The Regular Consumption of Nuts Is Associated with a Lower Prevalence of Abdominal Obesity and Metabolic Syndrome in Older People from the North of Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
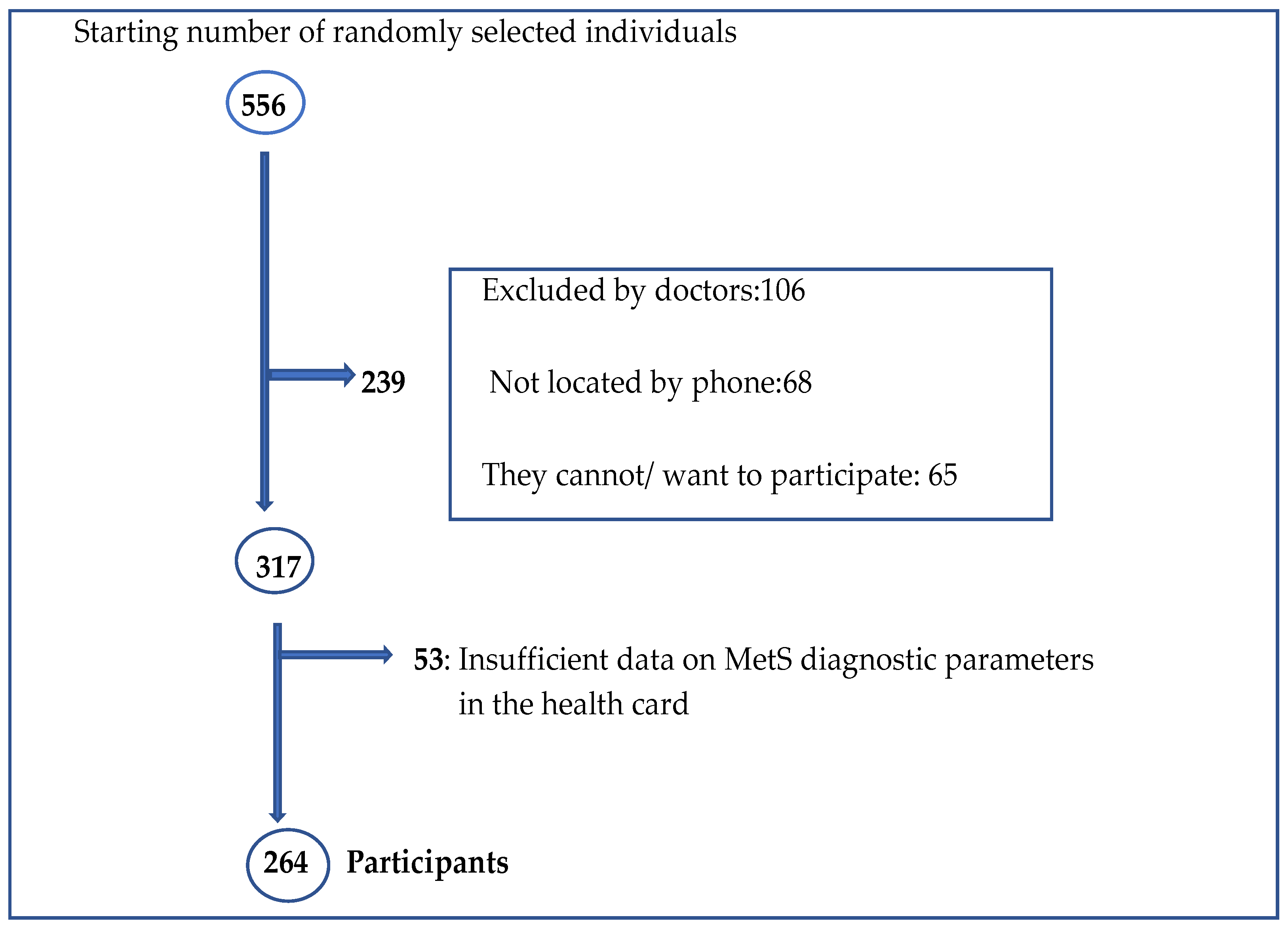
2.2. Participants
2.3. Sociodemographic Variables
2.4. Body Mass Index Levels
2.5. Diagnosis of Metabolic Syndrome according to IDF Criteria
2.6. Instruments
2.6.1. Adherence to Recommendations for Nut Consumption
2.6.2. Body Mass Index Assessment
2.6.3. Assessment of Diagnostic Parameters for Metabolic Syndrome
2.7. Procedure
2.7.1. Nut Consumption
2.7.2. Assessment of Nutritional Status Using BMI
2.7.3. Assessment of Diagnostic Parameters for Metabolic Syndrome
2.8. Statistical Analysis
3. Results
3.1. Adherence to Recommended Nut Consumption
3.2. Prevalence of Abdominal Obesity
3.3. Prevalence of Nutritional Status according to BMI
3.4. Prevalence of Metabolic Syndrome
3.5. Association between the Adherence to Recommended Nut Consumption and Prevalence of Abdominal Obesity
3.6. Association between Adherence to the Recommended Consumption of Nuts and Prevalence of Metabolic Syndrome
4. Discussion
4.1. Adherence to Recommended Nut Consumption
4.2. Prevalence of the Metabolic Syndrome
4.3. Relationship between Adherence to Recommended Nut Consumption and Prevalence of Abdominal Obesity and Metabolic Syndrome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Instituto Nacional de Estadística (INE). Proporción de Personas Mayores de Cierta de Edad por Provincia [Database on the Internet]; INE: Madrid, Spain, 2021. Available online: https://www.ine.es/jaxiT3/Tabla.htm?t=1488 (accessed on 30 September 2021).
