Living Lab for Citizens’ Wellness: A Case of Maintaining and Improving a Healthy Diet under the COVID-19 Pandemic
Abstract
:1. Introduction
1.1. Research Background
1.2. Diet and the Intestinal Environment
1.3. Libing Lab
1.4. Aim and Objectives
2. Materials and Methods
2.1. The Case
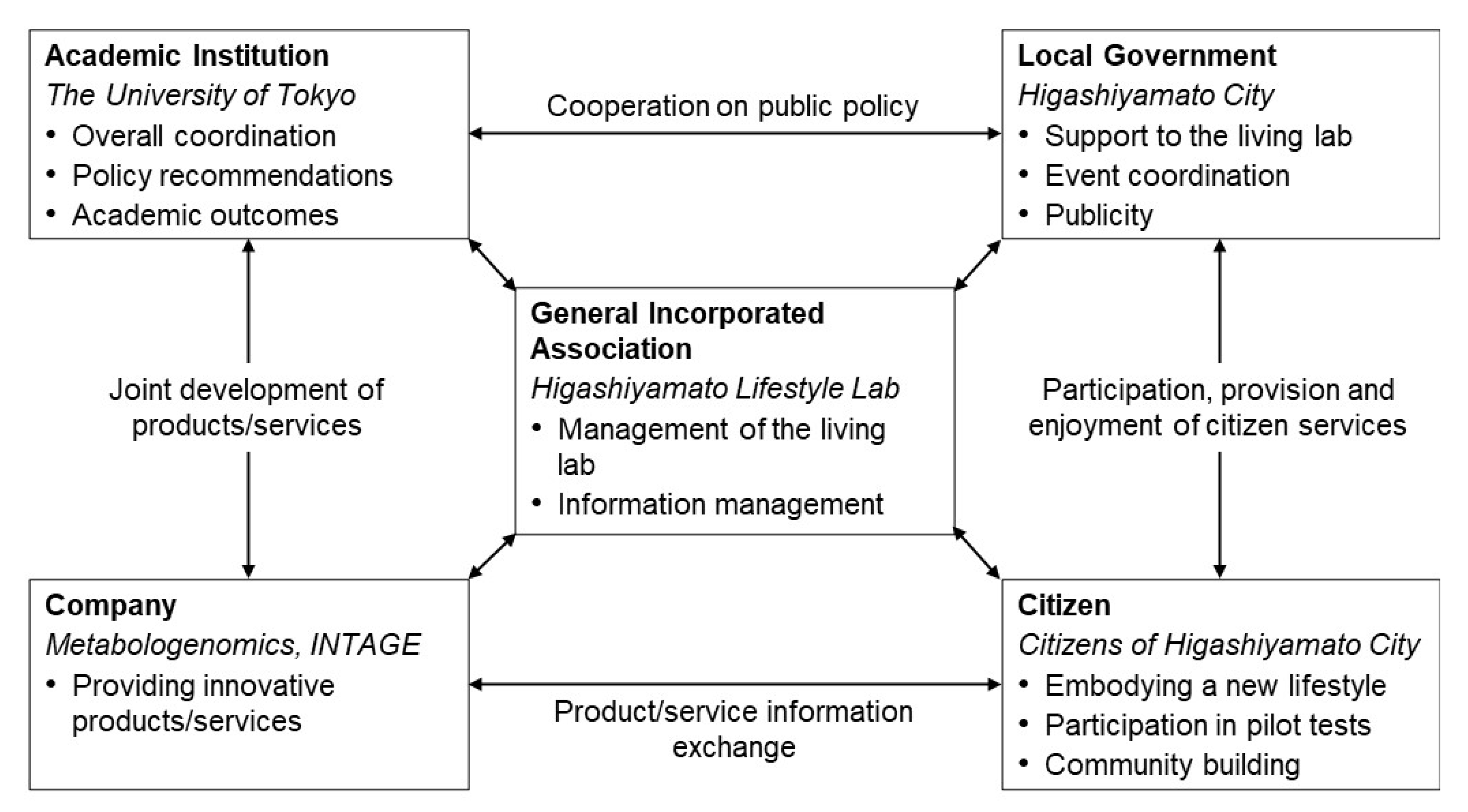
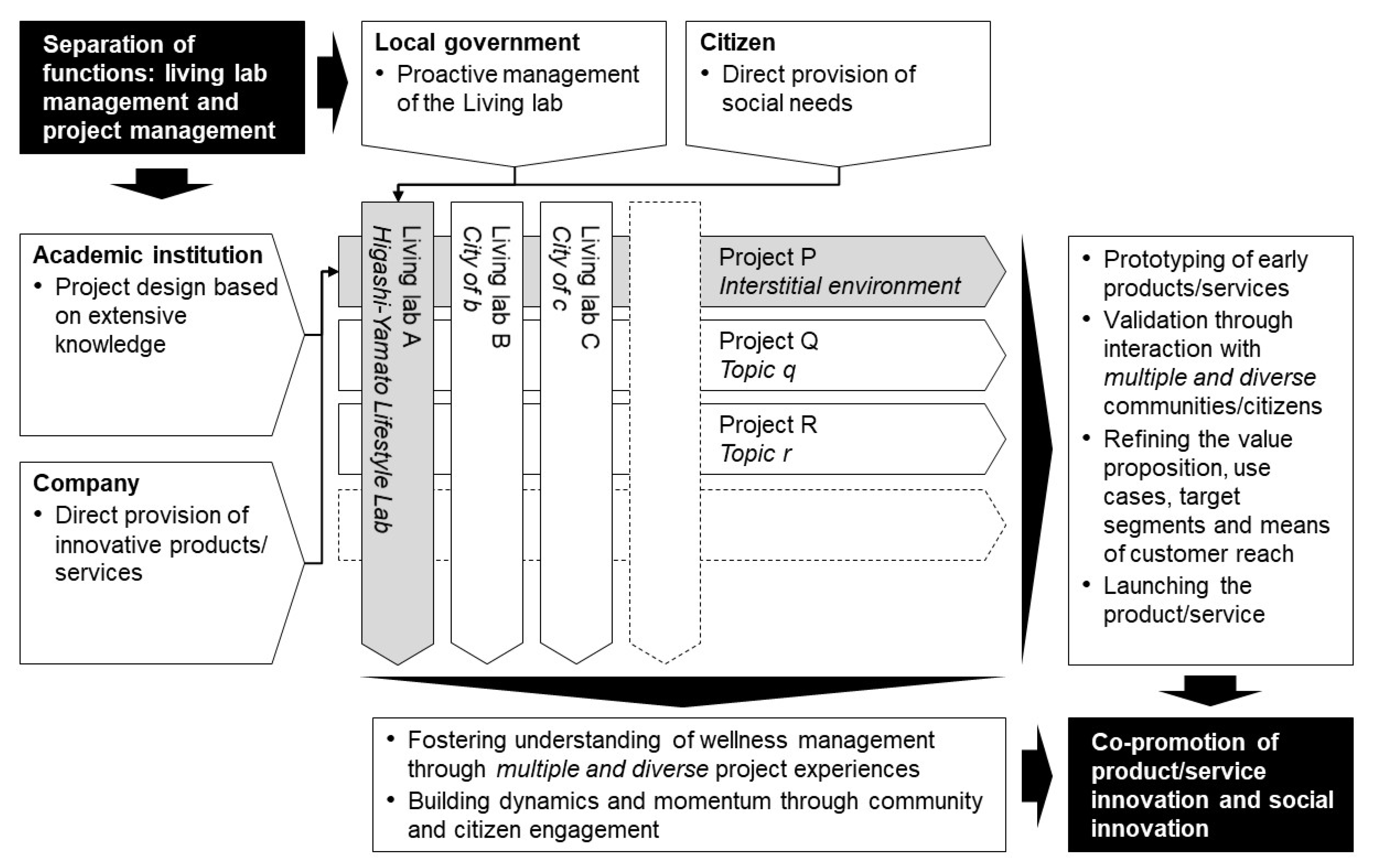
2.2. Organisations and Collaborative Scheme
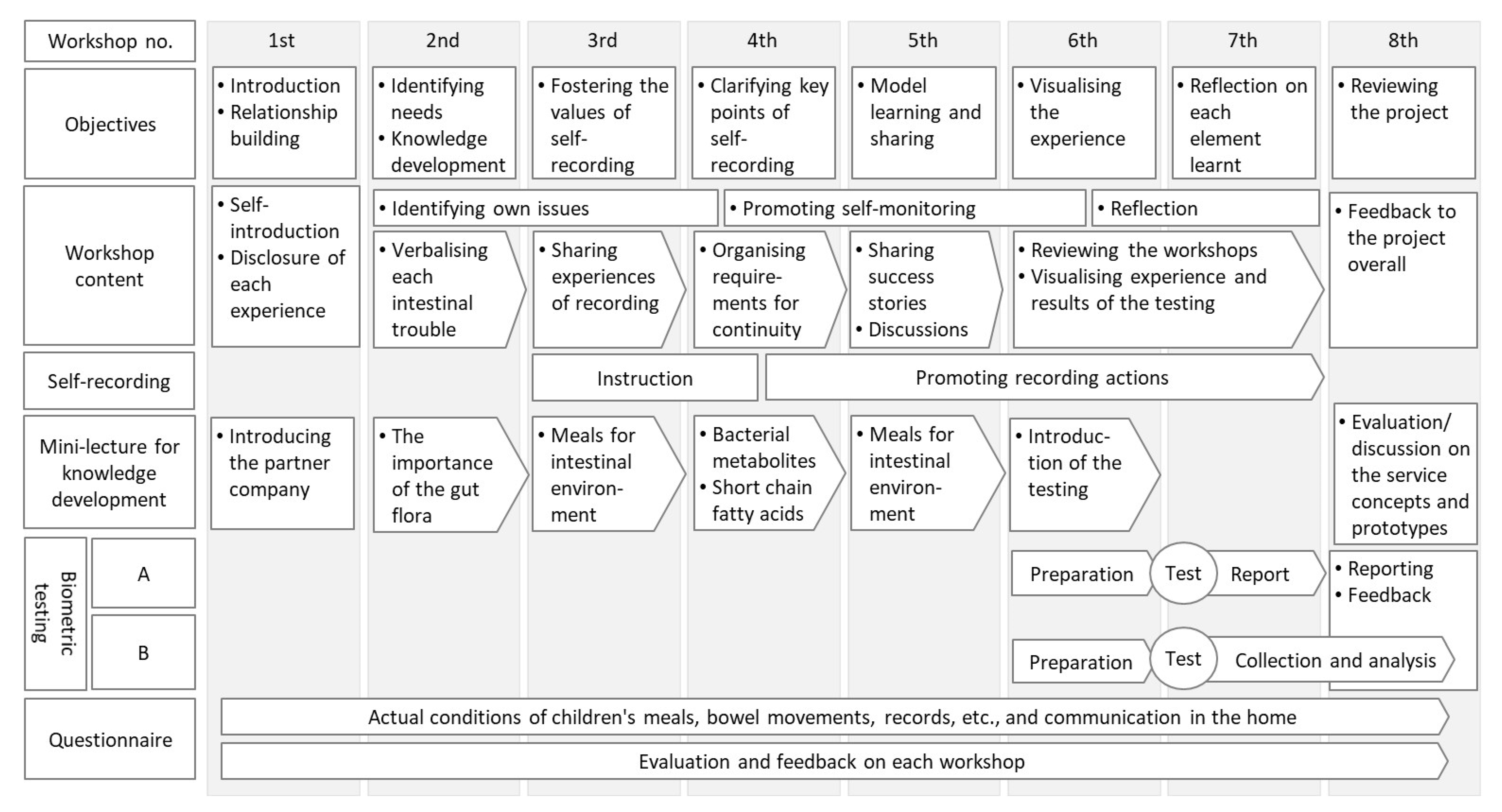
2.3. Project Design and Implementation
2.4. Measurement and Analytical Methods
2.4.1. Recruiting Test Subjects
2.4.2. Intervention Index
2.4.3. Testing Process
- Subject: Registered participants of the 6th workshop and their children (total 14 groups: 14 adults and 17 children), including those who could not participate on the day of the workshop but wished to register later.
- Requirements for participation: Participation in the 7th and 8th workshops, cooperation in questionnaires, and sharing of test result data.
- Test contents: Intestinal microbiota test (test A, sample submission), short-chain fatty acid simple test (test B, image submission).
