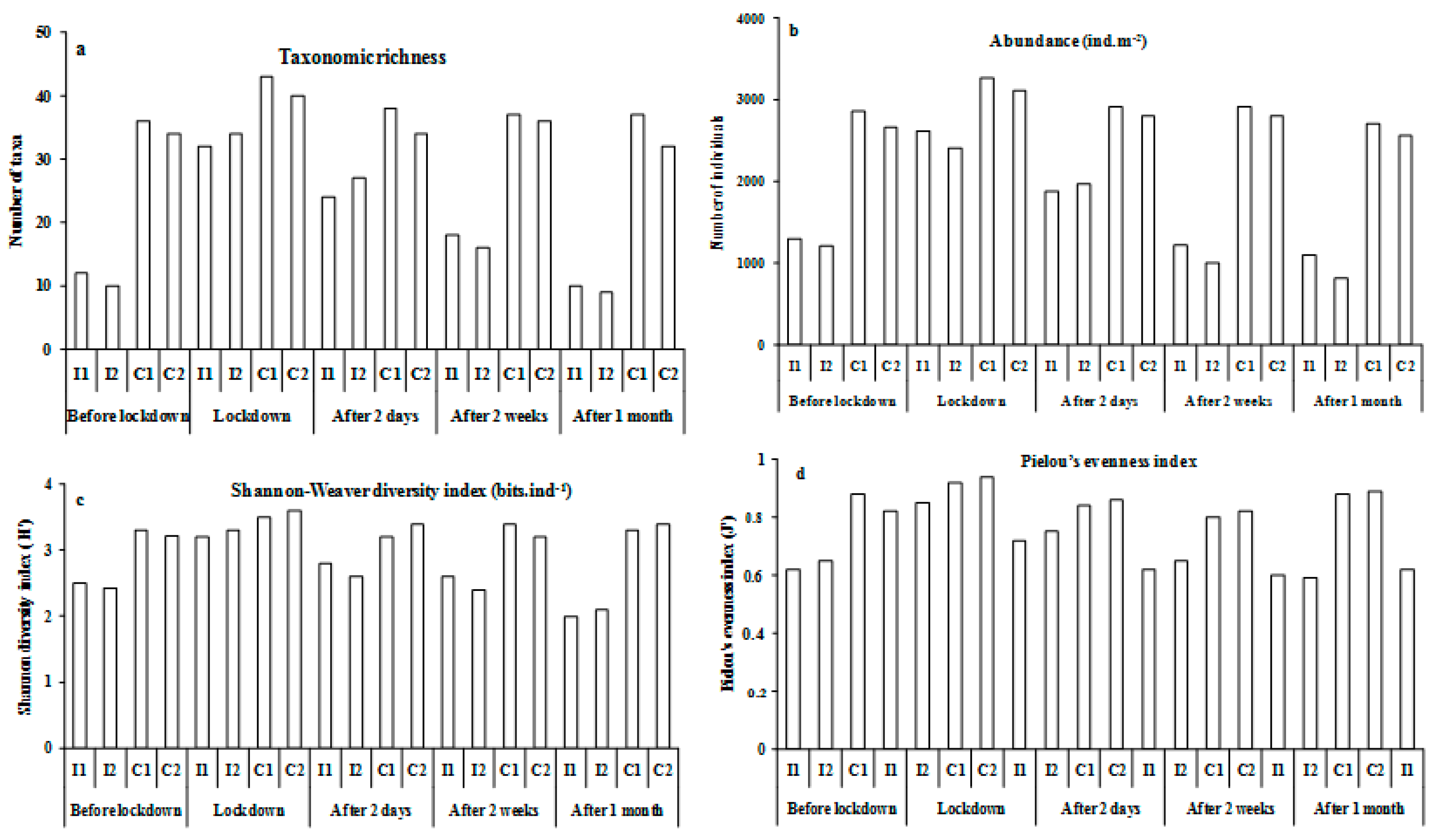
3.2. General Composition of the Benthic Macrofauna
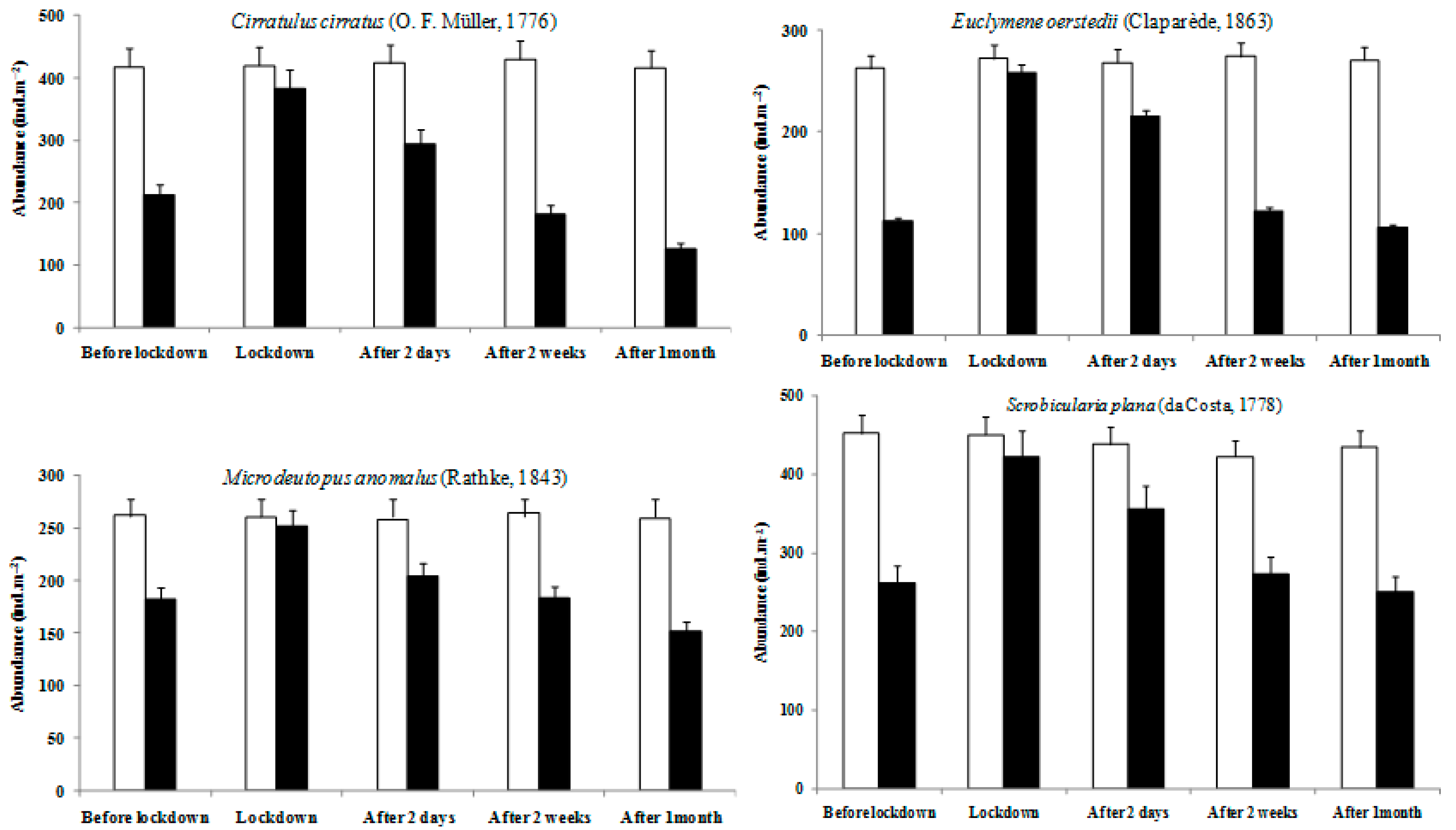
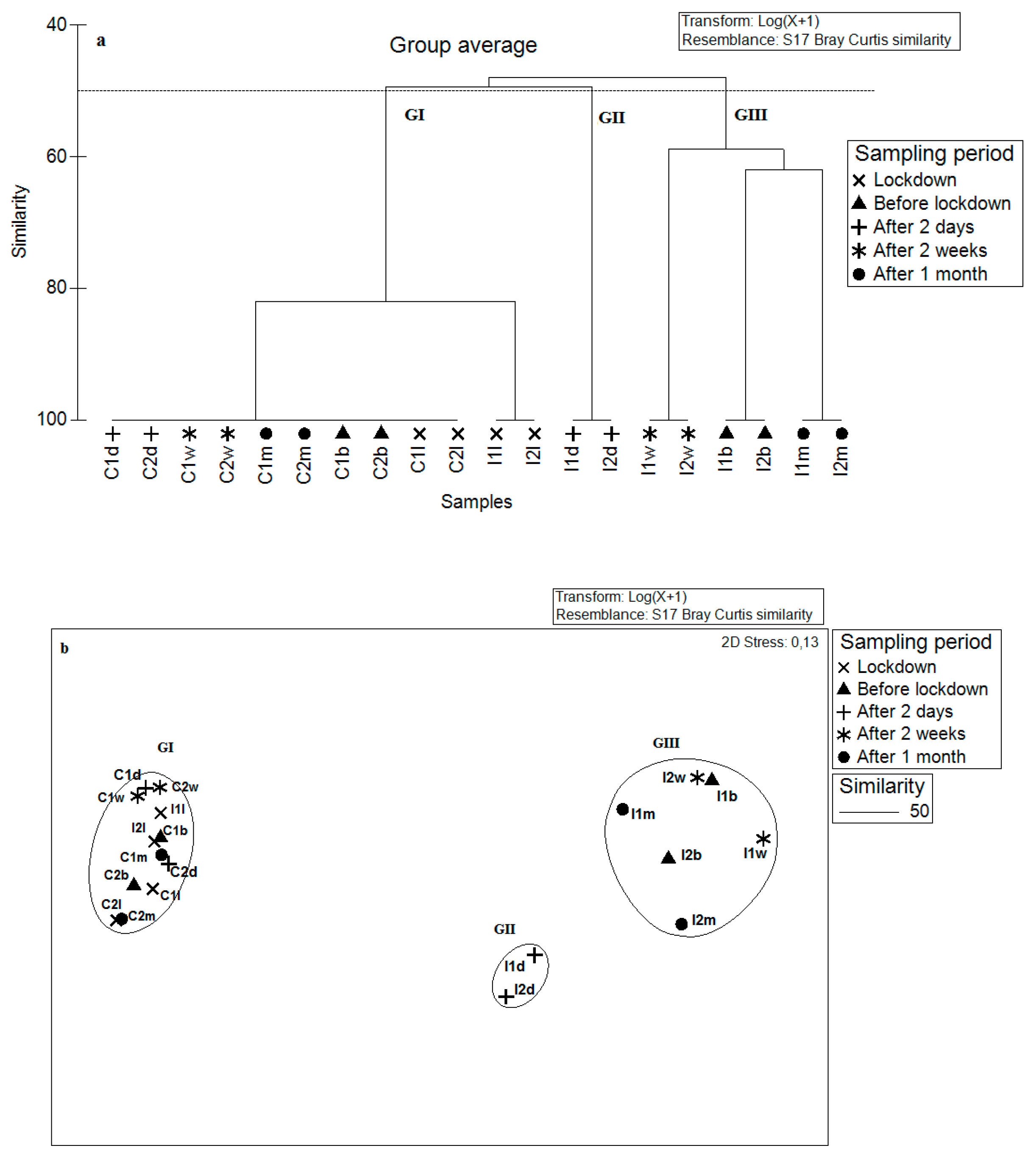
3.3. Impact of Bottom Trawling on the Macrofauna
4. Discussion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eigaard, O.R.; Bastardie, F.; Hintzen, N.; Buhl-Mortensen, L.; Mortensen, P.; Catarino, R.; Dinesen, G.E.; Egekvist, J.; Fock, H.O.; Geitner, K.; et al. The footprint of bottom trawling in European waters: Distribution, intensity, and seabed integrity. ICES J. Mar. Sci. 2017, 74, 847–865. [Google Scholar] [CrossRef]
- Puig, P.; Canals, M.; Company, J.B.; Martin, J.; Amblas, D.; Lastras, G.; Palanques, A.; Calafat, A. Ploughing the deep sea floor. Nature 2012, 489, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, A.; Bianchelli, S.; Martin, J.; Puig, P.; Palanques, A.; Masque, P.; Danovaro, R. Chronic and intensive bottom trawling impairs deep-sea biodiversity and ecosystem functioning. Proc. Natl. Acad. Sci. USA 2014, 111, 8861–8866. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Spencer, B.E. The Effects of Beam-Trawl Disturbance on Infaunal Communities in Different Habitats. J. Anim. Ecol. 1996, 65, 348. [Google Scholar] [CrossRef]
- Smith, C.J.; Papadopoulou, K.N.; DiLiberto, S. Impact of otter trawling on an eastern Mediterranean commercial trawl fishing ground. ICES J. Mar. Sci. 2000, 57, 1340–1351. [Google Scholar] [CrossRef]
- Hall-Spencer, J.M.; Froglia, C.; Atkinson, R.J.A.; Moore, P.G. The impact of Rapido trawling for scallops, Pecten jacobaeus (L.), on the benthos of the Gulf of Venice. ICES J. Mar. Sci. 1999, 56, 111–124. [Google Scholar] [CrossRef]
- Ardizzone, G.D.; Tucci, P.; Somaschini, A.; Belluscio, A.; Kaiser, M.J.; de Groot, J. Is the Bottom Trawling Partly Responsible for the Regression of Posidonia Oceanica Meadows in the Mediterranean Sea? The Effects of Fishing on Non-Target Species and Habitats; Blackwell Scientific: Oxford, UK, 2000; pp. 37–46. [Google Scholar]
