Isolation and Degradation Characteristics of PBAT Film Degrading Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Biodegradable Film and Source of Degradation Bacteria
2.1.2. Test Instrument
2.1.3. Culture Medium
2.2. Test Method
2.2.1. Soil Sampling and Film Pretreatment
2.2.2. Screening and Isolation of PBAT Film Degrading Bacteria
2.2.3. PBAT Film Degradation Test
2.2.4. Identification of Strain JZ1
2.2.5. Optimization of the Degradation Conditions
2.2.6. SEM-EDX Analysis
2.3. Quality Assurance and Quality Control
2.4. Statistical Analysis
3. Results
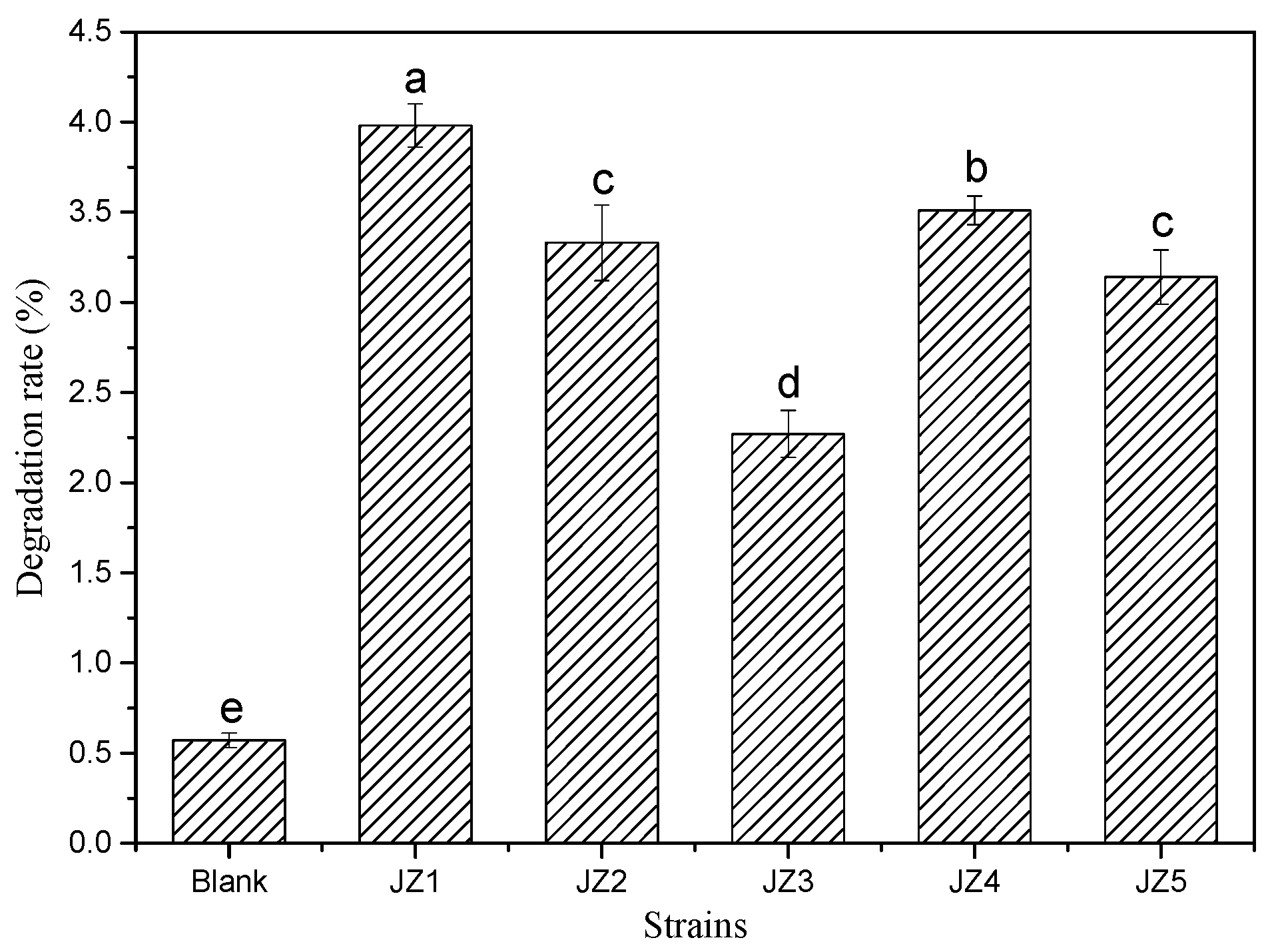
3.1. Degradation Capacity of Potential Degrading Bacteria to PBAT
3.2. Identification of PBAT Film Degrading Strain
3.3. Effect of Different Culture Conditions on the Degradation Rate of PBAT
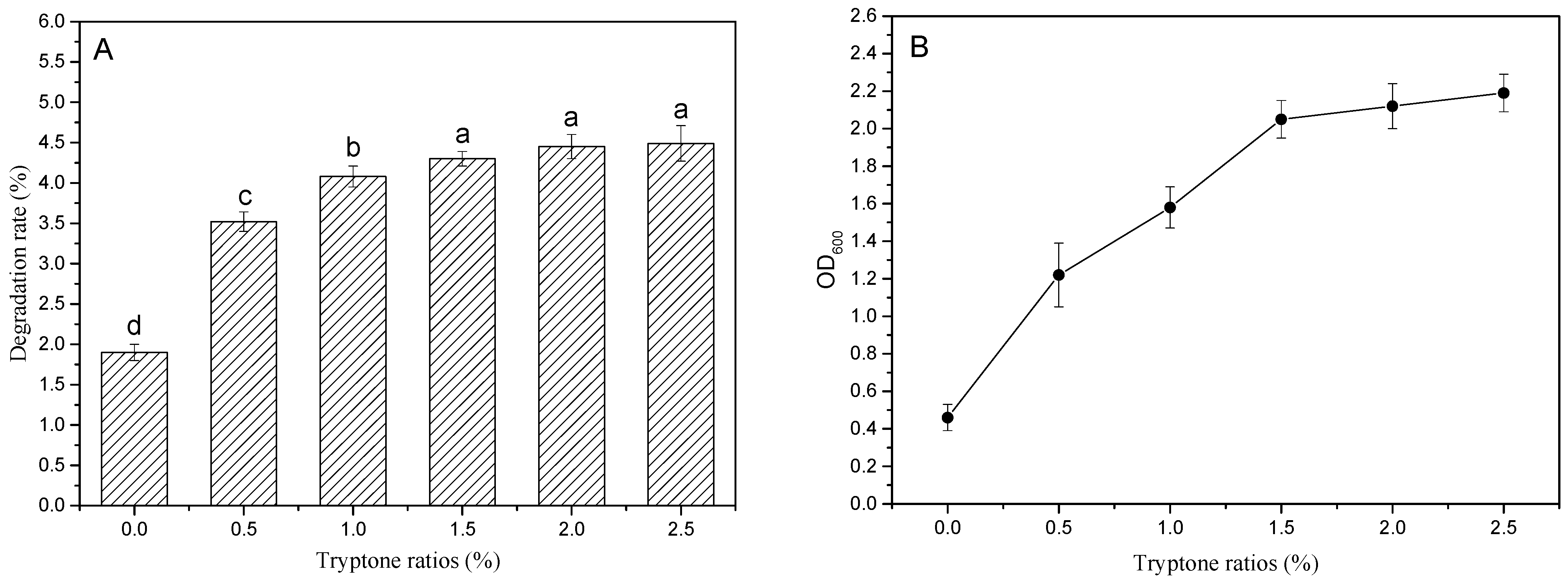
3.3.1. Effect of Different Tryptone Content on the Degradation Rate of PBAT Film
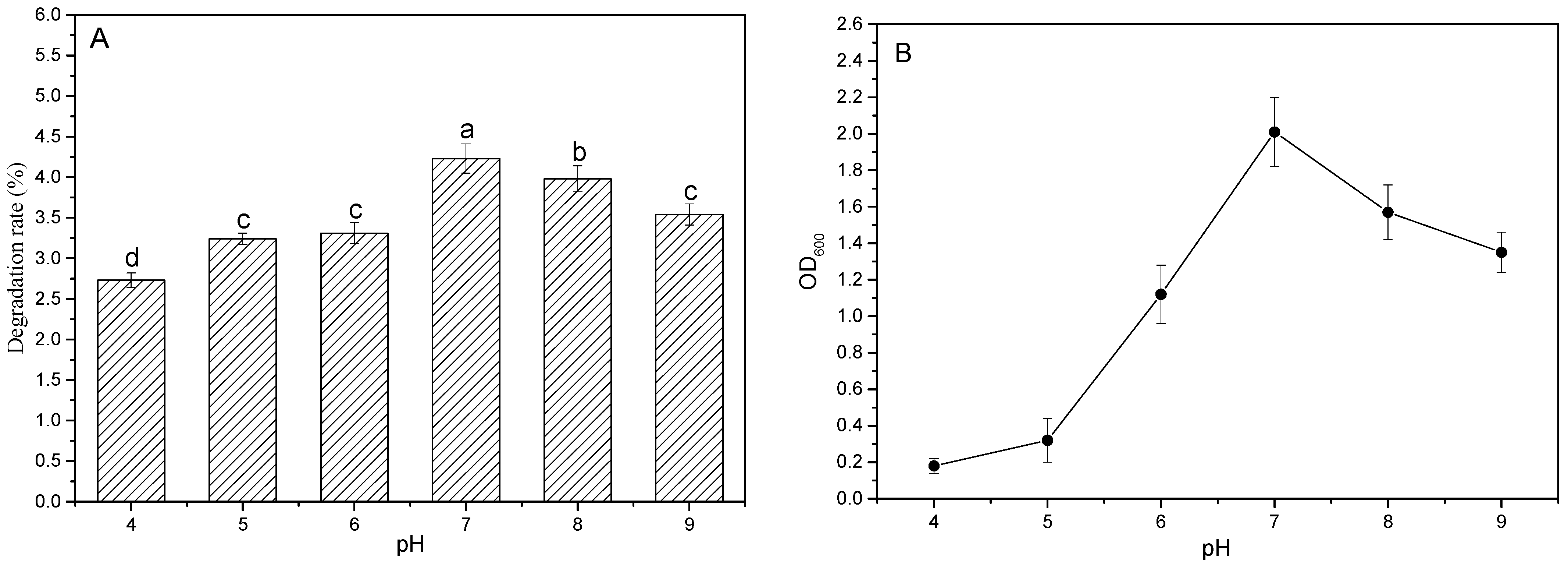
3.3.2. Effect of Different pH on Degradation Rate of PBAT Film
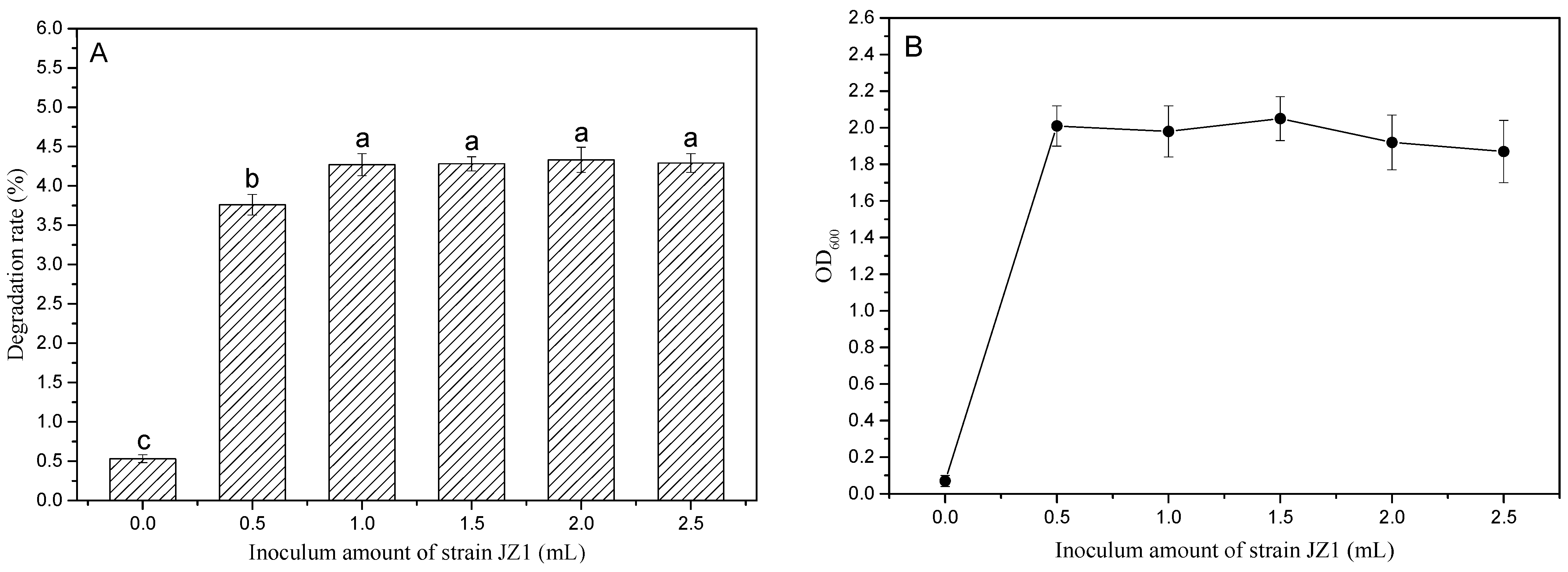
3.3.3. Effect of Different Inoculum Amounts on the Degradation Rate of PBAT Film
3.3.4. Effect of Strain Culture Time on PBAT Film Degradation Rate under Optimized Conditions
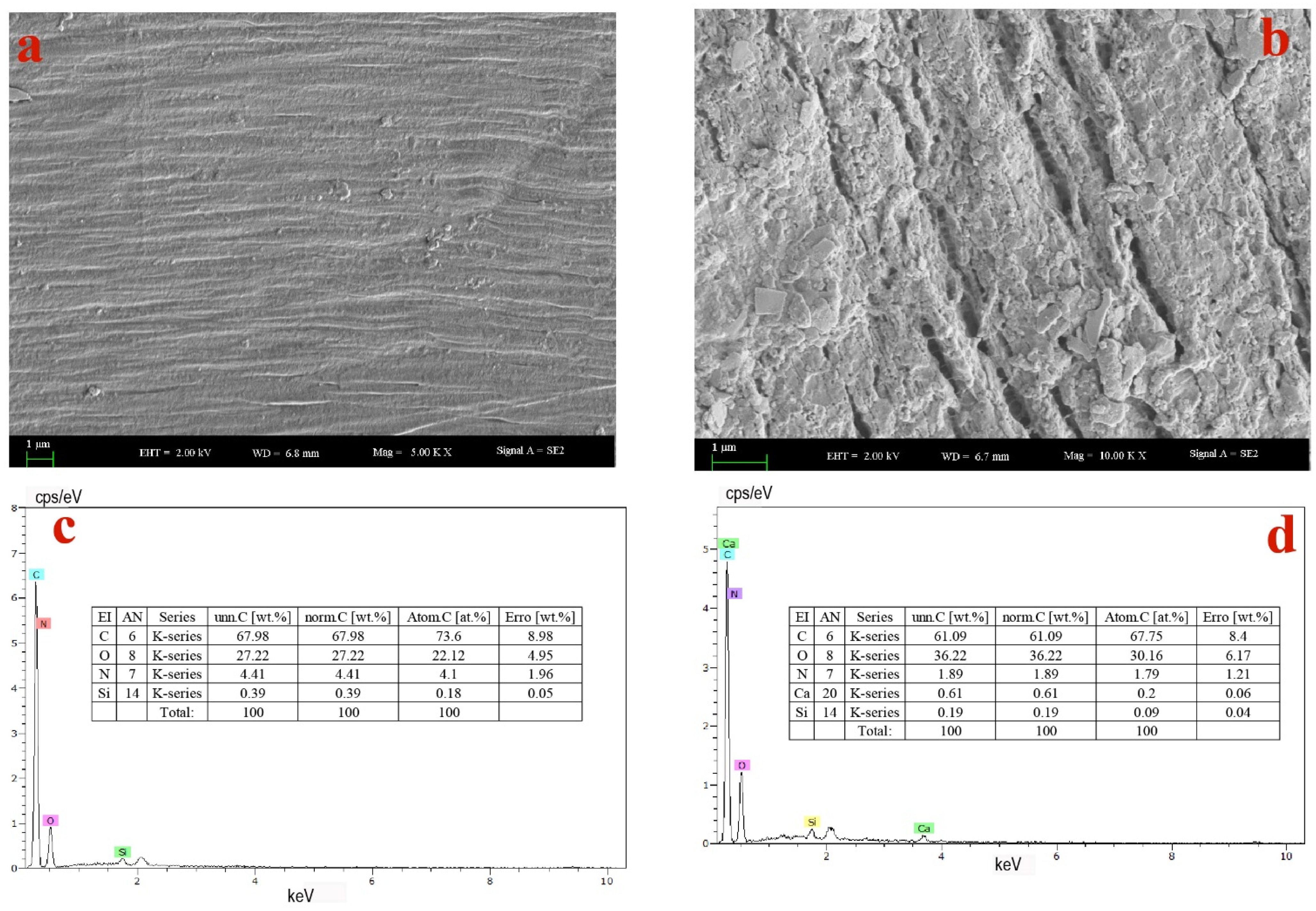
3.3.5. SEM-EDS Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Dong, X.; Li, F.; Chen, M. Research progress in biodegradable mulching film and its application. Sugarcane Sugar Ind. 2018, 3, 5. (In Chinese) [Google Scholar]
