Study of the Phytoextraction and Phytodegradation of Sulfamethoxazole and Trimethoprim from Water by Limnobium laevigatum
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards, Chemicals, and Materials
2.2. Characteristics and Preparation of Limnobium Laevigatum Cultivation in Hydroponic Conditions
2.3. Sampling and Preparation of the Samples
2.3.1. SMX and TRI Extraction from Water Samples
2.3.2. SMX and TRI Extraction from Plant Samples
2.4. Instrumentation and Analytical Conditions
2.5. Determination of SMX and TRI and Their TPs Using LC-MS/MS
2.6. Method Validation
3. Results and Discussion
3.1. Phytoremediation Potential of L. laevigatum
3.1.1. Determination of SMX and TRI in Water
3.1.2. Bioaccumulation Study of SMX and TRI in L. laevigatum Tissues
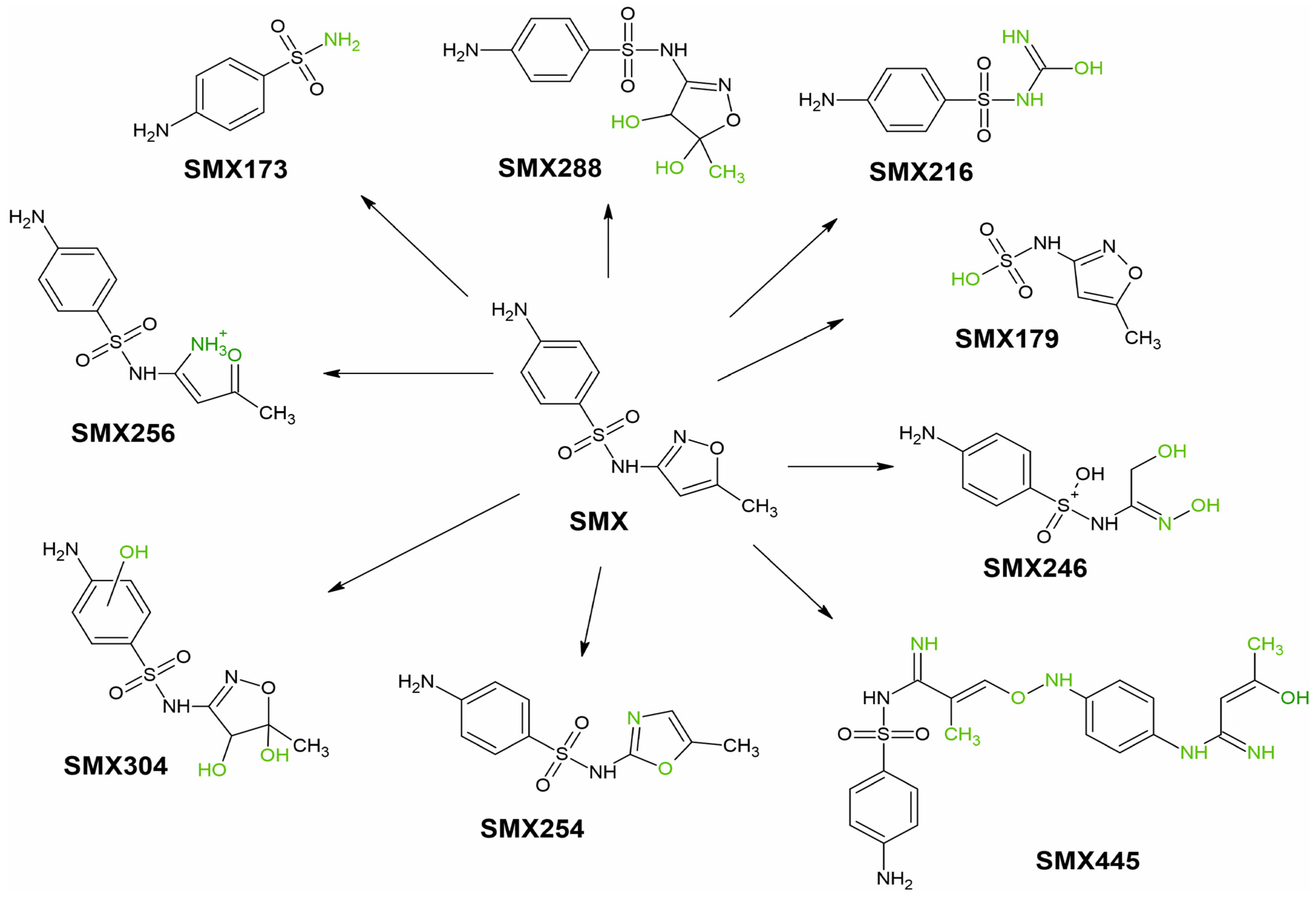
3.2. Identification of SMX and TRI TPs in Water and Plant Tissues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Xu, J.; Zhong, Z.; Guo, C.; Li, L.; He, Y.; Fan, W.; Chen, Y. Degradation of Sulfonamides Antibiotics in Lake Water and Sediment. Environ. Sci. Pollut. Res. 2013, 20, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Kokoszka, K.; Wilk, J.; Felis, E.; Bajkacz, S. Application of UHPLC-MS/MS Method to Study Occurrence and Fate of Sulfonamide Antibiotics and Their Transformation Products in Surface Water in Highly Urbanized Areas. Chemosphere 2021, 283, 131189. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Liu, Z.; Yin, H.; Dang, Z.; Wu, P.; Zhu, N.; Lin, Z. Trace Determination of Sulfonamide Antibiotics and Their Acetylated Metabolites via SPE-LC-MS/MS in Wastewater and Insights from Their Occurrence in a Municipal Wastewater Treatment Plant. Sci. Total Environ. 