- Foden, J.; Rogers, S.; Jones, A. Recovery of UK seabed habitats from benthic fishing and aggregate extraction—Towards a cumulative impact assessment. Mar. Ecol. Prog. Ser. 2010, 411, 259–270. [Google Scholar] [CrossRef]
- Muntadas, O.A. Benthic Communities’ Response to Different Trawling Impact Levels: Generalization towards Developing a Mediterranean Model. Ph.D. Thesis, Barcelona University, Barcelona, Spain, 2015; p. 480. Available online: http://hdl.handle.net/10261/126878 (accessed on 4 December 2015).
- Sciberras, M.; Parker, R.; Powell, C.; Robertson, C.; Kröger, S.; Bolam, S.; Geert Hiddink, J. Impacts of bottom fishing on the sediment infaunal community and biogeochemistry of cohesive and non-cohesive sediments: Trawling impacts on ecosystem processes. Limnol. Oceanogr. 2016, 61, 2076–2089. [Google Scholar] [CrossRef]
- Sammari, C.; Koutitonsky, V.G.; Moussa, M. Sea level variability and tidal resonance in the Gulf of Gabes, Tunisia. Cont. Shelf Res. 2006, 26, 338–350. [Google Scholar] [CrossRef]
- Posey, M.H.; Alphin, T.D.; Cahoon, L.B.; Lindquist, D.G.; Mallin, M.A.; Nevers, M.B. Top-down versus bottom-up limitation in benthic infaunal communities: Direct and indirect effects. Estuaries 2002, 25, 999–1014. [Google Scholar] [CrossRef]
- Mallin, M.A.; Lewitus, A.J. The importance of tidal creek ecosystems. J. Exp. Mar. Biol. Ecol. 2004, 298, 145–149. [Google Scholar] [CrossRef]
- Hattab, T.; Lasram, F.B.R.; Albouy, C.; Romdhane, M.S.; Jarboui, O.; Halouani, G.; Cury, P.; Le Loc’H, F. An ecosystem model of an exploited southern Mediterranean shelf region (Gulf of Gabes, Tunisia) and a comparison with other Mediterranean ecosystem model properties. J. Mar. Syst. 2013, 128, 159–174. [Google Scholar] [CrossRef]