- Explanation and training procedure: The whole testing procedure was explained to the participants in the 6th workshop by a demonstration of the actual kits and a slide presentation. These kits are designed for general consumer use and, thus, do not require any special skill or expertise.
- Distribution of the tools: The test kit was distributed in the 6th workshop after collecting the application and consent form from those who wished to participate. Those who wished to participate but were absent were individually explained at a later date in the same way.
2.4.4. Testing Flow: Test A (Intestinal Microbiota Test)
- The participants collected their child’s stool from diapers or wipes at home in a revalved container (MG Kit) and sent it to the company in a special envelope. All the storage and transport processes were carried out at room temperature.
- The results were returned to the participants individually in a paper form in the 8th workshop. The sample itself was disposed of by the testing company after the analysis in accordance with the defined regulations.
- The moderator of the company gave a lecture on the overall trend, followed by discussions and Q&A, in the same workshop. The detail of the relevant workshop was described in Appendix A.
2.4.5. Test Flow: Test B (Short-Chain Fatty Acid Test)
- The participants prepared image samples at home through the following procedure: (i) Collected stool from diaper using swab; (ii) suspended stool in the designated container, attached the cap with nozzle, and dripped the stool suspension onto the sensor through the nozzle; (iii) read and recorded the discoloration of the sensor; (iv) took a picture of the sensor, and (v) sent to the specified destination via a Google Form. The sample itself was disposed of by the participant themselves as a normal waste.
- The results were returned to the participants individually in a paper form in the 7th workshop.
- The moderator of the company gave a lecture on the overall trend, followed by discussion and Q&A, in the same workshop. The detail of the relevant workshop was described in Appendix A.