2019, 653, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Botitsi, E.; Frosyni, C.; Tsipi, D. Determination of Pharmaceuticals from Different Therapeutic Classes in Wastewaters by Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2007, 387, 1317–1327. [Google Scholar] [CrossRef]
- Gaffney, V.d.J.; Cardoso, V.V.; Benoliel, M.J.; Almeida, C.M.M. Chlorination and Oxidation of Sulfonamides by Free Chlorine: Identification and Behaviour of Reaction Products by UPLC-MS/MS. J. Environ. Manag. 2016, 166, 466–477. [Google Scholar] [CrossRef]
- Gómez-Ramos, M.d.M.; Mezcua, M.; Agüera, A.; Fernández-Alba, A.R.; Gonzalo, S.; Rodríguez, A.; Rosal, R. Chemical and Toxicological Evolution of the Antibiotic Sulfamethoxazole under Ozone Treatment in Water Solution. J. Hazard. Mater. 2011, 192, 18–25. [Google Scholar] [CrossRef]
- Llorca, M.; Lucas, D.; Ferrando-Climent, L.; Badia-Fabregat, M.; Cruz-Morató, C.; Barceló, D.; Rodríguez-Mozaz, S. Suspect Screening of Emerging Pollutants and Their Major Transformation Products in Wastewaters Treated with Fungi by Liquid Chromatography Coupled to a High Resolution Mass Spectrometry. J. Chromatogr. A 2016, 1439, 124–136. [Google Scholar] [CrossRef]
- Kennedy Neth, N.L.; Carlin, C.M.; Keen, O.S. Emerging Investigator Series: Transformation of Common Antibiotics during Water Disinfection with Chlorine and Formation of Antibacterially Active Products. Environ. Sci. 2019, 5, 1222–1233. [Google Scholar] [CrossRef]
- PHARMINDEX. Available online: https://pharmindex.pl/listalekow# (accessed on 4 July 2022).
