Higher Infection Risk among Health Care Workers and Lower Risk among Smokers Persistent across SARS-CoV-2 Waves—Longitudinal Results from the Population-Based TiKoCo Seroprevalence Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Participants, and Setting
2.2. Smoking and Occupational Status Assessed by Questionnaire
2.3. Assessment of Other Covariates by Questionnaire at BL
2.4. Assessment of Known Infections and Vaccination Status among Participants
2.5. Blood Processing, Storage, and Serum Antibody Measurements
2.6. Statistical Analyses
3. Results
3.1. Analyzed Participants
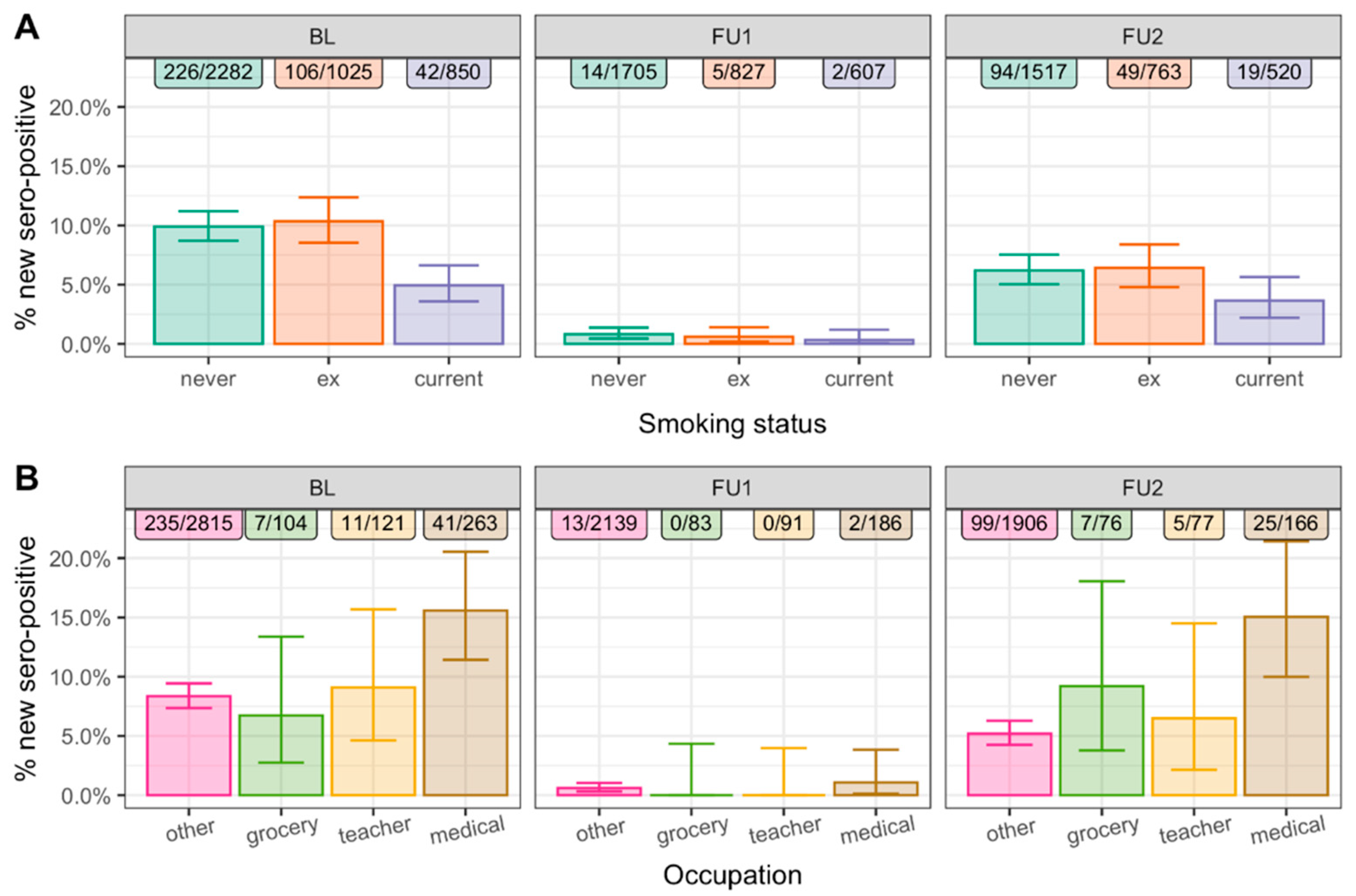
3.2. New N-Seropositivity Was Decreased for Smokers and Increased for Medical Personnel in Each of the Three Observation Periods
3.3. Associations Were Robust upon Adjustment by Other Factors and across Subgroups
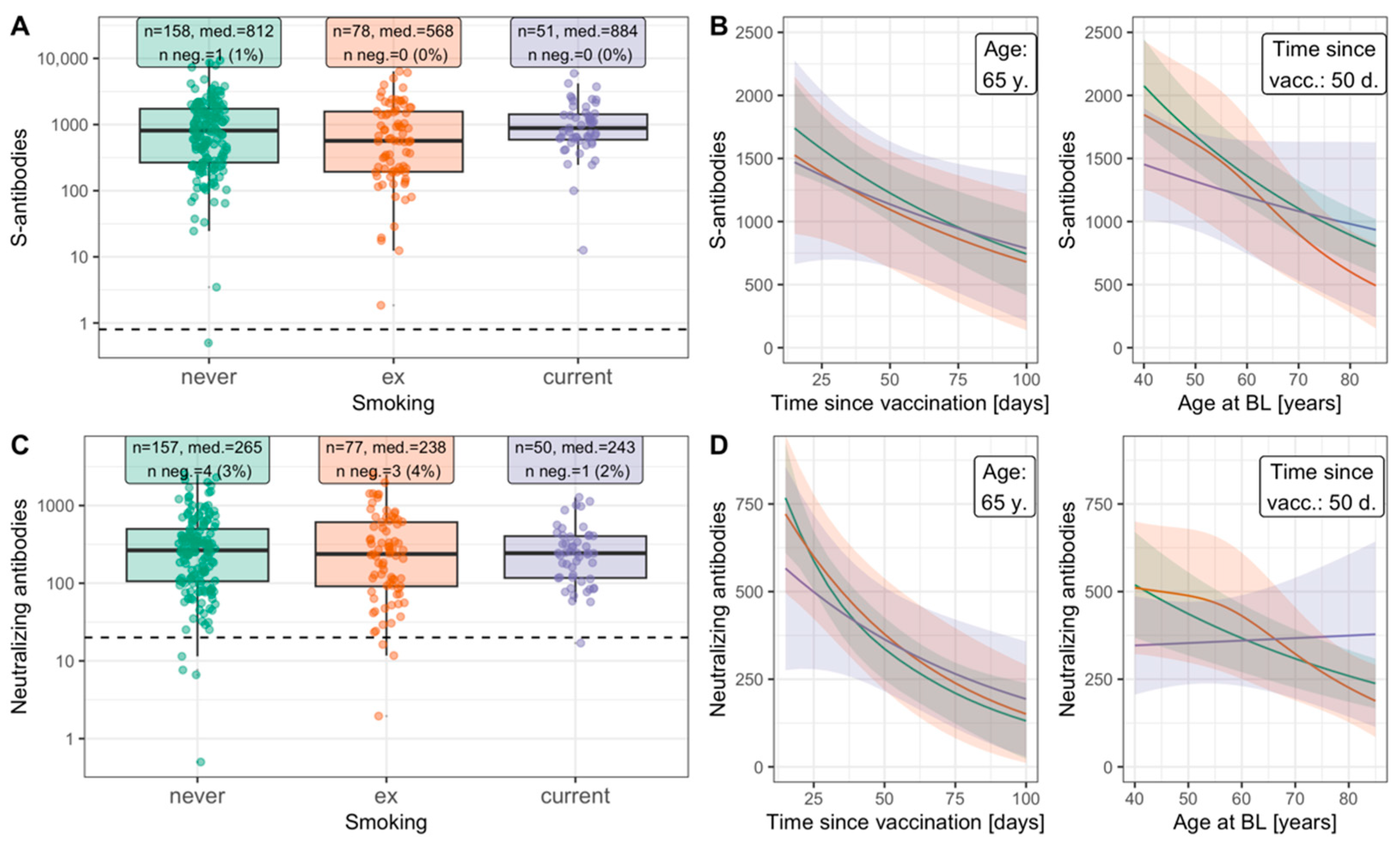
3.4. Similar Antibody Response to Infection or to Vaccination for Smokers and Non-Smokers
3.5. Fewer Infected among Current Smokers Than among Non-Smokers in Each of the Three Observation Periods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, H.; Atchison, C.; Whitaker, M.; Ainslie, K.E.C.; Elliott, J.; Okell, L.; Redd, R.; Ashby, D.; Donnelly, C.A.; Barclay, W.; et al. SARS-CoV-2 antibody prevalence in England following the first peak of the pandemic. Nat. Commun. 2021, 12, 905. [Google Scholar] [CrossRef] [PubMed]
- Carrat, F.; de Lamballerie, X.; Rahib, D.; Blanché, H.; Lapidus, N.; Artaud, F.; Kab, S.; Renuy, A.; de Edelenyi, F.S.; Meyer, L.; et al. Antibody status and cumulative incidence of SARS-CoV-2 infection among adults in three regions of France following the first lockdown and associated risk factors: A multicohort study. Int. J. Epidemiol. 2021, 50, 1458–1472. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.; Wisniak, A.; Perez-Saez, J.; Garrison-Desany, H.; Petrovic, D.; Piumatti, G.; Baysson, H.; Picazio, A.; Pennacchio, F.; De Ridder, D.; et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies, risk factors for infection and associated symptoms in Geneva, Switzerland: A population-based study. Scand. J. Public Health 2021, 50, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Prinelli, F.; Bianchi, F.; Drago, G.; Ruggieri, S.; Sojic, A.; Jesuthasan, N.; Molinaro, S.; Bastiani, L.; Maggi, S.; Noale, M.; et al. Association between Smoking and SARS-CoV-2 Infection: Cross-sectional Study of the EPICOVID19 Internet-Based Survey. JMIR Public Health Surveill. 2021, 7, e27091. [Google Scholar] [CrossRef] [PubMed]