- Pan, Y.; Zheng, M.; Zhou, Z.; Pei, Y.; Xu, S. Research Status and Development of Biodegradable Agricultural Film. Guangzhou Chem. Ind. 2020, 48, 37–39. (In Chinese) [Google Scholar]
- Dong, H.; Liu, T.; Li, Y.; Liu, H.; Wang, D. Effects of plastic film on cotton yield and soil physical and chemical properties in Xinjiang. Trans. China Soc. Agric. Eng. 2013, 29, 91–99. (In Chinese) [Google Scholar]
- Liao, Y.C.; Nazygul, J.; Li, M.; Wang, X.L.; Jiang, L.J. Effects of microplastics on the growth, physiology, and biochemical characteristics of wheat (Triticum aestivum). Environ. Sci. 2019, 40, 4661–4667. (In Chinese) [Google Scholar]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, C.; Song, X.; Weng, Y. Progress in preparation and application of PBAT thin films. China Plast. 2021, 35, 115–125. (In Chinese) [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, A.; Yang, J.; Ren, J. Preparation and properties of PBAT. Plastics 2010, 39, 98–101. (In Chinese) [Google Scholar]
- He, X. Analysis of the development status of degradable plastics. High Technol. Commer. 2020, 2, 56–62. (In Chinese) [Google Scholar]
- Wang, L.; He, X.; Hu, C.; Wang, X.; Liu, Q.; Yan, C.; Ding, J. Effects of Biodegradable Plastic Film on Soil Temperature and Humidity and Cotton Yield in South Xinjiang. Agric. Res. Arid. Areas 2021, 39, 8. (In Chinese) [Google Scholar]
- Cui, Y.; Wei, J.; Gou, L.; Han, C.; Han, Y.; Zhu, J.; Liu, K.; Xie, Y. Shule County 2021 fully biodegradable plastic film test demonstration and appropriate promotion and application. Agric. Technol. Equip. 2022, 5, 44–46. (In Chinese) [Google Scholar]