- Kokoszka, K.; Zieliński, W.; Korzeniewska, E.; Felis, E.; Harnisz, M.; Bajkacz, S. Suspect Screening of Antimicrobial Agents Transformation Products in Environmental Samples Development of LC-QTrap Method Running in Pseudo MRM Transitions. Sci. Total Environ. 2022, 808, 152114. [Google Scholar] [CrossRef]
- Girdhar, M.; Sharma, N.R.; Rehman, H.; Kumar, A.; Mohan, A. Comparative Assessment for Hyperaccumulatory and Phytoremediation Capability of Three Wild Weeds. 3 Biotech 2014, 4, 579–589. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of Heavy Metals: Mechanisms, Methods and Enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Carvalho, P.N.; Basto, M.C.P.; Almeida, C.M.R.; Brix, H. A Review of Plant–Pharmaceutical Interactions: From Uptake and Effects in Crop Plants to Phytoremediation in Constructed Wetlands. Environ. Sci. Pollut. Res. 2014, 21, 11729–11763. [Google Scholar] [CrossRef] [PubMed]
- Kotyza, J.; Soudek, P.; Kafka, Z.; Vaněk, T. Phytoremediation of Pharmaceuticals-Preliminary Study. Int. J. Phytoremediat. 2010, 12, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Jeevanantham, S.; Saravanan, A.; Hemavathy, R.V.; Kumar, P.S.; Yaashikaa, P.R.; Yuvaraj, D. Removal of Toxic Pollutants from Water Environment by Phytoremediation: A Survey on Application and Future Prospects. Environ. Technol. Innov. 2019, 13, 264–276. [Google Scholar] [CrossRef]
- Wei, Z.; van Le, Q.; Peng, W.; Yang, Y.; Yang, H.; Gu, H.; Lam, S.S.; Sonne, C. A Review on Phytoremediation of Contaminants in Air, Water and Soil. J. Hazard. Mater. 2021, 403, 123658. [Google Scholar] [CrossRef]
- Mustafa, H.M.; Hayder, G. Recent Studies on Applications of Aquatic Weed Plants in Phytoremediation of Wastewater: A Review Article. Ain. Shams Eng. J. 2021, 12, 355–365. [Google Scholar] [CrossRef]
- Ng, Y.S.; Chan, D.J.C. Wastewater Phytoremediation by Salvinia Molesta. J. Water Process Eng. 2017, 15, 107–115. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Jayawardana, C.K. Potential of Aquatic Macrophytes Eichhornia Crassopes, Pistia Stratiotes and Salvinia Molesta in Phytoremediation of Textile Wastewater. J. Water Secur. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- European Comission List of Invasive Alien Species of Union Concern. Available online: https://ec.europa.eu/environment/nature/invasivealien/list/index_en.htm (accessed on 5 July 2022).
- Arán, D.S.; Harguinteguy, C.A.; Fernandez-Cirelli, A.; Pignata, M.L. Phytoextraction of Pb, Cr, Ni, and Zn Using the Aquatic Plant Limnobium Laevigatum and Its Potential Use in the Treatment of Wastewater. Environ. Sci. Pollut. Res. 2017, 24, 18295–18308. [Google Scholar] [CrossRef]
- Fernández San Juan, M.R.; Albornoz, C.B.; Larsen, K.; Najle, R. Bioaccumulation of Heavy Metals in Limnobium Laevigatum and Ludwigia Peploides: Their Phytoremediation Potential in Water Contaminated with Heavy Metals. Environ. Earth Sci. 2018, 77, 404. [Google Scholar] [CrossRef]
- Polińska, W.; Kotowska, U.; Kiejza, D.; Karpińska, J. Insights into the Use of Phytoremediation Processes for the Removal of Organic Micropollutants from Water and Wastewater; a Review. Water 2021, 13, 2065. [Google Scholar] [CrossRef]
- Stando, K.; Korzeniewska, E.; Felis, E.; Harnisz, M.; Bajkacz, S. Uptake of Pharmaceutical Pollutants and Their Metabolites from Soil Fertilized with Manure to Parsley Tissues. Molecules 2022, 27, 4378. [Google Scholar] [CrossRef] [PubMed]
- University of Hertfordshire. VSDB: Veterinary Substances DataBase. Available online: http://sitem.herts.ac.uk/aeru/vsdb/Reports/1762.htm (accessed on 1 November 2022).