2.4.6. Questionnaire Survey
3. Results
3.1. Implementation Theme and Approaches
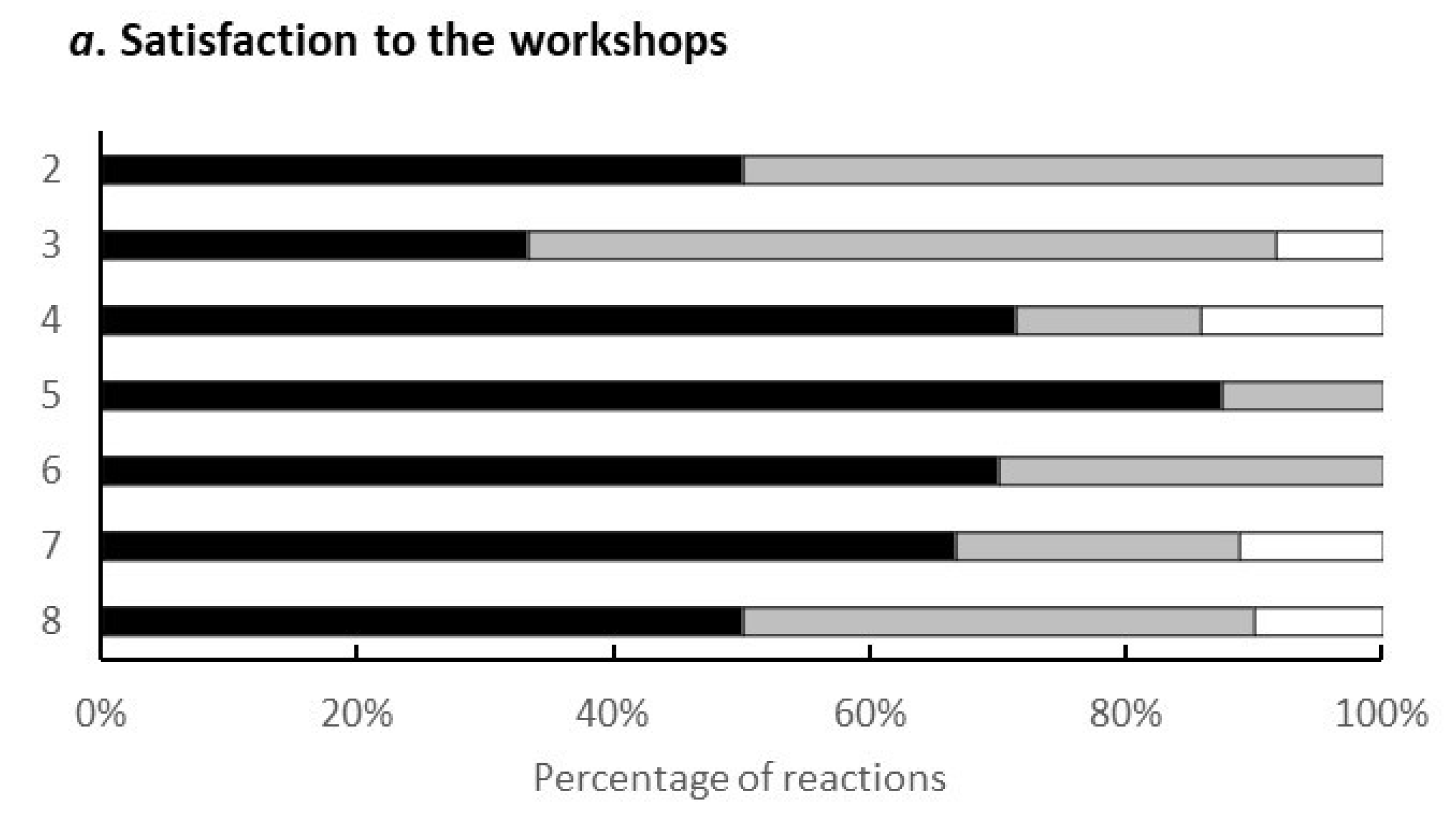
3.2. Workshops
3.3. Outcomes
4. Discussion
4.1. Features of the Case Study
4.2. Significance and Utility under the COVID-19 Pandemic
4.2.1. Appeal to the Significance of Health Promotion under the COVID-19 Pandemic
4.2.2. Local Government-Led Programme Coordination
4.2.3. Adapting to the Potential Needs of Citizens
4.2.4. Optimised Environment Development for Participants
4.3. Significance and Utility of Technology and Innovative Elements
4.4. Limitations and Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A. The Contents of the Workshops
- Introduction and explanation of the main idea
- ○
- Explanation of the purpose of the project
- ▪
- Higashiyamato City’s approach
- ▪
- What is a Living lab?
- ▪
- Background of the theme, etc.
- ○
- Discussed concerns during the COVID-19 self-restraint period
- Presentation on participants’ concerns
- Tummy Health Needs Deep Dive
- ○
- Case work
- ▪
- Write down the theme of ‘tummy troubles’ on a sticky note and present it to the group
- ▪
- Name a tummy problem that you use as a reference
- ○
- Presentation by 2 groups
- ▪
- Affixing the empathy seal
- Digging deeper into the solution image
- ○
- Review of last time
- ▪
- Do you keep any ‘records’? What’s holding you from keeping a record? Filling in stickies and presentations in the group
- ▪
- First impressions of existing tools: ‘Looks good’ and ‘Doesn’t look good’ on sticky notes and presentation in the group
- ▪
- Fill-in the sticky notes of ‘My impression ranking’ and announce it to the group
- ○
- Presentation by 2 groups
- ▪
- Affixing the empathy seal
- ○
- Homework for participants
- ▪
- Let us try it before the next time: ‘Record Challenge (declared)!’