- Su, H.; Bao, Z.; Liu, Q.; Dong, D.; Yan, C.; Lei, H.; Xue, Y.; Xu, Z. Feasibility of applying PBAT fully biodegradable mulching film to processing tomatoes in Xinjiang. Agric. Environ. Dev. 2020, 37, 615–622. (In Chinese) [Google Scholar]
- Wu, Q.; Wang, Z.; Zheng, X.; Zhang, J.; Li, W. Effects of biodegradation film mulching on soil temperature, moisture and yield of cotton under drip irrigation in typical oasis area. Trans. China Soc. Agric. Eng. 2017, 33, 135–143. (In Chinese) [Google Scholar]
- Wang, H.; Wei, D.; Zheng, A.; Xiao, H. Soil burial biodegradation of antimicrobial biodegradable PBAT films. Polym. Degrad. Stab. 2015, 116, 14–22. [Google Scholar] [CrossRef]
- Trinh Tan, F.; Cooper, D.G.; Marić, M.; Nicell, J.A. Biodegradation of a synthetic co-polyester by aerobic mesophilic microorganisms. Polym. Degrad. Stab. 2008, 93, 1479–1485. [Google Scholar] [CrossRef]
- Sekhar, V.C.; Nampoothiri, K.M.; Mohan, A.J.; Nair, N.R.; Bhaskar, T.; Pandey, A. Microbial degradation of high impact polystyrene (HIPS), an e-plastic with decabromodiphenyl oxide and antimony trioxide. J. Hazard. Mater. 2016, 318, 347–354. [Google Scholar] [CrossRef]
- Zhang, M.; Jia, H.; Weng, Y.; Li, C. Effects of polylactide/polybutylene adipate-co-terephthalate on bacterial community structure of soil and isolation of degrading bacteria. Microbiol. China 2020, 47, 420–430. (In Chinese) [Google Scholar]
- Wu, Q.; Fu, L.; Lu, Z. Experimental Study on Separation, Purification and Molecular Identification of Soil Microbes. J. Anyang Inst. Technol. 2016, 15, 27–29. (In Chinese) [Google Scholar]