- Barchuk, A.; Skougarevskiy, D.; Titaev, K.; Shirokov, D.; Raskina, Y.; Novkunkskaya, A.; Talantov, P.; Isaev, A.; Pomerantseva, E.; Zhikrivetskaya, S.; et al. Seroprevalence of SARS-CoV-2 antibodies in Saint Petersburg, Russia: A population-based study. Sci. Rep. 2021, 11, 12930. [Google Scholar] [CrossRef] [PubMed]
- Vial, P.; González, C.; Icaza, G.; Ramirez-Santana, M.; Quezada-Gaete, R.; Núñez-Franz, L.; Apablaza, M.; Vial, C.; Rubilar, P.; Correa, J.; et al. Seroprevalence, spatial distribution, and social determinants of SARS-CoV-2 in three urban centers of Chile. BMC Infect. Dis. 2022, 22, 99. [Google Scholar] [CrossRef] [PubMed]
- Karachaliou, M.; Moncunill, G.; Espinosa, A.; Castaño-Vinyals, G.; Jiménez, A.; Vidal, M.; Santano, R.; Barrios, D.; Puyol, L.; Carreras, A.; et al. Infection induced SARS-CoV-2 seroprevalence and heterogeneity of antibody responses in a general population cohort study in Catalonia Spain. Sci. Rep. 2021, 11, 21571. [Google Scholar] [CrossRef]
- Weber, S.; Didelot, A.; Agrinier, N.; Peyrin-Biroulet, L.; Schvoerer, E.; Rabaud, C.; Jeulin, H. SARS-CoV-2 seroprevalence in healthcare workers and risk factors. Infect. Dis. Health 2022, 27, 203–210. [Google Scholar] [CrossRef]
- Hausfater, P.; Boutolleau, D.; Lacombe, K.; Beurton, A.; Dumont, M.; Constantin, J.-M.; Ghosn, J.; Combes, A.; Cury, N.; Guedj, R.; et al. Cumulative incidence of SARS-CoV-2 infection and associated risk factors among frontline health care workers in Paris: The SEROCOV cohort study. Sci. Rep. 2022, 12, 7211. [Google Scholar] [CrossRef]
- Tomaselli, V.; Ferrara, P.; Cantone, G.G.; Romeo, A.C.; Rust, S.; Saitta, D.; Caraci, F.; Romano, C.; Thangaraju, M.; Zuccarello, P.; et al. The effect of laboratory-verified smoking on SARS-CoV-2 infection: Results from the Troina sero-epidemiological survey. Intern. Emerg. Med. 2022, 17, 1617–1630. [Google Scholar] [CrossRef]