- ○
- Intestinal Environment Mini Lecture
- Experiences and deep dives
- ○
- Review of last time
- ▪
- For the recording method you chose, write on sticky notes: (i) How long did you keep the record, (ii) What was good or bad about the method, and (iii) Good/noticeable points about the record?
- ▪
- Thinking with keyword cards, ‘If I do it this way, the record will last.’
- ○
- Card game
- ▪
- Filling in stickies and presentation in the group
- ○
- Presentation by 2 groups
- ▪
- Affixing the empathy seal
- ○
- ‘This is the way I’d like to record it!’ Declaration
- ○
- Intestinal Environment Mini Lecture
- Record awareness search and deep investigation of gut-environment needs
- ○
- Case work 1: ‘Hear your stories about how you have used the records.’
- ▪
- Presentation by three participants who are recording well
- ▪
- Write your findings and opinions on sticky notes and attach them to the graphic recording
- ○
- Case work 2: ‘Keyword Card: Children’s Tummy Trouble’
- ▪
- While discussing with the group whether or not they have had any experience with the contents of the ‘Tummy Trouble Card’ and their opinions, fill-in the sticky notes and attach them to a piece of paper.
- ○
- Intestinal Environment Mini Lecture
- Explanation of the Intestinal Environment Test and creation of the Customer Journey Map
- ○
- Explanation of the Intestinal Environment Test
- ▪
- pH test, bacterial flora test, and testing system for children’s stool
- ▪
- Fill-out the test consent form
- ▪
- Test kit distribution
- ○
- Case work
- ▪
- ‘Visualising Your Experience in the Lifestyle Lab.’
- ▪
- Individual work: filling-out the My Experience Summary Sheet
- ▪
- Group work: ‘Let’s summarise everyone’s experiences into one story!’
- Sharing of simple test results and opinions, sharing of experiences
- ○
- Results of the Intestinal Environment Test B (short chain-fatty acid)
- ▪
- Introduction of opinions that lead to improvement
- ▪
- What are high levels of short-chain fatty acids associated with?
- ▪
- Results summary
- ○
- Case work
- ▪
- Will this change your behaviour? Yes or no.
- ○
- Presentation by group representatives
- Sharing the results of the intestinal microbiota test, sharing opinions, and reflecting on the results
- ○
- Results of the Intestinal Environment Test A (intestinal microbiota test)
- ▪
- ‘How to read the test results?’
- ○
- Case work
- ▪
- ‘Reading through the test results.’
- ▪
- ‘Did your participation in the lab change anything? Has it helped you?’
- ○
- Presentation by group representatives
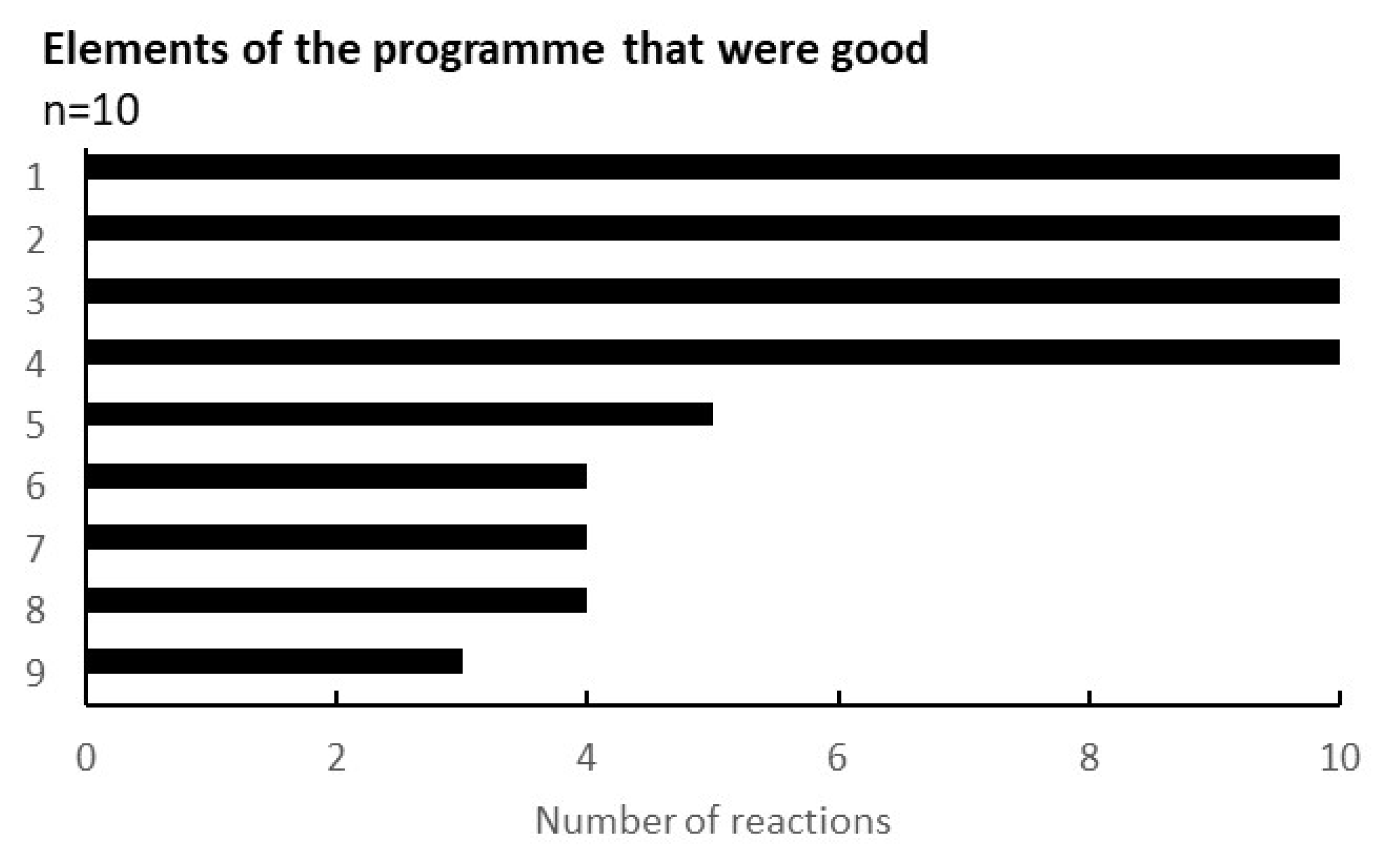
Appendix B. Questionnaire Survey Results

- Because my child tends to be constipated.
- It’s nice to be able to look it up because even though I am a little worried, to this day I still do not know if my kids’ poop is good or bad.
- Because I was always worried about my son’s intestinal environment (constipation since he was 0 years old)
- I wanted to have a chance to think about my child’s health. They can take care of my children.
- The reason I decided to participate was to learn about stool.
- I was worried at first because I had to take care of my child at the same time, but I was able to do it without any problems.
- I was approached by the people involved and it came with childcare.
- I am sure you have faced enormous trouble holding these meetings during the COVID-19 pandemic, and I thank you for making it possible for us to attend each meeting with peace of mind. Participating in these meetings, even if only once a month, is a good refresher for me and my son, and it has given us a chance to seriously consider food and the intestinal environment, which we have never done before. I am glad that I have participated. Thank you very much.