- Instituto Cántabro de Estadística (ICANE). Padrón Municipal de Habitantes de 2020: Por Grupos Quinquenales y Sexo [Database on the Internet]; ICANE: Santander, Spain, 28 January 2021. Available online: https://www.icane.es/data/municipal-register-quinquennial-age-group-gender#timeseries (accessed on 1 September 2021).
- Daengtern, L.; Thojampa, S.; Kumpeera, K.; Wannapornsiri, C.; Boonpracom, R. Factors affecting quality of life and longevity in the elderly people in Phrae city, Thailand. Asian Pac. Isl. Nurs. J. 2020, 5, 48–54. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud (OMS). Informe Mundial Sobre el Envejecimiento y la Salud [Monograph on the Internet]; OMS: Ginebra, Switzerland, 2015; Available online: https://apps.who.int/iris/bitstream/handle/10665/186471/WHO_FWC_ALC_15.01_spa.pdf%3Bjsessionid=58559B079FC2220A8FE6767E9BE0DEF2?sequence=1 (accessed on 27 November 2021).
- Organización Mundial de la Salud. Envejecimiento y Salud [Monograph on the Internet]; OMS: Ginebra, Switzerland, 2018; Available online: http://www.who.int/es/news-room/fact-sheets/detail/envejecimiento-y-salud (accessed on 4 October 2021).
- Organización Mundial de la Salud [Web Site]. Organismos Internacionales y Envejecimiento; OMS: Ginebra, Switzerland, 2009; Available online: http://traballoxunta.es/export/sites/default/Biblioteca/Documentos/Publicacions/congreso_envellecemento/congreso-envejecimiento_activo.pdf. (accessed on 15 November 2021).