- Yuan, R.; Zhu, Y.; Zhou, B.; Hu, J. Photocatalytic Oxidation of Sulfamethoxazole in the Presence of TiO2: Effect of Matrix in Aqueous Solution on Decomposition Mechanisms. Chem. Eng. J. 2019, 359, 1527–1536. [Google Scholar] [CrossRef]
- Długosz, M.; Zmudzki, P.; Kwiecień, A.; Szczubiałka, K.; Krzek, J.; Nowakowska, M. Photocatalytic Degradation of Sulfamethoxazole in Aqueous Solution Using a Floating TiO2-Expanded Perlite Photocatalyst. J. Hazard. Mater. 2015, 298, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Białk-Bielińska, A.; Stolte, S.; Matzke, M.; Fabiańska, A.; Maszkowska, J.; Kołodziejska, M.; Liberek, B.; Stepnowski, P.; Kumirska, J. Hydrolysis of Sulphonamides in Aqueous Solutions. J. Hazard. Mater. 2012, 221–222, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Malchi, T.; Maor, Y.; Tadmor, G.; Shenker, M.; Chefetz, B. Irrigation of Root Vegetables with Treated Wastewater: Evaluating Uptake of Pharmaceuticals and the Associated Human Health Risks. Environ. Sci. Technol. 2014, 48, 9325–9333. [Google Scholar] [CrossRef]
- Bergh, J.J.; Breytenbach, J.C.; Wessels, P.L. Degradation of Trimethoprim. J. Pharm. Sci. 1989, 78, 348–350. [Google Scholar] [CrossRef]
- Sirtori, C.; Agüera, A.; Gernjak, W.; Malato, S. Effect of Water-Matrix Composition on Trimethoprim Solar Photodegradation Kinetics and Pathways. Water Res. 2010, 44, 2735–2744. [Google Scholar] [CrossRef]
- Chen, H.R.; Rairat, T.; Loh, S.H.; Wu, Y.C.; Vickroy, T.W.; Chou, C.C. Assessment of Veterinary Drugs in Plants Using Pharmacokinetic Approaches: The Absorption, Distribution and Elimination of Tetracycline and Sulfamethoxazole in Ephemeral Vegetables. PLoS ONE 2017, 12, e0183087. [Google Scholar] [CrossRef]
- Ryan, C.C.; Tan, D.T.; Arnold, W.A. Direct and Indirect Photolysis of Sulfamethoxazole and Trimethoprim in Wastewater Treatment Plant Effluent. Water Res. 2011, 45, 1280–1286. [Google Scholar] [CrossRef]
- Herklotz, P.A.; Gurung, P.; vanden Heuvel, B.; Kinney, C.A. Uptake of Human Pharmaceuticals by Plants Grown under Hydroponic Conditions. Chemosphere 2010, 78, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.; Reinhold, D. Metabolism of Sulfamethoxazole by the Model Plant Arabidopsis Thaliana. Environ. Sci. Technol. 2019, 53, 4901–4911. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ying, G.G.; Tao, R.; Zhao, J.L.; Yang, J.F.; Zhao, L.F. Effects of Six Selected Antibiotics on Plant Growth and Soil Microbial and Enzymatic Activities. Environ. Pollut. 2009, 157, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Mikes, O.; Trapp, S. Acute Toxicity of the Dissociating Veterinary Antibiotics Trimethoprim to Willow Trees at Varying PH. Bull. Environ. Contam. Toxicol. 2010, 85, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Borecka, M.; Białk-Bielińska, A.; Haliński, Ł.P.; Pazdro, K.; Stepnowski, P.; Stolte, S. The Influence of Salinity on the Toxicity of Selected Sulfonamides and Trimethoprim towards the Green Algae Chlorella Vulgaris. J. Hazard. Mater. 2016, 308, 179–186. [Google Scholar] [CrossRef]
- Alharbi, S.K.; Kang, J.; Nghiem, L.D.; van de Merwe, J.P.; Leusch, F.D.L.; Price, W.E. Photolysis and UV/H2O2 of Diclofenac, Sulfamethoxazole, Carbamazepine, and Trimethoprim: Identification of Their Major Degradation Products by ESI–LC–MS and Assessment of the Toxicity of Reaction Mixtures. Process Saf. Environ. Prot. 2017, 112, 222–234. [Google Scholar] [CrossRef]
- Ji, Y.; Xie, W.; Fan, Y.; Shi, Y.; Kong, D.; Lu, J. Degradation of Trimethoprim by Thermo-Activated Persulfate Oxidation: Reaction Kinetics and Transformation Mechanisms. Chem. Eng. J. 2016, 286, 16–24. [Google Scholar] [CrossRef]
- Lecours, M.-A.; Eysseric, E.; Yargeau, V.; Lessard, J.; Brisard, G.; Segura, P. Electrochemistry-High Resolution Mass Spectrometry to Study Oxidation Products of Trimethoprim. Environments 2018, 5, 18. [Google Scholar] [CrossRef]
- Malchi, T.; Eyal, S.; Czosnek, H.; Shenker, M.; Chefetz, B. Plant Pharmacology: Insights into in-Planta Kinetic and Dynamic Processes of Xenobiotics. Crit. Rev. Environ. Sci. Technol. 2021, 52, 3525–3546. [Google Scholar] [CrossRef]
- Psutka, J.M.; Dion-Fortier, A.; Dieckmann, T.; Campbell, J.L.; Segura, P.A.; Hopkins, W.S. Identifying Fenton-Reacted Trimethoprim Transformation Products Using Differential Mobility Spectrometry. Anal. Chem. 2018, 90, 5352–5357. [Google Scholar] [CrossRef]
- Jewell, K.S.; Castronovo, S.; Wick, A.; Falås, P.; Joss, A.; Ternes, T.A. New Insights into the Transformation of Trimethoprim during Biological Wastewater Treatment. Water Res. 2016, 88, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Tadić, Đ.; Gramblicka, M.; Mistrik, R.; Bayona, J.M. Systematic Identification of Trimethoprim Metabolites in Lettuce. Anal. Bioanal. Chem. 2022, 414, 3121–3135. [Google Scholar] [CrossRef] [PubMed]