- Huo, X.; Gao, Y.; Lin, Q.; Zeng, J.; Zhang, T.; Chu, M.; Yang, H.; Shi, Y.; Wang, B.; Sun, J.; et al. Isolation and identification of poly (butylene adipate-co-terephthalate)-degrading Bacteria. Xinjiang Agric. Sci. 2017, 54, 2086–2091. (In Chinese) [Google Scholar]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Alvarado, E.; Camacho Montero, J.R.; Rosales, J.M. Atmospheric and soil degradation of aliphatie-aromatic polyester films. Polym. Degrad. Stab. 2010, 95, 99–107. [Google Scholar] [CrossRef]
- Kunlere, I.O.; Fagade, O.E.; Nwadike, B.I. Biodegradation of low-density polyethylene (LDPE) by certain indigenous bacteria and fungi. Int. J. Environ. Stud. 2019, 76, 428–440. [Google Scholar] [CrossRef]
- Ojha, N.; Pradhan, N.; Singh, S.; Barla, A.; Shrivastava, A.; Khatua, P.; Rai, V.; Bose, S. Evaluation of HDPE and LDPE degradation by fungus, implemented by statistical optimization. Sci. Rep. 2017, 7, 39515. [Google Scholar] [CrossRef] [PubMed]
- Restrepo-Flórez, J.-M.; Bassi, A.; Thompson, M.R. Microbial degradation and deterioration of polyethylene: A review. Int. Biodeterior. Biodegrad. 2014, 88, 83–90. [Google Scholar] [CrossRef]
- Perz, V.; Hromic, A.; Baumschlager, A.; Steinkellner, G.; Pavlov-Keller, T.; Gruber, K.; Bleymaier, K.; Zitzenbacher, S.; Zankel, A.; Mayrhofer, C.; et al. An esterase from anaerobic Clostridium hathewayi can hydrolyze aliphatic-aromatic polyesters. Environ. Sci. Technol. 2016, 50, 2899–2907. [Google Scholar] [CrossRef] [PubMed]