- I really enjoyed participating in each session. I learned a lot, did the tests, and my child seemed to enjoy playing with the nursery staff and friends. The preparations in advance were meticulous, and we spent a lot of time together. Thank you very much.
References
- Xiang, M.; Zhang, Z.; Kuwahara, K. Impact of COVID-19 pandemic on children and adolescents’ lifestyle behavior larger than expected. Prog. Cardiovasc. Dis. 2020, 63, 531. [Google Scholar] [CrossRef] [PubMed]
- Rishi, P.; Thakur, K.; Vij, S.; Rishi, L.; Singh, A.; Kaur, I.P.; Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Diet, gut microbiota and COVID-19. Indian J. Microbiol. 2020, 60, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dietz, W.; Santos-Burgoa, C. Obesity and its implications for COVID-19 mortality. Obesity 2020, 28, 1005. [Google Scholar] [CrossRef] [Green Version]
- Pfefferbaum, B.; North, C.S. Mental health and the Covid-19 pandemic. N. Engl. J. Med. 2020, 383, 510–512. [Google Scholar] [CrossRef]
- Cullen, W.; Gulati, G.; Kelly, B.D. Mental health in the COVID-19 pandemic. QJM Int. J. Med. 2020, 113, 311–312. [Google Scholar] [CrossRef]
- Usher, K.; Durkin, J.; Bhullar, N. The COVID-19 pandemic and mental health impacts. Int. J. Ment. Health Nurs. 2020, 29, 315. [Google Scholar] [CrossRef] [Green Version]
- Conversano, C.; Di Giuseppe, M.; Miccoli, M.; Ciacchini, R.; Gemignani, A.; Orrù, G. Mindfulness, age and gender as protective factors against psychological distress during Covid-19 pandemic. Front. Psychol. 2020, 11, 1900. [Google Scholar] [CrossRef]
- Nitschke, J.P.; Forbes, P.A.; Ali, N.; Cutler, J.; Apps, M.A.; Lockwood, P.L.; Lamm, C. Resilience during uncertainty? Greater social connectedness during COVID-19 lockdown is associated with reduced distress and fatigue. Br. J. Health Psychol. 2021, 26, 553–569. [Google Scholar] [CrossRef]
- Niwa, M.; Hara, Y.; Sengoku, S.; Kodama, K. Effectiveness of social measures against COVID-19 outbreaks in selected Japanese regions analyzed by system dynamic modeling. Int. J. Environ. Res. Public Health 2020, 17, 6238. [Google Scholar] [CrossRef]
- Niwa, M.; Hara, Y.; Matsuo, Y.; Narita, H.; Yeongjoo, L.; Sengoku, S.; Kodama, K. Superiority of mild interventions against COVID-19 on public health and economic measures. J. Pers. Med. 2021, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurokawa, K.; Itoh, T.; Kuwahara, T.; Oshima, K.; Toh, H.; Toyoda, A.; Takami, H.; Morita, H.; Sharma, V.K.; Srivastava, T.P.; et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007, 14, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldasano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4578–4585. [Google Scholar] [CrossRef] [Green Version]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [Green Version]
- Cho, I.; Yamanishi, S.; Cox, L.; Methé, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the intestinal microbiota during a critical develop-mental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef] [Green Version]
- Kostic, A.D.; Gevers, D.; Siljander, H.; Vatanen, T.; Hyötyläinen, T.; Hämäläinen, A.M.; Peet, A.; Tillmann, V.; Pöhö, P.; Mattila, I.; et al. The dynamics of the human infant gut microbiome in devel-opment and in progression toward type 1 diabetes. Cell Host Microbe 2015, 17, 260–273. [Google Scholar] [CrossRef] [Green Version]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Segal, J.P.; Mak, J.W.; Mullish, B.H.; Alexander, J.L.; Ng, S.C.; Marchesi, J.R. The gut microbiome: An under-recognised contributor to the COVID-19 pandemic? Ther. Adv. Gastroenterol. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilan, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Xu, Y.; Wu, P.; Zhou, H.; Lasanajak, Y.; Fang, Y.; Tang, L.; Ye, L.; Li, X.; Cai, Z.; et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol. Res. 2019, 148, 104403. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; Deroos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Hosseini, E.; Grootaert, C.; Verstraete, W.; Van de Wiele, T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 2011, 69, 245–258. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Matsuki, T.; Yahagi, K.; Mori, H.; Matsumoto, H.; Hara, T.; Tajima, S.; Ogawa, E.; Kodama, H.; Yamamoto, K.; Yamada, T.; et al. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat. Commun. 2016, 7, 11939. [Google Scholar] [CrossRef]
- Tsukuda, N.; Yahagi, K.; Hara, T.; Watanabe, Y.; Matsumoto, H.; Mori, H.; Higashi, K.; Tsuji, H.; Matsumoto, S.; Kurokawa, K.; et al. Key bacterial taxa and metabolic pathways affecting gut short-chain fatty acid profiles in early life. ISME J. 2021, 15, 2574–2590. [Google Scholar] [CrossRef] [PubMed]
- Følstad, A. Towards a living lab for the development of online community services. Electron. J. Virtual Org. Netw. 2008, 10, 47–58. [Google Scholar]
- Dekker, R.; Franco Contreras, J.; Meijer, A. The living lab as a methodology for public administration research: A systematic literature review of its applications in the social sciences. Int. J. Public Admin. 2020, 43, 1207–1217. [Google Scholar] [CrossRef]
- Chesbrough, H.W. Open Innovation: The New Imperative for Creating and Profiting from Technology; Harvard Business Review Press: Brighton, MA, USA, 2003. [Google Scholar]
- Curley, M.; Salmelin, B. Open Innovation 2.0: A New Mode of Digital Innovation for Prosperity and Sustainability; Springer: Berlin, Germany, 2017. [Google Scholar]
- Hossain, M.; Leminen, S.; Westerlund, M. A systematic review of the living lab literature. J. Clean. Prod. 2019, 213, 976–988. [Google Scholar] [CrossRef]
- Edwards-Schachter, M.E.; Matti, C.E.; Alcántara, E. Fostering quality of life through social innovation: A living lab methodology study case. Rev. Pol. Res. 2021, 29, 672–692. [Google Scholar] [CrossRef] [Green Version]
- Krieg-Brückner, B.; Röfer, T.; Shi, H.; Gersdorf, B. Mobility assistance in the Bremen ambient assisted living lab. GeroPsych 2010. [Google Scholar] [CrossRef]
- Liedtke, C.; Welfens, M.J.; Rohn, H.; Nordmann, J. LIVING LAB: User-driven innovation for sustainability. Int. J. Sustain. High. Educ. 2012, 13, 106–118. [Google Scholar] [CrossRef]
- Bergvall-Kåreborn, B.; Howcroft, D.; Ståhlbröst, A.; Wikman, A.M. Participation in living lab: Designing systems with users. In IFIP Working Conference on Human Benefit through the Diffusion of Information Systems Design Science Research; Springer: Heidelberg, Germany, 2010; pp. 317–326. [Google Scholar]
- Canzler, W.; Engels, F.; Rogge, J.C.; Simon, D.; Wentland, A. from “living lab” to strategic action field: Bringing together energy, mobility, and Information Technology in Germany. Energy Res. Soc. Sci. 2017, 27, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Swinkels, I.C.; Huygens, M.W.; Schoenmakers, T.M.; Nijeweme-D’Hollosy, W.O.; Van Velsen, L.; Vermeulen, J.; Schoone-Harmsen, M.; Jansen, Y.J.; Van Schayck, O.C.; Friele, R.; et al. Lessons learned from a living lab on the broad adoption of eHealth in primary health care. J. Med. Internet Res. 2018, 20, e9110. [Google Scholar] [CrossRef]
- Kimura, A.; Akasaka, F. Potentials and Challenges of Living Lab for Social Issues. Serviceol 2018, 5, 4–11. [Google Scholar]
- Ministry of Economy, Trade and Industry (METI). Research Report on the Creation of Innovative Social Problem-Solving Services in Living Labs. Available online: https://www.meti.go.jp/meti_lib/report/2019FY/000256.pdf (accessed on 22 November 2021).