- Gornyk, D.; Harries, M.; Glöckner, S.; Strengert, M.; Kerrinnes, T.; Heise, J.-K.; Maaß, H.; Ortmann, J.; Kessel, B.; Kemmling, Y.; et al. SARS-CoV-2 seroprevalence in Germany. Dtsch. Arztebl. Int. 2021, 118, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Radon, K.; Bakuli, A.; Pütz, P.; Le Gleut, R.; Noller, J.M.G.; Olbrich, L.; Saathoff, E.; Garí, M.; Schälte, Y.; Frahnow, T.; et al. From first to second wave: Follow-up of the prospective COVID-19 cohort (KoCo19) in Munich (Germany). BMC Infect. Dis. 2021, 21, 925. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Peterhoff, D.; Beileke, S.; Günther, F.; Berr, M.; Einhauser, S.; Schütz, A.; Niller, H.H.; Steininger, P.; Knöll, A.; et al. Estimates and Determinants of SARS-CoV-2 Seroprevalence and Infection Fatality Ratio Using Latent Class Analysis: The Population-Based Tirschenreuth Study in the Hardest-Hit German County in Spring 2020. Viruses 2021, 13, 1118. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Wang, J.-H.; Hsueh, P.-R. Population-based seroprevalence surveys of anti-SARS-CoV-2 antibody: An up-to-date review. Int. J. Infect. Dis. 2020, 101, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Simons, D.; Shahab, L.; Brown, J.; Perski, O. The association of smoking status with SARS-CoV-2 infection, hospitalization and mortality from COVID-19, a living rapid evidence review with Bayesian meta-analyses (version 7). Addiction 2021, 116, 1319–1368. [Google Scholar] [CrossRef]
- Paleiron, N.; Mayet, A.; Marbac, V.; Perisse, A.; Barazzutti, H.; Brocq, F.-X.; Janvier, F.; Dautzenberg, B.; Bylicki, O. Impact of Tobacco Smoking on the Risk of COVID-19: A Large Scale Retrospective Cohort Study. Nicotine Tob. Res. 2021, 23, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Liang, C.-L.; Liu, H.; Zeng, Y.-Q.; Hou, S.; Huang, S.; Lai, X.; Dai, Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017, 8, 268–284. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Shabani, Z.; Hassanabadi, M.; Rezayati, M.; Nemati, M.; Sayadi, A.; Sheikhi, A.; Vazirinejad, R.; Immunology, O.; Diseases, O.I.; et al. Lower immunity to tetanus in cigarette smoker subjects. J. Occup. Health Epidemiol. 2012, 1, 124–131. [Google Scholar] [CrossRef]
- Petráš, M.; Oleár, V. Predictors of the immune response to booster immunisation against tetanus in Czech healthy adults. Epidemiol. Infect. 2018, 146, 2079–2085. [Google Scholar] [CrossRef]