- Halouani, G.; Lasram, B.R.; Shin, Y.-J.; Velez, L.; Verley, P.; Hattab, T.; Oliveros-Ramos, R.; Diaz, F.; Ménard, F.; Baklouti, M.; et al. Modelling food web structure using an end-to-end approach in the coastal ecosystem of the Gulf of Gabes (Tunisia). Ecol. Model. 2016, 339, 45–57. [Google Scholar] [CrossRef]
- Dauvin, J.-C.; Fersi, A.; Pezy, J.-P.; Bakalem, A.; Neifar, L. Macrobenthic communities in the tidal channels around the Gulf of Gabès, Tunisia. Mar. Pollut. Bull. 2021, 162, 111846. [Google Scholar] [CrossRef]
- Hattab, T.; Albouy, C.; Ben Rais Lasram, F.; Somot, S.; Le Loc’h, F.; Leprieur, F. Towards a better understanding of potential impacts of climate change on marine species distribution: A Multiscale modelling approach. Glob. Ecol. Biog. 2014, 23, 1417–1429. [Google Scholar] [CrossRef]
- Mosbahi, N.; Serbaji, M.M.; Pezy, J.-P.; Neifar, L.; Dauvin, J.-C. Response of benthic macrofauna to multiple anthropogenic pressures in the shallow coastal zone south of Sfax (Tunisia, central Mediterranean Sea). Environ. Pollut. 2019, 253, 474–487. [Google Scholar] [CrossRef]
- Bali, M.; Gueddari, M. Les chenaux de marée autour des îles de Kneiss, Tunisie: Sédimentologie et évolution. Hydrol. Sci. J. 2011, 56, 498–506. [Google Scholar] [CrossRef]
- Mosbahi, N.; Pezy, J.-P.; Dauvin, J.-C.; Neifar, L. Short-term impact of bait digging on intertidal macrofauna of tidal mudflats around the Kneiss Islands (Gulf of Gabès, Tunisia). Aquat. Living Resour. 2016, 28, 111–118. [Google Scholar] [CrossRef]
- Zerelli, S. Investigating Illegal Bottom Trawling in the Gulf of Gabès, Tunisia. Rapport Final, Fishact Tunisia 2018. Available online: https://fishact.org/2018/12/investigating-illegal-bottom-trawling-in-the-gulf-of-gabes-tunisia/ (accessed on 2 November 2018).