- Marten, E.; Muller, R.J.; Deckwer, W.-D. Studies on the enzymatic hydrolysis of polyesters. II. Aliphatic–aromatic copolyesters. Polym. Degrad. Stab. 2005, 88, 371–381. [Google Scholar] [CrossRef]
- Andrady, A.L.; Hamid, S.H.; Torikai, A. Effects of climate change and UV-B on materials. Photochem. Photobiol. 2003, 2, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Huang, G. Review on development actualities of degradable plastics. Sci. Technol. LCIC 2001, 19, 276–281. (In Chinese) [Google Scholar]
- Yakabe, Y.; Nohara, K.; Hara, T.; Fujino, Y. Factors affecting the biodegradability of biodegradable polyester in soil. Chemosphere 1992, 25, 1879–1888. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Guo, J.; Feng, Q.; Liang, T. Comparison of spatial interpolation methods for precipitation distribution in Xinjiang. Grassl. Sci. 2018, 35, 521–529. (In Chinese) [Google Scholar]
- Hu, M.; Xu, Y.; Shao, W.; Wang, H.; Du, Y.; Zhou, J. Current situation and countermeasures of research, development, promotion and application of biodegradable mulching film. Zhejiang Agric. Sci. 2019, 60, 4. (In Chinese) [Google Scholar]
- Kasuya, K.; Ishii, N.; Inoue, Y.; Yazawa, K.; Tagaya, T.; Yotsumoto, T.; Kazahaya, J.; Nagai, D. Characterization of a meso philicaliphatic-aromatic copolyester-degrading fungus. Polym. Degrad. Stab. 2009, 94, 1190–1196. [Google Scholar] [CrossRef]
- Muroi, F.; Tachibana, Y.; Soulenthone, P.; Yamamoto, K.; Mizuno, T.; Sakurai, T.; Kobayashi, Y.; Kasuya, K. Characterization of apoly (butyleneadipate-co-terephthalate) hydrolase from the aerobic mesophilic bacterium Bacillus pumilus. Polym. Degrad. Stab. 2017, 137, 11–12. [Google Scholar] [CrossRef]