- Ferrara, P.; Gianfredi, V.; Tomaselli, V.; Polosa, R. The Effect of Smoking on Humoral Response to COVID-19 Vaccines: A Systematic Review of Epidemiological Studies. Vaccines 2022, 10, 303. [Google Scholar] [CrossRef]
- Einhauser, S.; Peterhoff, D.; Beileke, S.; Günther, F.; Niller, H.-H.; Steininger, P.; Knöll, A.; Korn, K.; Berr, M.; Schütz, A.; et al. Time Trend in SARS-CoV-2 Seropositivity, Surveillance Detection- and Infection Fatality Ratio until Spring 2021 in the Tirschenreuth County—Results from a Population-Based Longitudinal Study in Germany. Viruses 2022, 14, 1168. [Google Scholar] [CrossRef] [PubMed]
- Kellam, P.; Barclay, W. The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J. Gen. Virol. 2020, 101, 791–797. [Google Scholar] [CrossRef] [PubMed]
- an der Heiden, M.; Hamouda, O. Schätzung der aktuellen Entwicklung der SARS-CoV-2-Epidemie in Deutschland—Nowcasting. Epidemiol. Bull. 2020, 17, 10–18. [Google Scholar]
- Einhauser, S.; Peterhoff, D.; Niller, H.H.; Beileke, S.; Günther, F.; Steininger, P.; Burkhardt, R.; Heid, I.M.; Pfahlberg, A.B.; Überla, K.; et al. Spectrum Bias and Individual Strengths of SARS-CoV-2 Serological Tests—A Population-Based Evaluation. Diagnostics 2021, 11, 1843. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models; Chapman and Hall/CRC: London, UK, 2006. [Google Scholar]
- Peterhoff, D.; Einhauser, S.; Beileke, S.; Niller, H.-H.; Günther, F.; Schachtner, M.; Asbach, B.; Steininger, P.; Tenbusch, M.; Peter, A.S.; et al. Comparative Immunogenicity of COVID-19 Vaccines in a Population-Based Cohort Study with SARS-CoV-2-Infected and Uninfected Participants. Vaccines 2022, 10, 324. [Google Scholar] [CrossRef]
- Kupfer, D.M.; White, V.L.; Jenkins, M.C.; Burian, D. Examining smoking-induced differential gene expression changes in buccal mucosa. BMC Med. Genom. 2010, 3, 24. [Google Scholar] [CrossRef]
- Caruso, M.; Distefano, A.; Emma, R.; Rosa, M.D.; Carota, G.; Rust, S.; Polosa, R.; Zuccarello, P.; Ferrante, M.; Raciti, G.; et al. Role of Cigarette Smoke on Angiotensin-Converting Enzyme-2 Protein Membrane Expression in Bronchial Epithelial Cells Using an Air-Liquid Interface Model. Front. Pharmacol. 2021, 12, 652102. [Google Scholar] [CrossRef]