- Cabinet Office of Japan (CAO). 5th Science and Technology Basic Plan. Available online: https://www8.cao.go.jp/cstp/kihonkeikaku/index5.html (accessed on 22 November 2021).
- Santonen, T.; Julin, M. Empirical evaluation of health and wellbeing living lab business models. In Proceedings of the ISPIM Connects Ottawa, Innovation for Local and Global Impact-2019, Ottawa, ON, Canada, 7–10 April 2019. [Google Scholar]
- Parveen, S.; Senin, A.A.; Umar, A. Organization culture and open innovation: A quadruple helix open innovation model approach. Int. J. Econ. Financ. Issues 2015, 335–342. [Google Scholar]
- Holopainen, A.; Kämäräinen, P.; Kaunisto, M.; Kekäläinen, H.; Metsävainio, K. Living Lab services promoting health in the community through participation. Finnish J. eHealth eWelfare 2018, 10, 373–380. [Google Scholar] [CrossRef]
- Akasaka, F.; Yasuoka, M.; Nakatani, M.; Kimura, A.; Ihara, M. Patterns for living lab practice: Describing key know-how to promote service co-creation with users. Int. J. Autom. Tech. 2020, 14, 769–778. [Google Scholar] [CrossRef]
- Hakkarainen, L.; Hyysalo, S. How do we keep the living laboratory alive? Learning and conflicts in living lab collaboration. Technol. Innov. Manag. Rev. 2013, 3, 16–22. [Google Scholar] [CrossRef]
- Michie, S.; Abraham, C.; Whittington, C.; McAteer, J. Effective Techniques in Healthy Eating and Physical Activity Interventions: A Meta- Regression. Health Psychol. 2009, 28, 690–701. [Google Scholar] [CrossRef] [Green Version]
- Becker, M.H.; Drachman, R.H.; Kirscht, J.P. A new approach to explaining sick-role behavior in low-income populations. Am. J. Public Health 1974, 64, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Kimura, A.; Kusano, K.; Akasaka, F.; Watanabe, H.; Masayuki, I. Community-based Living Lab: Sustainable co-creation among citizens, community comprehensive care centers and companies. Bull. JSSD 2018. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabata, N.; Tsukada, M.; Kubo, K.; Inoue, Y.; Miroku, R.; Odashima, F.; Shiratori, K.; Sekiya, T.; Sengoku, S.; Shiroyama, H.; et al. Living Lab for Citizens’ Wellness: A Case of Maintaining and Improving a Healthy Diet under the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 1254. https://doi.org/10.3390/ijerph19031254
Tabata N, Tsukada M, Kubo K, Inoue Y, Miroku R, Odashima F, Shiratori K, Sekiya T, Sengoku S, Shiroyama H, et al. Living Lab for Citizens’ Wellness: A Case of Maintaining and Improving a Healthy Diet under the COVID-19 Pandemic. International Journal of Environmental Research and Public Health. 2022; 19(3):1254. https://doi.org/10.3390/ijerph19031254
Chicago/Turabian StyleTabata, Natsuko, Mai Tsukada, Kozue Kubo, Yuri Inoue, Reiko Miroku, Fumihiko Odashima, Koichiro Shiratori, Takashi Sekiya, Shintaro Sengoku, Hideaki Shiroyama, and et al. 2022. "Living Lab for Citizens’ Wellness: A Case of Maintaining and Improving a Healthy Diet under the COVID-19 Pandemic" International Journal of Environmental Research and Public Health 19, no. 3: 1254. https://doi.org/10.3390/ijerph19031254
APA StyleTabata, N., Tsukada, M., Kubo, K., Inoue, Y., Miroku, R., Odashima, F., Shiratori, K., Sekiya, T., Sengoku, S., Shiroyama, H., & Kimura, H. (2022). Living Lab for Citizens’ Wellness: A Case of Maintaining and Improving a Healthy Diet under the COVID-19 Pandemic. International Journal of Environmental Research and Public Health, 19(3), 1254. https://doi.org/10.3390/ijerph19031254