- Ben Brahim, M.; Hamza, A.; Hannachi, I.; Rebai, A.; Jarboui, O.; Bouain, A.; Aleya, L. Variability in the structure of epiphytic assemblages of Posidonia oceanica in relation to human interferences in the Gulf of Gabes, Tunisia. Mar. Environ. Res. 2010, 70, 411–421. [Google Scholar] [CrossRef]
- Blanchet, H.; Lavesque, N.; Ruellet, T.; Dauvin, J.; Sauriau, P.-G.; Desroy, N.; Desclaux, C.; Leconte, M.; Bachelet, G.; Janson, A.-L.; et al. Use of biotic indices in semi-enclosed coastal ecosystems and transitional waters habitats—Implications for the implementation of the European Water Framework Directive. Ecol. Indic. 2008, 8, 360–372. [Google Scholar] [CrossRef]
- Hale, S.S.; Heltshe, J.F. Signals from the benthos: Development and evaluation of a benthic index for the nearshore Gulf of Maine. Ecol. Indic. 2008, 8, 338–350. [Google Scholar] [CrossRef]
- Dauvin, J.C.; Alizier, S.; Rolet, C.; Bakalem, A.; Bellan, G.; Gomez Gesteira, J.L.; Grimes, S.; De-La-Ossa-Carretero, J.A.; Del-Pilar-Ruso, Y. Response of the different indices to the diverse human pressures. Ecol. Ind. 2012, 12, 143–153. [Google Scholar] [CrossRef]
- Dauvin, J.; Andrade, H.; De-La-Ossa-Carretero, J.; Del-Pilar-Ruso, Y.; Riera, R. Polychaete/amphipod ratios: An approach to validating simple benthic indicators. Ecol. Indic. 2016, 63, 89–99. [Google Scholar] [CrossRef]
- Reiss, H.; Kröncke, I. Seasonal variability of benthic indices: An approach to test the applicability of different indices for ecosystem quality assessment. Mar. Pollut. Bull. 2005, 50, 1490–1499. [Google Scholar] [CrossRef]
- Dauvin, J.C. Le benthos: Témoin des variations de l’environnement. Océanis 1993, 19, 25–53. [Google Scholar]
- Dauvin, J.-C. Paradox of estuarine quality: Benthic indicators and indices, consensus or debate for the future. Mar. Pollut. Bull. 2007, 55, 271–281. [Google Scholar] [CrossRef]
- Cucinotta, D.; Vanelli, M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- McKay, B.; Calfas, J.; Ansari, T.; Coronavirus Declared Pandemic by World Health Organization. Wall St J. Archived from the Original on 11 March 2020. Available online: https://www.wsj.com/articles/u-s-coronavirus-cases-top-1-000-11583917794 (accessed on 12 March 2020).
- Sheahan, T.P.; Frieman, M.B. The continued epidemic threat of SARS-CoV-2 and implications for the future of global public health. Curr. Opin. Virol. 2020, 40, 37–40. [Google Scholar] [CrossRef]
- Briz-Redón, Á.; Belenguer-Sapiña, C.; Serrano-Aroca, Á. Changes in air pollution during COVID-19 lockdown in Spain: A multi-city study. J. Environ. Sci. 2021, 101, 16–26. [Google Scholar] [CrossRef]
- Nigam, R.; Pandya, K.; Luis, A.J.; Sengupta, R.; Kotha, M. Positive effects of COVID-19 lockdown on air quality of industrial cities (Ankleshwar and Vapi) of Western India. Sci. Rep. 2021, 11, 4285. [Google Scholar] [CrossRef]
- Khan, I.; Shah, D.; Shah, S.S. COVID-19 pandemic and its positive impacts on environment: An updated review. Int. J. Environ. Sci. Technol. 2020, 18, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Bates, A.E.; Primack, R.B.; Moraga, P.; Duarte, C.M. COVID-19 pandemic and associated lockdown as a “Global Human Confinement Experiment” to investigate biodiversity conservation. Biol. Conserv. 2020, 248, 108665. [Google Scholar] [CrossRef] [PubMed]
- Mosbahi, N.; Pezy, J.P.; Dauvin, J.C.; Neifar, L. Immediate Effect of Clam Harvesting on Intertidal Benthic Communities in the Mudflat Zones of Kneiss Islands (Central Mediterranean Sea). J. Aquac. Res. Dev. 2016, 7, 2155. [Google Scholar] [CrossRef]
- Shannon, F.P.; Weaver, W. The Mathematical Theory of Communication; University Illinois Press: Champaign, IL, USA, 1963; p. 117. [Google Scholar]
- Pielou, E.C. Shannon’s Formula as a Measure of Specific Diversity: Its Use and Misuse. Am. Nat. 1966, 100, 463–465. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2006. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- McLaverty, C.; Eigaard, O.R.; Gislason, H.; Bastardie, F.; Brooks, M.E.; Jonsson, P.; Lehmann, A.; Dinesen, G.E. Using large benthic macrofauna to refine and improve ecological indicators of bottom trawling disturbance. Ecol. Indic. 2020, 110, 105811. [Google Scholar] [CrossRef]
- Sánchez-Jerez, P.; Esplá, A.A.R. Detection of environmental impacts by bottom trawling on Posidonia oceanica (L.) Delile meadows: Sensitivity of fish and macroinvertebrate communities. J. Aquat. Ecosyst. Heal. 1996, 5, 239–253. [Google Scholar] [CrossRef]
- Sacchi, J. The use of trawling nets in the Mediterranean. Problems and selectivity options. In The Mediterranean Fisheries Sector. A Reference Publication for The VII Meeting of Ministers of Agriculture and Fisheries of CIHEAM Member Countries (Zaragoza, Spain, 4 February 2008); Basurco, B., Ed.; CIHEAM/FAO/GFCM: Zaragoza, Spain, 2008; pp. 87–96, Options Méditerranéennes: Série B. Etudes et Recherches (N 62); Available online: http://om.ciheam.org/article.php?IDPDF=800739 (accessed on 12 March 2020).