- COVID-19 Host Genetics Initiative. Mapping the human genetic architecture of COVID-19. Nature 2021, 600, 472–477. [Google Scholar] [CrossRef]
- Thibord, F.; Chan, M.V.; Chen, M.-H.; Johnson, A.D. A year of COVID-19 GWAS results from the GRASP portal reveals potential genetic risk factors. Hum. Genet. Genom. Adv. 2022, 3, 100095. [Google Scholar] [CrossRef]
- Merikallio, H.; Kaarteenaho, R.; Lindén, S.; Padra, M.; Karimi, R.; Li, C.-X.; Lappi-Blanco, E.; Wheelock, .M.; Sköld, M.C. Smoking-associated increase in mucins 1 and 4 in human airways. Respir. Res. 2020, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.P.; Zhao, J.; Harper, R. Cigarette Smoke Induces MUC5AC Protein Expression through the Activation of Sp1. J. Biol. Chem. 2012, 287, 27948–27958. [Google Scholar] [CrossRef] [PubMed]
- Ditz, B.; Mudiyanselage, S.R.; Van Nijnatten, J.; Brandsma, C.-A.; Hiemstra, P.; Timens, W.; Kerstjens, H.; Berge, M.V.D.; Faiz, A. Bronchial airway expression of mucin-related, ENaC and chloride channel genes in COPD and non-COPD smokers compared to former and never smokers. Eur. Respir. J. 2020, 56, 908. [Google Scholar] [CrossRef]
- Plante, J.A.; Plante, K.S.; Gralinski, L.E.; Beall, A.; Ferris, M.T.; Bottomly, D.; Green, R.; McWeeney, S.K.; Heise, M.T.; Baric, R.S.; et al. Mucin 4 Protects Female Mice from Coronavirus Pathogenesis. bioRxiv 2020. [Google Scholar] [CrossRef]
- Chatterjee, M.; van Putten, J.P.M.; Strijbis, K. Defensive Properties of Mucin Glycoproteins during Respiratory Infections—Relevance for SARS-CoV-2. mBio 2020, 11, e02374-20. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Choi, A.H.; Deng, Z.; Yang, Y.; Zhao, J.; Wang, Y.; Hardwidge, P.R.; Zhu, G. Cell membrane-anchored MUC4 promotes tumorigenicity in epithelial carcinomas. Oncotarget 2017, 8, 14147–14157. [Google Scholar] [CrossRef]
- He, Y.-F.; Zhang, M.-Y.; Wu, X.; Sun, X.-J.; Xu, T.; He, Q.-Z.; Di, W. High MUC2 Expression in Ovarian Cancer Is Inversely Associated with the M1/M2 Ratio of Tumor-Associated Macrophages and Patient Survival Time. PLoS ONE 2013, 8, e79769. [Google Scholar] [CrossRef]


| BL [N = 4181] | FU1 [N = 3513] | FU2 [N = 3374] | |
|---|---|---|---|
| Age, sex (BL) | [N = 4181] | [N = 3513] | [N = 3374] |
| Median age (IQR) [yrs] | 52.0 (35.0–64.0) | 53.0 (37.0–64.0) | 53.0 (37.0–64.0) |