- Ribera, J.M. Microbiota intestinal y envejecimiento: ¿un nuevo camino de intervención? Rev. Española Geriatr. Gerontol. 2016, 51, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jenning, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Rev. Española Cardiol. 2017, 70, 115.e1–115.e64. [Google Scholar] [CrossRef]
- Gurka, M.J.; Guo, Y.; Filipp, S.L.; DeBoer, M.D. Metabolic syndrome severity is significantly associated with future coronary heart disease in type 2 diabetes. Cardiovasc. Diabetol. 2018, 17, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Salas-Salvadó, J.; Ros, E. Bioactives and health benefits of nuts and dried fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Chang, S.K.; Shahidi, F. Nuts: Nutrients, natural antioxidants, fat-soluble bioactives, and phenolics. In Health Benefits of Nuts and Dried Fruits, 1st ed.; Alasalvar, C., Salas-Salvadó, J., Ros, E., Sabaté, J., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 13–57. [Google Scholar] [CrossRef]
- Lainas, K.; Alasalvar, C.; Bolling, B.W. Effects of roasting on proanthocyanidins contents of Turkish Tombul hazelnut and its skin. J. Funct. Foods 2016, 23, 647–653. [Google Scholar] [CrossRef]
- Tan, S.Y.; Tey, S.L.; Brown, R. Can nuts mitigate malnutrition in older adults? A conceptual framework. Nutrients 2018, 10, 1448. [Google Scholar] [CrossRef]
- Neale, E.P.; Tapsell, L.C.; Guan, V.; Batterham, M.J. The effect of nut consumption on markers of inflammation and endothelial function: A systematic review and meta-analysis of randomized controlled trials. BMJ Open 2017, 7, e016863-1–e016863-14. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Krauss, R.M.; Taubes, G.; Willett, W. Dietary fat and cardiometabolic health: Evidence, controversies, and consensus for guidance. BMJ 2018, 361, k2139. [Google Scholar] [CrossRef]
- Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Bompadre, S.; Mezzetti, B.; Quiles, J.L.; Giampieri, F.; Battino, M. The healthy effects of strawberry polyphenols: Which strategy behind antioxidant capacity? Crit. Rev. Food Sci. Nutr. 2016, 56, S46–S59. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.Z. Relationship between nut consumption and metabolic syndrome: A meta-analysis of observational studies. J. Am. Coll. Nutr. 2019, 38, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; Estruch, R.; Salas-Salvadó, J.; San Julian, B.; Sánchez-Tainta, A.; Ros, C.; Valls-Pedret, C.; Martínez-González, M.A. Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Iglesias, R.C.; Ruíz, R.; Aparicio, S.; Crespo, J.; Lopez, L.D.; Manna, P.P.; Giampieri, F.; Maurizio, B. Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer´s disease: A focus on human studies. Pharmacol. Res. 2018, 131, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Institut Hospital del Mar d´Investigacions Médiques (IMIM) [Web Site]. IMIM-Hospital del Mar: Barcelona, Spain, 2012. Available online: https://imim.cat/ofertadeserveis/software-public/granmo/ (accessed on 25 December 2021).
- Martínez de la Iglesia, J.; Dueñas Herrero, R.; Onís Vilches, M.C.; Aguado Taberné, C.; Albert Colomer, C.; Luque Luque, R. Adaptación y validación al castellano del cuestionario de Pfeiffer (SPMSQ) para detectar la existencia de deterioro cognitivo en personas mayores de 65 años. Med. Clin. 2001, 117, 129–134. [Google Scholar] [CrossRef]
- International Diabetes Federation. The IDF Consensus Worldwide Definition of Metabolic Syndrome [Monograph on Internet]; IDF: Brussels, Belgium, 2005; Available online: https://www.pitt.edu/~super1/Metabolic/IDF1.pdf (accessed on 24 December 2021).
- Sociedad Española para el Estudio de la Obesidad (SEEDO). Consenso SEEDO´2000 para la evaluación del sobrepeso y la obesidad y el establecimiento de criterios de intervención terapéutica. Med. Clin. 2000, 115, 587–597. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing mediterranean diet adherence among older spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Chumlea, W.; Roche, A.; Steinbaugh, M. Estimating stature from knee height for persons 60 to 90 years of age. J. Am. Geriatr. Soc. 1985, 33, 116–120. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; WHO Technical Report Series 894; World Health Organization: Geneva, Switzerland, 2000; Available online: https://apps.who.int/iris/handle/10665/42330 (accessed on 15 October 2021).