- De Juan, S.; Thrush, S.; Demestre, M. Functional changes as indicators of trawling disturbance on a benthic community located in a fishing ground (NW Mediterranean Sea). Mar. Ecol. Prog. Ser. 2007, 334, 117–129. [Google Scholar] [CrossRef]
- Baldrighi, E.; Lavaleye, M.; Aliani, S.; Conversi, A.; Manini, E. Large Spatial Scale Variability in Bathyal Macrobenthos Abundance, Biomass, α- and β-Diversity along the Mediterranean Continental Margin. PLoS ONE 2014, 9, e107261. [Google Scholar] [CrossRef]
- Fersi, A.; Dauvin, J.C.; Pezy, J.P.; Neifar, L. Amphipods from tidal channels of the Gulf of Gabès (central Mediterranean Sea). Mediterr. Mar. Sci. 2018, 19, 430–443. [Google Scholar] [CrossRef]
- Madricardo, F.; Montereale-Gavazzi, G.; Sigovini, M.; Kruss, A.; Toso, C.; Foglini, F. Seafloor Morphology and Habitats of Tidal Channels in the Venice Lagoon, Italy Tidal Channel Habitats. Available online: https://www.sciencedirect.com/science/article/pii/B9780128149607000099?via%3Dihub (accessed on 12 March 2020).
- Collie, J.S.; Hall, S.J.; Kaiser, M.J.; Poiner, I.R. A quantitative analysis of fishing impacts on shelf-sea benthos. J. Anim. Ecol. 2000, 69, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Sciberras, M.; Hiddink, J.G.; Jennings, S.; Szostek, C.L.; Hughes, K.M.; Kneafsey, B.; Clarke, L.J.; Ellis, N.; Rijnsdorp, A.D.; McConnaughey, R.A.; et al. Response of benthic fauna to experimental bottom fishing: A global meta-analysis. Fish Fish. 2018, 19, 698–715. [Google Scholar] [CrossRef]
- Mosbahi, N.; Pezy, J.-P.; Dauvin, J.-C.; Neifar, L. Spatial and Temporal Structures of the Macrozoobenthos from the Intertidal Zone of the Kneiss Islands (Central Mediterranean Sea). Open J. Mar. Sci. 2016, 6, 223–237. [Google Scholar] [CrossRef]
- van Denderen, D.; Bolam, S.G.; Friedland, R.; Hiddink, J.G.; Norén, K.; Rijnsdorp, A.D.; Sköld, M.; Törnroos, A.; Virtanen, E.; Valanko, S. Evaluating impacts of bottom trawling and hypoxia on benthic communities at the local, habitat, and regional scale using a modelling approach. ICES J. Mar. Sci. 2020, 77, 278–289. [Google Scholar] [CrossRef]
- Kaiser, M.J. Significance of Bottom-Fishing Disturbance. Conserv. Biol. 1998, 12, 1230–1235. [Google Scholar] [CrossRef]
- Craeymeersch, J.A.; Piet, G.J.; Rijnsdorp, A.D.; Buijs, J. Distribution of macrofauna in relation to the micro distribution of trawling effort. In The Effects of Fishing on Non-Target Species and Habitats: Biological, Conservation and Socio-Economic Issues; Kaiser, M.J., de Groot, S.J., Eds.; Blackwell Science: Oxford, UK, 2000; pp. 187–197. [Google Scholar]
- Meenakumari, B.; Bhagirathan, U.; Pravin, P. Impact of bottom trawling on benthic communities: A Review. Fish. Techn. 2008, 45, 1–22. [Google Scholar]
- Dauvin, J.; Bakalem, A.; Baffreau, A.; Grimes, S. Benthic ecological status of Algerian harbours. Mar. Pollut. Bull. 2017, 125, 378–388. [Google Scholar] [CrossRef]
- Foveau, A.; Dauvin, J.-C. Surprisingly diversified macrofauna in mobile gravels and pebbles from high-energy hydrodynamic environment of the ‘Raz Blanchard’ (English Channel). Reg. Stud. Mar. Sci. 2017, 16, 188–197. [Google Scholar] [CrossRef]
- Petović, S.; Marković, O.; Ikica, Z.; Đurović, M.; Joksimović, A. Effects of bottom trawling on the benthic assemblages in the south Adriatic Sea (Montenegro). Acta Adriat. 2016, 57, 81–92. [Google Scholar]