- Liu, J.; Hou, L.; Liu, T.; Wang, P.; Gao, X.; Lin, Y. Isolation of PBAT plastic-degrading bacteria and their degradation characteristics. J. Agro-Environ. Sci. 2021, 40, 129–136. (In Chinese) [Google Scholar]
- Lin, J.; Zhou, J.; Kang, Z.; Du, G.; Chen, J. Isolation, identification of poly lactic acid degrading microorganisms and optimization of the degradation process. Microbiol. China 2013, 40, 1560–1569. (In Chinese) [Google Scholar]
- Onraedt, A.; Soetaert, W.; Vandamme, E. Industrial importance of the genus Brevibacterium. Biotechnol. Lett. 2005, 27, 527–533. [Google Scholar] [CrossRef]
- Montecillo, J.A.V.; Bae, H. Reclassification of Brevibacterium frigoritolerans as Peribacillus frigoritolerans comb. nov. based on phylogenomics and multiple molecular synapomorphies. Int. J. Syst. Evol. Microbiol. 2022, 72, 5389. [Google Scholar] [CrossRef]
- Jariyal, M.; Jindal, V.; Mandal, K.; Gupta, V.K.; Singh, B. Bioremediation of organophosphorus pesticide phorate in soil by microbial consortia. Ecotoxicol. Environ. Saf. 2018, 159, 310–316. [Google Scholar] [CrossRef]
- Behera, L.; Datta, D.; Kumar, S.; Kumar, S.; Sravani, B.; Chandra, R. Role of microbial consortia in remediation of soil, water and environmental pollution caused by indiscriminate use of chemicals in agriculture: Opportunities and challenges. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, H.B., Vaishnav, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 18, pp. 399–418. [Google Scholar] [CrossRef]