| Min, max age [yrs] | 14.0, 102.0 | 14.0, 102.0 | 14.0, 102.0 |
| Age 14–19 [yrs]: % (n) | 5.4 (225) | 5.0 (176) | 5.2 (177) |
| Age 20–49 [yrs]: % (n) | 40.8 (1707) | 38.3 (1345) | 38.1 (1284) |
| Age 50–69 [yrs]: % (n) | 38.8 (1624) | 41.2 (1449) | 41.2 (1389) |
| Age 70+ [yrs]: % (n) | 14.9 (625) | 15.5 (543) | 15.5 (524) |
| Women: % (n) | 51.6 (2158) | 53.0 (1861) | 53.7 (1813) |
| Chronic diseases | [N = 4081] | [N = 3435] | [N = 3300] |
| Autoimmune: % (n) | 7.1 (289) | 7.3 (250) | 7.4 (243) |
| Cancer: % (n) | 4.9 (202) | 5.2 (178) | 5.0 (164) |
| Type 2 diabetes: % (n) | 7.6 (312) | 7.5 (259) | 7.4 (245) |
| Cardiovascular: % (n) | 9.9 (402) | 9.6 (331) | 9.5 (314) |
| None of these: % (n) | 75.8 (3093) | 75.6 (2596) | 76.0 (2507) |
| Education | [N = 4085] | [N = 3433] | [N = 3301] |
| Median (IQR) [yrs] | 11.0 (10.0–14.0) | 11.0 (10.0–13.0) | 11.0 (10.0–14.0) |
| ≥13 yrs: % (n) | 30.0 (1225) | 29.5 (1013) | 29.8 (985) |
| Occupation (20–69 years) | [N = 3303] | [N = 2773] | [N = 2652] |
| Curr. working (BL): % (n) | 74.0 (2444) | 73.8 (2046) | 74.1 (1965) |
| Medical: % (n) | 8.0 (263) | 8.1 (224) | 8.1 (216) |
| Education: % (n) | 3.7 (121) | 3.6 (101) | 3.6 (95) |
| Grocery: % (n) | 3.1 (104) | 3.2 (90) | 3.3 (87) |
| Smoking | [N = 4157] | [N = 3493] | [N = 3356] |
| Never smoking: % (n) | 54.9 (2282) | 56.7 (1981) | 57.3 (1923) |
| Ex-smoker: % (n) | 24.7 (1025) | 24.6 (860) | 24.6 (827) |
| Current smoker: % (n) | 20.4 (850) | 18.7 (652) | 18.1 (606) |
| Other lifestyle factors | |||
| Alc. drinks, daily: median (IQR) | 0.2 (0.0–0.6) [N = 4049] | 0.2 (0.0–0.6) [N = 3412] | 0.2 (0.0–0.6) [N = 3280] |
| >2 alc. drinks, daily: % (n) | 6.5 (262) [N = 4049] | 6.3 (216) [N = 3412] | 6.2 (204) [N = 3280] |
| BMI: Median (IQR) | 26.6 (23.7–30.4) [N = 4134] | 26.6 (23.7–30.3) [N = 3474] | 26.6 (23.7–30.4) [N = 3339] |
| N-seropositive (BL) | |||
| % (n) | 8.9 (374) [N = 4181] | 10.0 (351) [N = 3513] | 10.3 (349) [N = 3374] |
| % (n), age 14–19 yrs | 10.7 (24) [N = 225] | 13.6 (24) [N = 176] | 13.0 (23) [N = 177] |
| % (n), age 20–49 yrs | 8.6 (146) [N = 1707] | 10.0 (135) [N = 1345] | 10.4 (133) [N = 1284] |
| % (n), age 50–69 yrs | 9.3 (151) [N = 1624] | 9.8 (142) [N = 1449] | 10.3 (143) [N = 1389] |
| % (n), age 70+ yrs | 8.5 (53) [N = 625] | 9.2 (50) [N = 543] | 9.5 (50) [N = 524] |