- Jennings, S.; Kaiser, M.J. The Effects of Fishing on Marine Ecosystems. Adv. Mar. Biol. 1998, 34, 201–352. [Google Scholar] [CrossRef]
- McConnaughey, R.A.; Syrjala, S.E. Short-term effects of bottom trawling and a storm event on soft-bottom benthos in the eastern Bering Sea. ICES J. Mar. Sci. 2014, 71, 2469–2483. [Google Scholar] [CrossRef]
- Giovanardi, O.; Pranovi, F.; Franceschini, G. “Rapido” trawl fishing in the Northern Adriatic: Preliminary observations of the effects on macrobenthic communities. Acta Adriat. 1998, 39, 37–52. [Google Scholar]
- Kaiser, M.J.; Hormbrey, S.; Booth, J.R.; Hinz, H.; Hiddink, J.G. Recovery linked to life history of sessile epifauna following exclusion of towed mobile fishing gear. J. Appl. Ecol. 2018, 55, 1060–1070. [Google Scholar] [CrossRef]
- Pranovi, F.; Raicevich, S.; Franceschini, G.; Farrace, M.G.; Giovanardi, O. Rapido trawling in the northern Adriatic Sea: Effects on benthic communities in an experimental area. ICES J. Mar. Sci. 2000, 57, 517–524. [Google Scholar] [CrossRef]
- Cowie, P.R.; Widdicombe, S.; Austen, M.C. Effects of physical disturbance on an estuarine intertidal community: Field and mesocosm results compared. Mar. Biol. 2000, 136, 485–495. [Google Scholar] [CrossRef]
- De Carvalho, A.N.; Vaz, A.S.L.; Sérgio, T.I.B.; Dos Santos, P.J.T. Sustainability of bait fishing harvesting in estuarine ecosystems—Case study in the Local Natural Reserve of Douro Estuary, Portugal. Rev. Gestão Costeira Integr. 2013, 13, 157–168. [Google Scholar] [CrossRef][Green Version]
- Collie, J.; Hiddink, J.G.; Van Kooten, T.; Rijnsdorp, A.D.; Kaiser, M.J.; Jennings, S.; Hilborn, R. Indirect effects of bottom fishing on the productivity of marine fish. Fish Fish. 2016, 18, 619–637. [Google Scholar] [CrossRef]
- Gilkinson, K.; Paulin, M.; Hurley, S.; Schwinghamer, P. Impacts of trawl door scouring on infaunal bivalves: Results of a physical trawl door model/dense sand interaction. J. Exp. Mar. Biol. Ecol. 1998, 224, 291–312. [Google Scholar] [CrossRef]
- Hermsen, J.; Collie, J.; Valentine, P. Mobile fishing gear reduces benthic megafaunal production on Georges Bank. Mar. Ecol. Prog. Ser. 2003, 260, 97–108. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Spencer, B. Fish scavenging behaviour in recently trawled areas. Mar. Ecol. Prog. Ser. 1994, 112, 41–49. [Google Scholar] [CrossRef]
- De Biasi, A.M. Impact of experimental trawling on the benthic assemblage along the Tuscany coast (north Tyrrhenian Sea, Italy). ICES J. Mar. Sci. 2004, 61, 1260–1266. [Google Scholar] [CrossRef]
- Jennings, S.; Nicholson, M.D.; Dinmore, T.; Lancaster, J. Effects of chronic trawling disturbance on the production of infaunal communities. Mar. Ecol. Prog. Ser. 2002, 243, 251–260. [Google Scholar] [CrossRef]
- Duplisea, D.E.; Jennings, S.; Warr, K.J.; Dinmore, T.A. A size-based model of the impacts of bottom trawling on benthic community structure. Can. J. Fish. Aquat. Sci. 2002, 59, 1785–1795. [Google Scholar] [CrossRef]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ecology: From Individuals to Ecosystems, 4th ed.; Blackwell Science: Oxford, UK, 2006. [Google Scholar]
- Kaiser, M.J.; Ramsay, K.; Richardson, C.A.; Spence, F.E.; Brand, A.R. Chronic fishing disturbance has changed shelf sea benthic community structure. J. Anim. Ecol. 2000, 69, 494–503. [Google Scholar] [CrossRef]