- Guerra, R.S.; Amaral, T.F.; Marques, E.A.; Mota, J.; Restivo, M.T. Anatomical location for waist circumference measurement in older adults: A preliminary study. Nutr. Hosp. 2012, 27, 1554–1561. [Google Scholar] [CrossRef]
- Sociedad Internacional para el Avance de la Kinantropometría. Estándares Internacionales Para la Valoración Antropométrica; Universidad Católica San Antonio de Murcia: Murcia, Spain, 2001. [Google Scholar]
- Silveira, B.K.S.; da Silva, A.; Hermsdorff, H.H.M.; Bressan, J. Effect of chronic consumption of nuts on oxidative stress: Systematic review of clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Naghshi, S.; Aune, D.; Beyene, J.; Mobarak, S.; Asadi, M.; Sadeghi, O. Dietary intake and biomarkers of alpha linolenic acid and risk of all cause, cardiovascular, and cancer mortality: A systematic review and dose-response meta-analysis of cohort studies. BMJ 2021, 375, n2213. [Google Scholar] [CrossRef]
- Escurriol, V.; Cofan, M.; Serra, M.; Bulo, M.; Basora, J.; Salas-Salvadó, J.; Corella, D.; Zazpe, I.; Martínez-González, M.A.; Ruíz-Gutiérre, V.; et al. Serum sterol responses to increasing plant sterol intake from natural foods in the Mediterranean diet. Eur. J. Nutr. 2009, 48, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.E.; Simedrea, R.; Gheldiu, A.M.; Mocan, A.; Vlase, L.; Popa, D.S.; Ferreira, I.C.F.R. Benefits of tree nut consumption on aging and age-related diseases: Mechanisms of actions. Trends Food Sci. Technol. 2019, 88, 104–120. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Rebordo-Rodríguez, P.; Varela López, A.; Quiles, J.L.; et al. Relevance of functional foods in the Mediterranean diet: The role of olive oil, berries and honey in the prevention of cancer and cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 893–920. [Google Scholar] [CrossRef] [PubMed]
- Nishi, S.K.; Viguiiouk, E.; Blanco Mejia, S.; Kendall, C.W.C.; Bazinet, R.; Hanley, A.J.; Comelli, E.M.; Salas Salvado, J.; Jenkins, D.J.A.; Sievenpiper, J.L. Are fatty nuts a weight concern? A systematic review and meta-analysis and dose-response meta-regression of prospective cohorts and randomized controlled trials. Obes. Rev. 2021, 22, e13330–e13347. [Google Scholar] [CrossRef]
- Vicinanza, R.; Troisi, G.; Cangemi, R.; Ulderico de Martino, M.; Pastori, D.; Bernardini, S.; Crisciotti, F.; Di Violante, F.; Frizza, A.; Cacciafesta, M.; et al. Aging and adherence to the Mediterranean diet: Relationship with cardiometabolic disorders and polypharmacy. J. Nutr. Health Aging 2018, 22, 73–81. [Google Scholar] [CrossRef]
- Micek, A.; Godos, J.; Cernigliaro, A.; Cincione, R.I.; Buscemi, S.; Libra, M.; Galvano, F.; Grosso, G. Total nut, tree nut, and peanut consumption and metabolic status in southern Italian adults. Int. J. Environ. Res. Public Health 2021, 18, 1847. [Google Scholar] [CrossRef]
- Ministerio de Agricultura, Pesca y Alimentación (MAPA). Informe de Consumo Alimentario en España 2020 [Internet Database]; MAPA: Madrid, Spain, 2021. Available online: https://www.mapa.gob.es/es/alimentacion/temas/consumo-tendencias/informe-anual-consumo-2020_baja-res_tcm30-562704.pdf (accessed on 15 September 2021).