| % (n), never-smoker | 9.9 (226) [N = 2282] | 10.8 (213) [N = 1981] | 11.0 (212) [N = 1923] |
| % (n), ex-smoker | 10.3 (106) [N = 1025] | 11.4 (98) [N = 860] | 11.7 (97) [N = 827] |
| % (n) current smoker | 4.9 (42) [N = 850] | 6.1 (40) [N = 652] | 6.6 (40) [N = 606] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Günther, F.; Einhauser, S.; Peterhoff, D.; Wiegrebe, S.; Niller, H.H.; Beileke, S.; Steininger, P.; Burkhardt, R.; Küchenhoff, H.; Gefeller, O.; et al. Higher Infection Risk among Health Care Workers and Lower Risk among Smokers Persistent across SARS-CoV-2 Waves—Longitudinal Results from the Population-Based TiKoCo Seroprevalence Study. Int. J. Environ. Res. Public Health 2022, 19, 16996. https://doi.org/10.3390/ijerph192416996
Günther F, Einhauser S, Peterhoff D, Wiegrebe S, Niller HH, Beileke S, Steininger P, Burkhardt R, Küchenhoff H, Gefeller O, et al. Higher Infection Risk among Health Care Workers and Lower Risk among Smokers Persistent across SARS-CoV-2 Waves—Longitudinal Results from the Population-Based TiKoCo Seroprevalence Study. International Journal of Environmental Research and Public Health. 2022; 19(24):16996. https://doi.org/10.3390/ijerph192416996
Chicago/Turabian StyleGünther, Felix, Sebastian Einhauser, David Peterhoff, Simon Wiegrebe, Hans Helmut Niller, Stephanie Beileke, Philipp Steininger, Ralph Burkhardt, Helmut Küchenhoff, Olaf Gefeller, and et al. 2022. "Higher Infection Risk among Health Care Workers and Lower Risk among Smokers Persistent across SARS-CoV-2 Waves—Longitudinal Results from the Population-Based TiKoCo Seroprevalence Study" International Journal of Environmental Research and Public Health 19, no. 24: 16996. https://doi.org/10.3390/ijerph192416996
APA StyleGünther, F., Einhauser, S., Peterhoff, D., Wiegrebe, S., Niller, H. H., Beileke, S., Steininger, P., Burkhardt, R., Küchenhoff, H., Gefeller, O., Überla, K., Heid, I. M., & Wagner, R. (2022). Higher Infection Risk among Health Care Workers and Lower Risk among Smokers Persistent across SARS-CoV-2 Waves—Longitudinal Results from the Population-Based TiKoCo Seroprevalence Study. International Journal of Environmental Research and Public Health, 19(24), 16996. https://doi.org/10.3390/ijerph192416996