- Palanques, A.; Puig, P.; Guillén, J.; Demestre, M.; Martin, J. Effects of bottom trawling on the Ebro continental shelf sedimentary system (NW Mediterranean). Cont. Shelf Res. 2014, 72, 83–98. [Google Scholar] [CrossRef]
- Villnäs, A.; Norkko, J.; Lukkari, K.; Hewitt, J.; Norkko, A. Consequences of Increasing Hypoxic Disturbance on Benthic Communities and Ecosystem Functioning. PLoS ONE 2012, 7, e44920. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Marine benthic hypoxia: A review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biology Annu. Rev. 1995, 33, 203–245. [Google Scholar]
- Hiddink, J.G.; Jennings, S.; Sciberras, M.; Bolam, S.G.; Cambiè, G.; McConnaughey, R.A.; Mazor, T.; Hilborn, R.; Collie, J.S.; Pitcher, C.R.; et al. Assessing bottom trawling impacts based on the longevity of benthic invertebrates. J. Appl. Ecol. 2018, 56, 1075–1084. [Google Scholar] [CrossRef]
- Diesing, M.; Stephens, D.; Aldridge, J. A proposed method for assessing the extent of the seabed significantly affected by demersal fishing in the Greater North Sea. ICES J. Mar. Sci. 2013, 70, 1085–1096. [Google Scholar] [CrossRef]
- Atkinson, L.; Field, J.; Hutchings, L. Effects of demersal trawling along the west coast of southern Africa: Multivariate analysis of benthic assemblages. Mar. Ecol. Prog. Ser. 2011, 430, 241–255. [Google Scholar] [CrossRef]
- Henniger, T.O.; Forenamen, P.W. Macrofaunal community structure in the littoral zone of a freshwater-deprived, permanently open Eastern Cape estuary. Afr. Zool. 2011, 46, 263–279. [Google Scholar] [CrossRef]
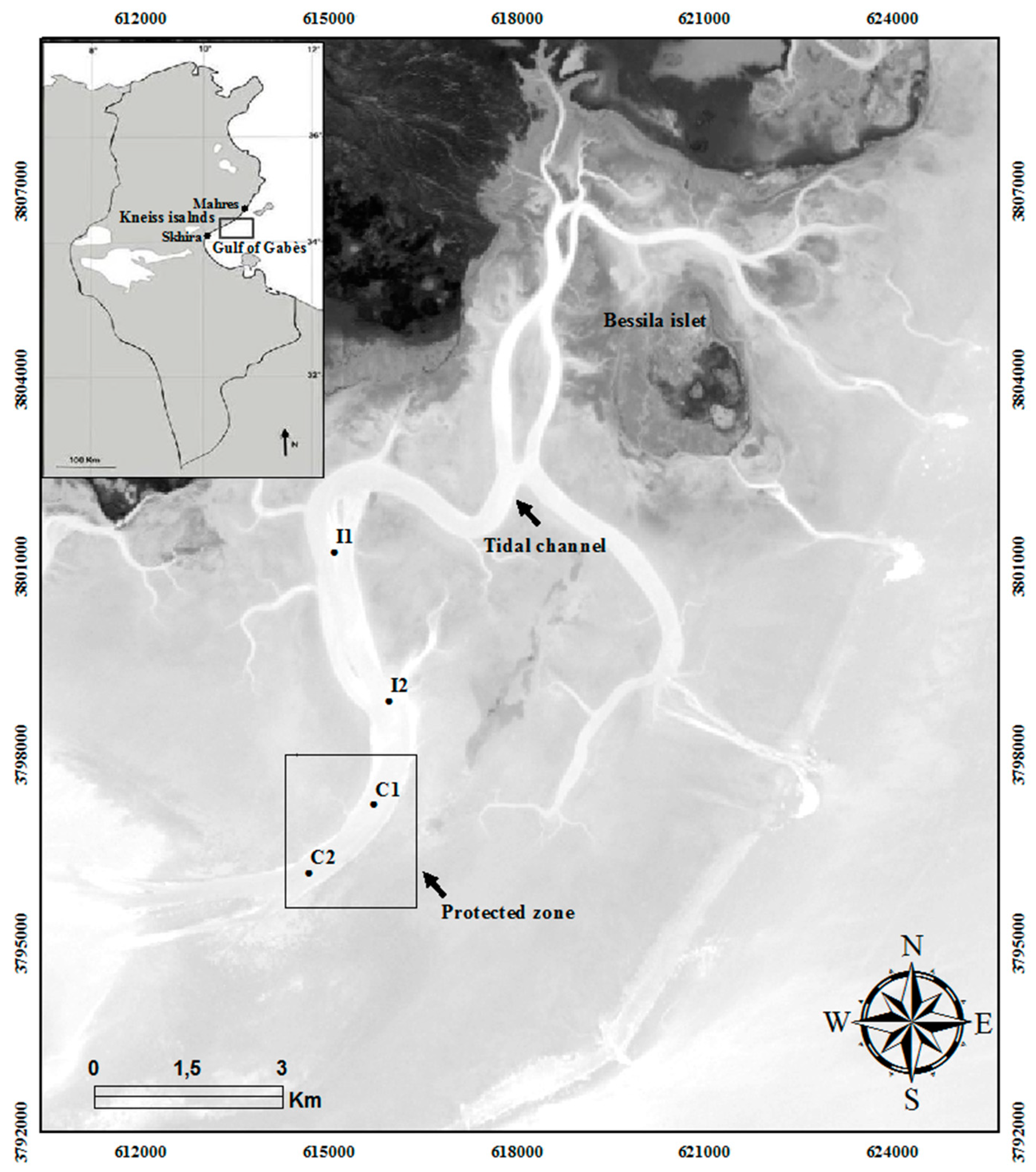
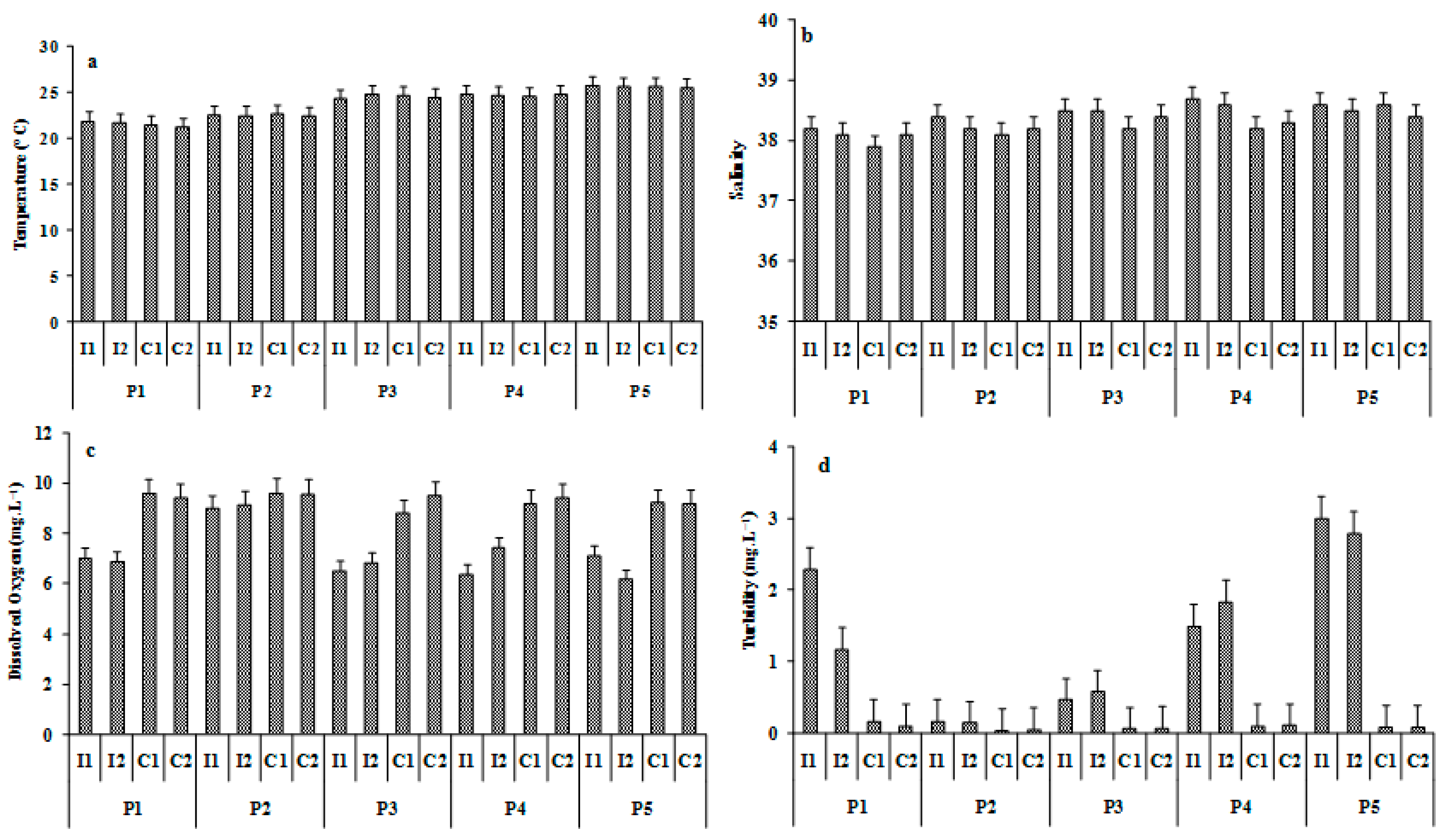
| Stations | Coordinates | Depth (m) | OM (%) | Q50 (mm) | Sandy (%) | Silt-Clay (%) | Sediment Type | |
|---|---|---|---|---|---|---|---|---|
| Latitude (N) | Longitude (E) | |||||||
| I1 | 38.00974 | 32.614844 | 5.60 | 4.1 | 0.79 | 83.5 ± 3.62 | 16.0 ± 5.18 | medium sand |
| I2 | 37.98597 | 32.615700 | 8.30 | 3.8 | 1.11 | 97.3 ± 4.10 | 2.6 ± 6.22 | coarse sand |
| C1 | 37.96937 | 32.615471 | 7.40 | 3.6 | 1.21 | 96.8 ± 3.12 | 3.2 ± 4.60 | coarse sand |
| C2 | 37.95854 | 32.614429 | 6.50 | 2.6 | 1.14 | 95.8 ± 2.64 | 3.4 ± 1.42 | coarse sand |
| Presence of BT Activity | Absence of BT Activity | Deconfinement; Presence of BT Activity | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Lockdown | Lockdown | After Two Days | After Two Weeks | After One Month | ||||||||||||||||
| C | I | C | I | C | I | C | I | C | I | |||||||||||
| A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | |
| Polychaetes | 47 | 35.1 | 27.0 | 14.4 | 42 | 34.3 | 37 | 24.9 | 36 | 35.4 | 30 | 21.8 | 43 | 31.7 | 26 | 16.2 | 49 | 31.3 | 19 | 13.6 |
| Crustaceans | 27 | 34.3 | 44 | 51.3 | 33 | 35.1 | 35 | 42.8 | 38 | 34.5 | 39 | 42.4 | 31 | 36.0 | 42 | 50.4 | 26 | 38.3 | 42 | 54.2 |
| Molluscs | 22 | 27.2 | 27 | 32.2 | 21 | 28.1 | 25 | 28.8 | 24 | 28.3 | 30 | 34.6 | 23 | 30.3 | 32 | 33.4 | 22 | 27.2 | 39 | 32.2 |
| Others groups | 4 | 3.4 | 2 | 2.1 | 4 | 2.4 | 3 | 3.5 | 2 | 1.8 | 1 | 1.2 | 3 | 2.0 | 0 | 0 | 3 | 3.2 | 0 | 0 |
| Mean taxa number | 35.0 ± 3.0 | 11.0 ± 2.1 | 41.5 ± 3.6 | 33.0 ± 4.2 | 36.0 ± 2.4 | 25.5 ± 2.6 | 36.5 ± 3.2 | 17.0 ± 1.8 | 34.5 ± 3.4 | 9.5 ± 2.2 | ||||||||||