- Kiki, C.; Rashid, A.; Wang, Y.; Li, Y.; Zeng, Q.; Yu, C.P.; Sun, Q. Dissipation of Antibiotics by Microalgae: Kinetics, Identification of Transformation Products and Pathways. J. Hazard. Mater. 2020, 387, 121985. [Google Scholar] [CrossRef] [PubMed]
- Anquandah, G.A.K.; Sharma, V.K.; Knight, D.A.; Batchu, S.R.; Gardinali, P.R. Oxidation of Trimethoprim by Ferrate(VI): Kinetics, Products, and Antibacterial Activity. Environ. Sci. Technol. 2011, 45, 10575–10581. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J. Trimethoprim Degradation by Fenton and Fe(II)-Activated Persulfate Processes. Chemosphere 2018, 191, 97–105. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, Y.; Huang, C.H.; Li, N.; Liu, H.; Zhao, L.; Sun, P. UV/H2O2 and UV/PDS Treatment of Trimethoprim and Sulfamethoxazole in Synthetic Human Urine: Transformation Products and Toxicity. Environ. Sci. Technol. 2016, 50, 2573–2583. [Google Scholar] [CrossRef]
- Xiong, Q.; Liu, Y.S.; Hu, L.X.; Shi, Z.Q.; Cai, W.W.; He, L.Y.; Ying, G.G. Co-Metabolism of Sulfamethoxazole by a Freshwater Microalga Chlorella Pyrenoidosa. Water Res. 2020, 175, 115656. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, Z.; Xu, Y.; Tian, J.; Qi, H.; Lin, W.; Cui, F. Oxidation of Sulfamethoxazole (SMX) by Chlorine, Ozone and Permanganate-A Comparative Study. J. Hazard. Mater. 2014, 274, 258–269. [Google Scholar] [CrossRef]
- Zheng, L.; Jin, H.; Yu, M.; Zhongwei, Q.; Zhang, L.; Shikun, C.; Li, Z. Degradation of Sulfamethoxazole by Electrochemically Activated Persulfate Using Iron Anode. Int. J. Chem. React. Eng. 2019, 17, 1–14. [Google Scholar] [CrossRef]
- Jia, Y.; Khanal, S.K.; Zhang, H.; Chen, G.H.; Lu, H. Sulfamethoxazole Degradation in Anaerobic Sulfate-Reducing Bacteria Sludge System. Water. Res. 2017, 119, 12–20. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Microbial Degradation of Sulfamethoxazole in the Environment. Appl. Microbiol. Biotechnol. 2018, 102, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, T.H.; Cha, S.M.; Yu, S. Degradation of Sulfamethoxazole by Ionizing Radiation: Identification and Characterization of Radiolytic Products. Chem. Eng. J. 2017, 313, 556–566. [Google Scholar] [CrossRef]