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Guallar-Castillón, P.; Pérez, R.F.; López García, E.; León-Muñoz, L.M.; Aguilera, M.T.; Graciani, A.; Gutiérrez-Fisac, J.L.; Banegas, J.R.; Rodríguez-Artalejo, F. Magnitud y manejo del síndrome metabólico en España 2008–2010: Estudio ENRICA. Rev. Española Cardiol. 2014, 67, 367–373. [Google Scholar] [CrossRef]
- Ortíz-Rodríguez, M.A.; Yáñez-Velasco, L.; Carnevale, A.; Romero-Hidalgo, S.; Bernal, D.; Aguilar-Salinas, C.; Rojas, R.; Villa, A.; Tur, J.A. Prevalence of metabolic syndrome among elderly Mexicans. Arch. Gerontol. Geriatr. 2017, 73, 288–293. [Google Scholar] [CrossRef]
- Shin, D.; Kongpakpaisarn, K.; Bohra, C. Trends in the prevalence of metabolic syndrome and its components in the United States 2007–2014. Int. J. Cardiol. 2018, 259, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Farmanfarma, K.K.; Kaykhaei, M.A.; Adineh, H.A.; Mohammadi, M.; Dabiri, S.; Ansari-Moghaddam, A. Prevalence of metabolic syndrome in Iran: A meta-analysis of 69 studies. Diabetes Metab. Syndr. 2019, 13, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Metelskaya, V.A.; Shkolnikova, M.A.; Shalnova, S.A.; Andreev, E.M.; Deev, A.D.; Jdanov, D.A.; Shkolnikov, V.M.; Vaupel, J.W. Prevalence, components, correlates of metabolic syndrome (MetS) among elderly Muscovites. Arch. Gerontol. Geriatr. 2012, 55, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bergés, D.; Félix-Redondo, F.J.; Lozano, L.; Pérez-Castán, J.F.; Sanz, H.; Cabrera De León, A.; Hidalgo, A.B.; Morcillo, Y.; Tejero, V.; Álvarez-Palacios, P. Prevalencia de síndrome metabólico según las nuevas recomendaciones de la OMS. Estudio HERMEX. Gac. Sanit. 2011, 25, 519–524. [Google Scholar] [CrossRef][Green Version]
- Lee, S.J.; Lee, E.Y.; Lee, J.H.; Kim, J.E.; Kim, K.J.; Rhee, Y.; Kim, H.C.; Youm, Y.; Kim, C.O. Associations of serum 25-hydroxyvitamin D with metabolic syndrome and its components in elderly men and women. The Korean Urban Rural Elderly cohort study. BMC Geriatr. 2019, 19, 102–109. [Google Scholar] [CrossRef]
- Yan, Z.; Fan, Y.; Meng, Z.; Huang, C.; Liu, M.; Zhang, Q.; Song, K.; Jia, Q. The relationship between red blood cell distribution width and metabolic syndrome in elderly Chinese: A cross-sectional study. Lipids Health Dis. 2019, 18, 34–42. [Google Scholar] [CrossRef]
- Sinha, N.; Bhattacharya, A.; Deshmuskh, P.R.; Panja, T.K.; Yasmin, S.; Arlappa, N. Metabolic syndrome among elderly care-home residents in southern India: A cross-sectional study. WHO South East Asia J. Public Health 2016, 5, 62–69. [Google Scholar] [CrossRef]
- Chimbo-Yung, J.M.; Chuchuca-Cajamarca, Á.J.; Wong, S.; Encalada-Torres, L.E. Metabolic syndrome and physical activity in elderly people from the Ecuadorian highlands. Rev. Salud Publica 2017, 19, 754–759. [Google Scholar] [CrossRef]
- Nogueira Saad, M.A.; Pérez Cardoso, G.; Martins, W.D.A.; Coca Velarde, L.G.; da Cruz Filho, R.A. Prevalence of metabolic syndrome in elderly and agreement among four diagnostic criteria. Arq. Bras Cardiol. 2014, 102, 263–269. [Google Scholar] [CrossRef]
- Teixeira de Paula, J.A.; Moreira, O.C.; Diniz da Silva, C.; Santos Silva, D.; dos Santos Amorim, P.R. Metabolic syndrome prevalence in elderly of urban and rural communities participants in the HIPERDIA in the city of Coimbra/MG, Brazil. Investig. Educ. Enferm. 2015, 33, 325–333. [Google Scholar] [CrossRef]