| Mean abundance | 2762 ± 202 | 1256 ± 112 | 3187 ± 348 | 2807 ± 211 | 2856 ± 262 | 1923 ± 104 | 2821 ± 301 | 1112 ± 96 | 2634 ± 182 | 958 ± 62 | ||||||||||
| Mean biomass | 113 ± 20 | 64 ± 9 | 186 ± 22 | 124 ± 12 | 194 ± 24 | 102 ± 13 | 198 ± 14 | 68 ± 8 | 211 ± 14 | 49 ± 6 | ||||||||||
| Factor | F | p | |
|---|---|---|---|
| Time | 3.9 | <0.01 * | |
| Richness species | Site | 1.14 | <0.01 * |
| Trawling | 0.23 | 0.01 * | |
| Time | 2.9 | <0.01 * | |
| Abundance | Site | 1.21 | <0.01 * |
| Trawling | 0.84 | <0.01 * | |
| Time | 21.4 | 0.01 * | |
| Biomass | Site | 0.86 | <0.01 * |
| Trawling | 12.2 | 0.01 * | |
| Time | 2.11 | <0.01 * | |
| H’ | Site | 2.18 | <0.01 * |
| Trawling | 1.14 | 0.01 * | |
| Time | 2.41 | 0.01 * | |
| J’ | Site | 3.11 | <0.01 * |
| Trawling | 1.04 | 0.01 * |
| GI | GII | GIII | |
|---|---|---|---|
| Similarity (%) | 64.54 | 49.43 | 27.82 |
| Main species | Cirratulus cirratus—52.3 | Cirratulus cirratus—48.6 | Microdeutopus gryllotalpa—51.6 |
| Euclymene oerstedii—46.0 | Hilbigneris gracilis—44.9 | Cymadusa filosa—48.2 | |
| Hediste diversicolor—38.1 | Perinereis cultrifera—42.0 | Dexamine spinosa—41.0 | |
| Amphitritides gracilis—34.2 | Cerithium scabridum—37.0 | Gibbula ardens—32.2 | |
| Leucothoe incisa—28.2 | Euclymene oerstedii—26.7 | Tellina tenius—28.1 | |
| Eulymene lumbricoides—26.6 | Scrobicularia plana—21.0 | Tritia cuvierii—21.4 | |
| Monocorophium acherusicum—22.7 | Melita palmata—18.2 | Gammarus insensibilis—20.8 | |
| Microdeutopus anomalus—18.6 | Monocorophium insidiosum—16.1 | Euclymene oerstedii—19.4 | |
| Scrobicularia plana—11.3 | Marphysa sanguinea—10.2 | Loripes orbiculatus—17.3 | |
| Melinna palmata—7.0 | Tricolia speciosa—8.6 | Calliostoma zizyphinum—15.2 | |
| Heteromastus filiformis—4.2 | Dexamine spinosa—3.2 | Pinctada (imbricata) radiata—7.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosbahi, N.; Pezy, J.-P.; Dauvin, J.-C.; Neifar, L. COVID-19 Pandemic Lockdown: An Excellent Opportunity to Study the Effects of Trawling Disturbance on Macrobenthic Fauna in the Shallow Waters of the Gulf of Gabès (Tunisia, Central Mediterranean Sea). Int. J. Environ. Res. Public Health 2022, 19, 1282. https://doi.org/10.3390/ijerph19031282
Mosbahi N, Pezy J-P, Dauvin J-C, Neifar L. COVID-19 Pandemic Lockdown: An Excellent Opportunity to Study the Effects of Trawling Disturbance on Macrobenthic Fauna in the Shallow Waters of the Gulf of Gabès (Tunisia, Central Mediterranean Sea). International Journal of Environmental Research and Public Health. 2022; 19(3):1282. https://doi.org/10.3390/ijerph19031282
Chicago/Turabian StyleMosbahi, Nawfel, Jean-Philippe Pezy, Jean-Claude Dauvin, and Lassad Neifar. 2022. "COVID-19 Pandemic Lockdown: An Excellent Opportunity to Study the Effects of Trawling Disturbance on Macrobenthic Fauna in the Shallow Waters of the Gulf of Gabès (Tunisia, Central Mediterranean Sea)" International Journal of Environmental Research and Public Health 19, no. 3: 1282. https://doi.org/10.3390/ijerph19031282
APA StyleMosbahi, N., Pezy, J.-P., Dauvin, J.-C., & Neifar, L. (2022). COVID-19 Pandemic Lockdown: An Excellent Opportunity to Study the Effects of Trawling Disturbance on Macrobenthic Fauna in the Shallow Waters of the Gulf of Gabès (Tunisia, Central Mediterranean Sea). International Journal of Environmental Research and Public Health, 19(3), 1282. https://doi.org/10.3390/ijerph19031282