- Trovó, A.G.; Nogueira, R.F.P.; Agüera, A.; Fernandez-Alba, A.R.; Sirtori, C.; Malato, S. Degradation of Sulfamethoxazole in Water by Solar Photo-Fenton. Chemical and Toxicological Evaluation. Water Res. 2009, 43, 3922–3931. [Google Scholar] [CrossRef] [PubMed]
- Trovó, A.G.; Nogueira, R.F.P.; Agüera, A.; Sirtori, C.; Fernández-Alba, A.R. Photodegradation of Sulfamethoxazole in Various Aqueous Media: Persistence, Toxicity and Photoproducts Assessment. Chemosphere 2009, 77, 1292–1298. [Google Scholar] [CrossRef]
- Petala, A.; Mantzavinos, D.; Frontistis, Z. Impact of Water Matrix on the Photocatalytic Removal of Pharmaceuticals by Visible Light Active Materials. Curr. Opin. Green Sustain. Chem. 2021, 28, 100445. [Google Scholar] [CrossRef]
- Mai, Z.; Wu, F.; Deng, N.; Vu, T.A. Photodegradation of Sulfamethoxazole in Water: Kinetics and Influences Factors. Res. J. Chem. Environ. 2010, 14, 5–10. [Google Scholar]
- Eichhorn, P.; Ferguson, P.L.; Pérez, S.; Aga, D.S. Application of Ion Trap-MS with H/D Exchange and QqTOF-MS in the Identification of Microbial Degradates of Trimethoprim in Nitrifying Activated Sludge. Anal. Chem. 2005, 77, 4176–4184. [Google Scholar] [CrossRef]


| Day | Factors | ||
|---|---|---|---|
| PWAMAv. Photolysis + Hydrolysis + Plant Sorption (RSD) [%] | WAMAv. Photolysis + Hydrolysis (RSD) [%] | PS Plant Sorption [%] | |
| 0 | 0.0 (7.3) | 0.0 (1.6) | 0.0 |
| 1 | 5.2 (0.9) | 0.7 (2.5) | 4.5 |
| 2 | 52.9 (7.6) | 33.5 (1.4) | 19.4 |
| 3 | 68.6 (5.4) | 40.9 (1.6) | 27.7 |
| 4 | 74.9 (7.1) | 50.7 (7.4) | 24.2 |
| 5 | 81.8 (3.9) | 53.0 (1.7) | 28.8 |
| 6 | 85.5 (8.7) | 61.8 (1.6) | 23.7 |
| 7 | 86.9 (6.7) | 65.7 (5.4) | 21.2 |
| 9 | 88.4 (2.5) | 74.4 (2.0) | 14.0 |
| 11 | 92.2 (6.7) | 80.8 (4.1) | 11.4 |
| 14 | 96.0 (8.0) | 83.8 (1.6) | 12.2 |
| Day | Factors | ||
|---|---|---|---|
| PWAMAv. Photolysis + Hydrolysis + Plant Sorption (RSD) [%] | WAMAv. Photolysis + Hydrolysis (RSD) [%] | PS Plant Sorption [%] | |
| 0 | 0.0 (6.9) | 0.0 (1.2) | 0.0 |
| 1 | 9.0 (5.0) | 1.6 (2.7) | 7.4 |
| 2 | 41.6 (3.6) | 5.1 (2.4) | 36.5 |
| 3 | 63.1 (6.2) | 7.5 (0.3) | 55.6 |
| 4 | 66.2 (8.8) | 13.5 (6.2) | 52.7 |
| 5 | 68.5 (5.0) | 14.2 (4.9) | 54.3 |
| 6 | 70.3 (9.7) | 18.8 (1.2) | 51.5 |