- Saukkonen, T.; Jokelainen, J.; Timonen, M.; Cederberg, H.; Laakso, M.; Härkönen, P.; Keinänen-Kiukaanniemi, S.; Rajala, U. Prevalence of metabolic syndrome components among the elderly using three different definitions: A cohort study in Finland. Scand. J. Prim. Health Care 2012, 30, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Tyrovolas, S.; Chalkias, C.; Morena, M.; Tsiligianni, I.; Zeimbekis, A.; Gotsis, E.; Metallinos, G.; Bountziouka, V.; Polychronopoulos, E.; Lionis, C.; et al. Health care access and prevalence of metabolic syndrome among elders living in high-altitude areas of the Mediterranean islands: The MEDIS study. Rev. Diabet. Stud. 2011, 8, 468–476. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernández-Bergés, D.; Cabrera de León, A.; Sanz, H.; Elosua, R.; Guembe, M.J.; Almazora, M.; Vega-Alonso, T.; Félix-Redondo, F.J.; Ortíz-Marrón, H.; Rigo, F.; et al. Síndrome metabólico en España: Prevalencia y riesgo coronario asociado a la definición armonizada y a la propuesta por la OMS. Estudio DARIOS. Rev. Esp. Cardiol. 2012, 65, 241–248. [Google Scholar] [CrossRef]
- Vernay, M.; Salanave, B.; de Peretti, C.; Druet, C.; Malon, A.; Deschamps, V.; Hercberg, S.; Castetbon, K. Metabolic syndrome and socioeconomic status in France: The French Nutrition and Health Survey (ENNS, 2006–2007). Int. J. Public Health 2013, 58, 855–864. [Google Scholar] [CrossRef]
- Slagter, S.N.; Van Waateringe, R.P.; Van Beek, A.P.; Van der Klauw, M.M.; Wolffenbutel, B.H.R.; Van Vliet-Ostaptchouk, J.V. Sex, BMI and age differences in metabolic síndrome: The Dutch Lifelines Cohort Study. Endocr. Connect. 2017, 6, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; D’Ardes, D.; Guagnano, M.T.; Davi, G. Metabolic syndrome: Sex-related cardiovascular risk and therapeutic approach. Curr. Med. Chem. 2017, 24, 2602–2627. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Carr, M.C.; Murdoch, S.J.; Mitchell, E.; Woods, N.F.; Wener, M.H.; Chandler, W.L.; Boyko, E.J.; Brunzell, J.D. Adipokines, inflammation, and visceral adiposity across the menopausal transition: A prospective study. J. Clin. Endocrinol. Metab. 2009, 94, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Smith, U. Abdominal obesity: A marker of ectopic fat accumulation. J. Clin. Investig. 2015, 125, 1790–1792. [Google Scholar] [CrossRef] [PubMed]
- Pucci, G.; Alcidi, R.; Tap, L.; Battista, F.; Mattace-Raso, F.; Schillaci, G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol. Res. 2017, 120, 34–42. [Google Scholar] [CrossRef]
- Christakis, N.A.; Fowler, J.M. The spread of obesity in a large social network over 32 years. N. Engl. J. Med. 2007, 357, 370–379. [Google Scholar] [CrossRef]