- Jariyal, M.; Gupta, V.K.; Mandal, K.; Jindal, V. Brevibacterium frigoritolerans as a Novel Organism for the Bioremediation of Phorate. Bull. Environ. Contam. Toxicol. 2015, 95, 680–686. [Google Scholar] [CrossRef]
- Jariyal, M.; Gupta, V.K.; Mandal, K.; Jindal, V.; Banta, G.; Singh, B. Isolation and characterization of novel phorate-degrading bacterial species from agricultural soil. Environ. Sci. Pollut. Res. 2014, 21, 2214–2222. [Google Scholar] [CrossRef]
- Palma, T.L.; Magno, G.; Costa, M.C. Biodegradation of Paracetamol by Some Gram-Positive Bacterial Isolates. Curr. Microbiol. 2021, 78, 2774–2786. [Google Scholar] [CrossRef]
- Jin, M.; Zhao, Q.; Zhou, Z.; Zhu, L.; Zhang, Z.; Jiang, L. Draft Genome Sequence of a Potential Organic Phosphorus-Degrading Bacterium Brevibacterium frigoritolerans GD44, Isolated from Radioactive Soil in Xinjiang, China. Curr. Microbiol. 2020, 77, 2896–2903. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Yin, L.; Liu, C.; Zou, H.; Wu, Z.; Zhang, Z. Analysis of the complete genome sequence of Brevibacterium frigoritolerans ZB201705 isolated from drought- and salt-stressed rhizosphere soil of maize. Ann. Microbiol. 2019, 69, 1489–1496. [Google Scholar] [CrossRef]
- Motoo, K.; Yuka, S.-Y.; Takashi, W.; Yukiko, S.; Hiroko, K. Phylloplane fungal enzyme accelerate decomposition of biodegradable plastic film in agricultural settings. Jpn. Agric. Res. Q. 2016, 50, 229–234. [Google Scholar] [CrossRef]