- Yu, Z.M.; Parker, L.; Dummer, T.J. Depressive symptoms, diet quality, physical activity, and composition among populations in Nova Scotia, Canada: Report from the Atlantic Partnership for Tomorrow´s Health. Prev. Med. 2014, 61, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ibarrola-Jurado, N.; Guasch-Ferré, M.; Ros, E.; Martínez-González, M.A.; Corella, D.; Fiol, M.; Wärnberg, J.; Estruch, R.; Román, P.; Arós, F.; et al. Cross-sectional assessment of nut consumption and obesity, metabolic syndrome and other cardiometabolic risk factors: The PREDIMED study. PLoS ONE 2013, 8, e57367. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Montero, A.; Bes-Rastrollo, M.; Beunza, J.J.; Barrio-López, M.T.; de la Fuente-Arrillaga, C.; Moreno-Galarraga, I.; Martínez-González, M.A. Nut consumption and incidence of metabolic syndrome after 6-year follow-up: The SUN (Seguimiento Universidad de Navarra, University of Navarra Follow-up) cohort. Public Health Nutr. 2013, 16, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour-Niazi, S.; Hosseini, S.; Mirmiran, P.; Azizi, F. Prospective study of nut consumption and incidence of metabolic syndrome: Tehran lipid and glucose study. Nutrients 2017, 9, 1056. [Google Scholar] [CrossRef] [PubMed]
- Hassannejad, R.; Mohamadifard, N.; Kazemi, I.; Manourian, M.; Sadeghi, M.; Roohafza, H.; Sarrafzadegan, N. Long-term nuts intake and metabolic syndrome: A 13-year longitudinal population-based study. Clin. Nutr. 2019, 38, 1246–1252. [Google Scholar] [CrossRef]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Chiva-Blanch, G.; Chiva-Blanch, G.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; et al. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: A prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, e6–e17. [Google Scholar] [CrossRef]
- Vadivel, V.; Kunyanga, C.N.; Biesalski, H.K. Health benefits of nut consumption with special reference to body weight control. Nutrition 2012, 28, 1089–1097. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.; Clifton, P.M. Nuts and cardio-metabolic disease: A review of meta-analyses. Nutrients 2018, 10, 1935. [Google Scholar] [CrossRef]
- Landsberg, L.; Aronne, L.J.; Beilin, L.J.; Burke, V.; Igel, L.I.; Lloyd-Jones, D.; Sowers, J. Obesity-related hypertension: Pathogenesis, cardiovascular risk, and treatment: A position paper of the Obesity Society and the American Society of Hypertension. J. Clin. Hypertens 2013, 15, 14–33. [Google Scholar] [CrossRef]
- Del Gobbo, L.C.; Falk, M.C.; Feldman, R.; Lewis, K.; Mozaffarian, D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: Systematic review, meta-analyisis, and dose-response of 61 controlled intervention trials. Am. J. Clin. Nutr. 2015, 102, 1347–1356. [Google Scholar] [CrossRef]
- Asghari, G.; Ghorbani, Z.; Mirmiran, P.; Azizi, F. Nut consumption is associated with lower incidence of type 2 diabetes: The Tehran Lipid and Glucose Study. Diabetes Metab. 2017, 43, 18–24. [Google Scholar] [CrossRef] [PubMed]
- O’Mahoney, L.L.; Mattu, J.; Price, O.J.; Birch, K.M.; Ajjan, R.A.; Farrar, D.; Tapp, R.; West, D.J.; Deighton, K.; Campbell, M.D. Omega-3 polyunsaturated fatty acid favourably modulate cardiometabolic biomarkers in type 2 diabetes: A meta-analysis and meta-regression of randomized controlled trials. Cardiovasc. Diabetol. 2018, 17, 98. [Google Scholar] [CrossRef] [PubMed]
| Low Nut Intake (<3 Ration/Week) (N = 158) | High Nut Intake (≥3 Ration/Week) (N = 106) | ||
|---|---|---|---|
| n (%) | n (%) | p-Value 1 | |
| Sex | 0.388 | ||
| Men | 65 (63.1) | 38 (36.9) | |
| Women | 93 (57.8) | 68 (42.2) | |
| Age groups | 0.899 | ||
| 65–69 | 51 (58.0) | 37 (42.0) | |
| 70–74 | 60 (61.2) | 38 (38.8) | |
| 75–79 | 47 (60.3) | 31 (39.7) | |
| Marital status | 0.744 | ||
| Married/partnered | 97 (58.1) | 70 (41.9) | |
| Separated | 9 (56.3) | 7 (43.8) | |
| Widowed | 32 (66.7) | 16 (33.3) | |
| Single | 20 (60.6) | 13 (39.4) | |
| Type of cohabitation | 0.803 | ||
| Couple | 96 (58.5) | 68(41.5) | |
| With relatives | 16 (55.2) | 13 (44.8) | |
| With a carer | 2 (66.7) | 1 (33.3) | |
| Alone | 43 (64.2) | 24 (35.8) | |
| Shared flat | 1 (100) | 0 (0.0) | |
| Educational level | 0.399 | ||
| University | 58 (54.2) | 49 (45.8) | |
| Secondary school | 47 (63.5) | 27 (36.5) | |
| Primary school | 50 (65.8) | 26 (34.2) | |
| Incomplete | 3 (42.9) | 4 (57.1) | |
| BMI categories | 0.035 | ||
| Normal weight | 31 (48.4) | 33 (51.6) | |
| Overweight | 79 (59.8) | 53 (40.2) | |
| Obesse | 48 (70.6) | 20 (29.4) | |
| Health status | |||
| Hypertension | 128 (61.0) | 82 (39.0) | 0.471 |
| Hyperglycemia | 52 (61.9) | 32 (38.1) | 0.642 |
| Hipertriglyceridemia | 43 (78.2) | 12 (21.8) | 0.002 |
| Los HDL-c | 30 (57.7) | 22 (42.3) | 0.723 |
| BMI | Men (N = 103) | Women (N = 161) | p-Value ¹ | Total p-Value ² (N = 264) |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Normal weight | 4 (30.8) | 7 (13.7) | <0.001 | 11 (17.2) |
| Overweight | 25 (40.3) | 27 (38.6) | 0.783 | 52 (39.4) |
| Obesity | 20 (71.4) | 23 (57.5) | 0.023 | 43 (63.2) |
| Low Nut Intake (<3 Ration/Week) (N = 158) | High Nut Intake (≥3 Ration/Week) (N = 106) | |||
|---|---|---|---|---|
| n (%) | n (%) | PR; (95% IC) | p-Value ¹ | |
| Abdominal obesity | 133 (84.2) | 75 (70.8) | 1.19 (1.03–1.37) | 0.015 |
| Metabolic syndrome | 74 (46.8) | 32 (30.2) | 1.61 (1.16–2.25) | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cubas-Basterrechea, G.; Elío, I.; Sumalla-Cano, S.; Aparicio-Obregón, S.; González-Antón, C.T.; Muñoz-Cacho, P. The Regular Consumption of Nuts Is Associated with a Lower Prevalence of Abdominal Obesity and Metabolic Syndrome in Older People from the North of Spain. Int. J. Environ. Res. Public Health 2022, 19, 1256. https://doi.org/10.3390/ijerph19031256
Cubas-Basterrechea G, Elío I, Sumalla-Cano S, Aparicio-Obregón S, González-Antón CT, Muñoz-Cacho P. The Regular Consumption of Nuts Is Associated with a Lower Prevalence of Abdominal Obesity and Metabolic Syndrome in Older People from the North of Spain. International Journal of Environmental Research and Public Health. 2022; 19(3):1256. https://doi.org/10.3390/ijerph19031256
Chicago/Turabian StyleCubas-Basterrechea, Gloria, Iñaki Elío, Sandra Sumalla-Cano, Silvia Aparicio-Obregón, Carolina Teresa González-Antón, and Pedro Muñoz-Cacho. 2022. "The Regular Consumption of Nuts Is Associated with a Lower Prevalence of Abdominal Obesity and Metabolic Syndrome in Older People from the North of Spain" International Journal of Environmental Research and Public Health 19, no. 3: 1256. https://doi.org/10.3390/ijerph19031256
APA StyleCubas-Basterrechea, G., Elío, I., Sumalla-Cano, S., Aparicio-Obregón, S., González-Antón, C. T., & Muñoz-Cacho, P. (2022). The Regular Consumption of Nuts Is Associated with a Lower Prevalence of Abdominal Obesity and Metabolic Syndrome in Older People from the North of Spain. International Journal of Environmental Research and Public Health, 19(3), 1256. https://doi.org/10.3390/ijerph19031256