- Aarthy, M.; Puhazhselvan, P.; Aparna, R.; George, A.S.; Gowthaman, M.K.; Ayyadurai, N.; Kazuo, M.; Toshiaki, N.-K.; Numbi, R.K. Growth associated degradation of aliphatic-aromatic copolyesters by cryptococcus sp. mtcc 5455. Polym. Degrad. Stab. 2018, 152, 20–28. [Google Scholar] [CrossRef]
- Hu, W.; Sun, J.; Shan, N.; Yan, J.; Wang, X. Effect of COPO degradable membrane on soil temperature and crop yield and analysis of its degradable characteristics. Xinjiang Agric. Sci. 2015, 52, 317–320. (In Chinese) [Google Scholar]
- Jie, N.; Xie, H. Study on the Effect of Photolysis Film mulching on Cotton Field. Shanxi Agric. Sci. 1994. (In Chinese) [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wufuer, R.; Li, W.; Wang, S.; Duo, J. Isolation and Degradation Characteristics of PBAT Film Degrading Bacteria. Int. J. Environ. Res. Public Health 2022, 19, 17087. https://doi.org/10.3390/ijerph192417087
Wufuer R, Li W, Wang S, Duo J. Isolation and Degradation Characteristics of PBAT Film Degrading Bacteria. International Journal of Environmental Research and Public Health. 2022; 19(24):17087. https://doi.org/10.3390/ijerph192417087
Chicago/Turabian StyleWufuer, Rehemanjiang, Wenfeng Li, Shuzhi Wang, and Jia Duo. 2022. "Isolation and Degradation Characteristics of PBAT Film Degrading Bacteria" International Journal of Environmental Research and Public Health 19, no. 24: 17087. https://doi.org/10.3390/ijerph192417087
APA StyleWufuer, R., Li, W., Wang, S., & Duo, J. (2022). Isolation and Degradation Characteristics of PBAT Film Degrading Bacteria. International Journal of Environmental Research and Public Health, 19(24), 17087. https://doi.org/10.3390/ijerph192417087