| 7 | 70.8 (6.2) | 19.4 (4.2) | 51.4 |
| 9 | 72.2 (2.7) | 24.1 (5.9) | 48.1 |
| 11 | 73.5 (8.5) | 33.6 (6.5) | 39.9 |
| 14 | 75.4 (7.0) | 37.4 (7.4) | 38.0 |
| Compound | Day | Average Concentration (SD) [ng g−1]FW | Average Concentration (SD) [ng g−1]DW | BAFDW | BAFFW |
|---|---|---|---|---|---|
| SMX | 7 | 4.4 (0.1) | 146.6 (3.2) | 0.147 | 0.004 |
| 14 | 1.3 (0.1) | 42.6 (0.5) | 0.043 | 0.001 | |
| TRI | 7 | 78.4 (2.3) | 2587.7 (113.3) | 2.588 | 0.078 |
| 14 | 72.0 (3.9) | 2368.9 (148.1) | 2.369 | 0.072 |
| Abbrev. | [M + H]+ (m/z) | Water | Plant | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 Day | 1 Day | 2 Day | 3 Day | 4 Day | 5 Day | 6 Day | 7 Day | 9 Day | 11 Day | 14 Day | 7 Day | 14 Day | |||||||||||||
| WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | ||||
| TRI171 | 171.0 | - | - | - | - | - | + | - | + | - | + | - | + | - | + | - | + | - | + | - | - | - | - | - | - |
| TRI325 | 325.0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | + | - | + | - | - | - | - | - |
| TRI291 | 291.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | + | - | + | - | - | - |
| TRI323 | 323.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + |
| TRI305 | 305.2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + |
| TRI307 | 307.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + |
| Abbrev. | [M + H]+ (m/z) | Water | Plant | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 Day | 1 Day | 2 Day | 3 Day | 4 Day | 5 Day | 6 Day | 7 Day | 9 Day | 11 Day | 14 Day | 7 Day | 14 Day | |||||||||||||
| WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | WAMs | PWAMs | ||||
| SMX173 | 173.0 | - | - | - | - | - | - | - | - | + | + | + | + | + | + | + | + | + | + | + | - | + | - | - | - |
| SMX256 | 256.1 | - | - | - | - | - | - | - | - | - | - | + | - | + | - | + | - | + | - | + | - | + | - | - | - |
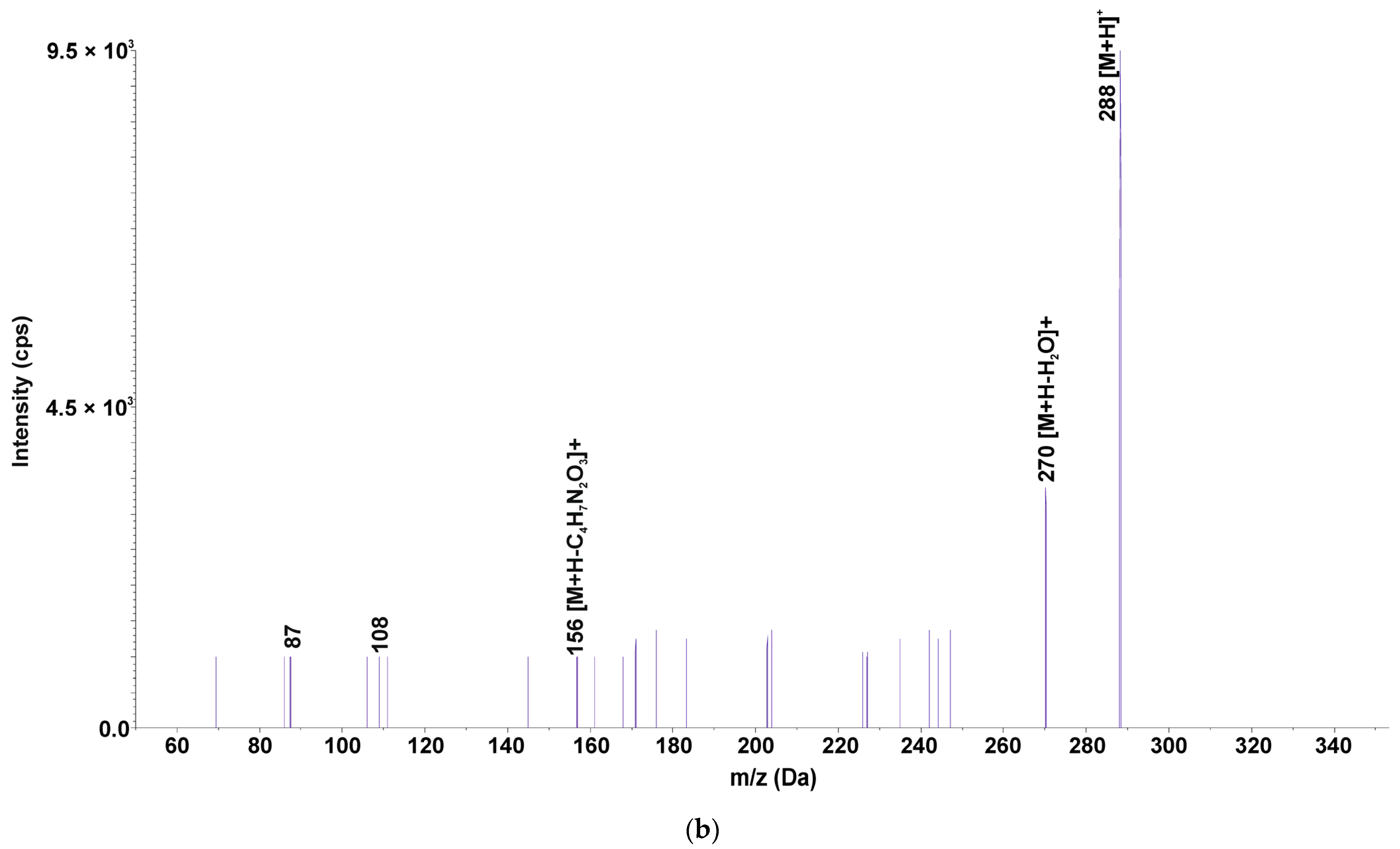
| SMX288 | 288.0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - | + | - | + | - | + | - | - |
| SMX304 | 304.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + |
| SMX254 | 254.0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | - |
| SMX445 | 445.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + |
| SMX216 | 216.0 | - | - | - | - | - | - | - | - | - | + | - | + | - | + | - | + | - | + | - | - | - | - | + | - |
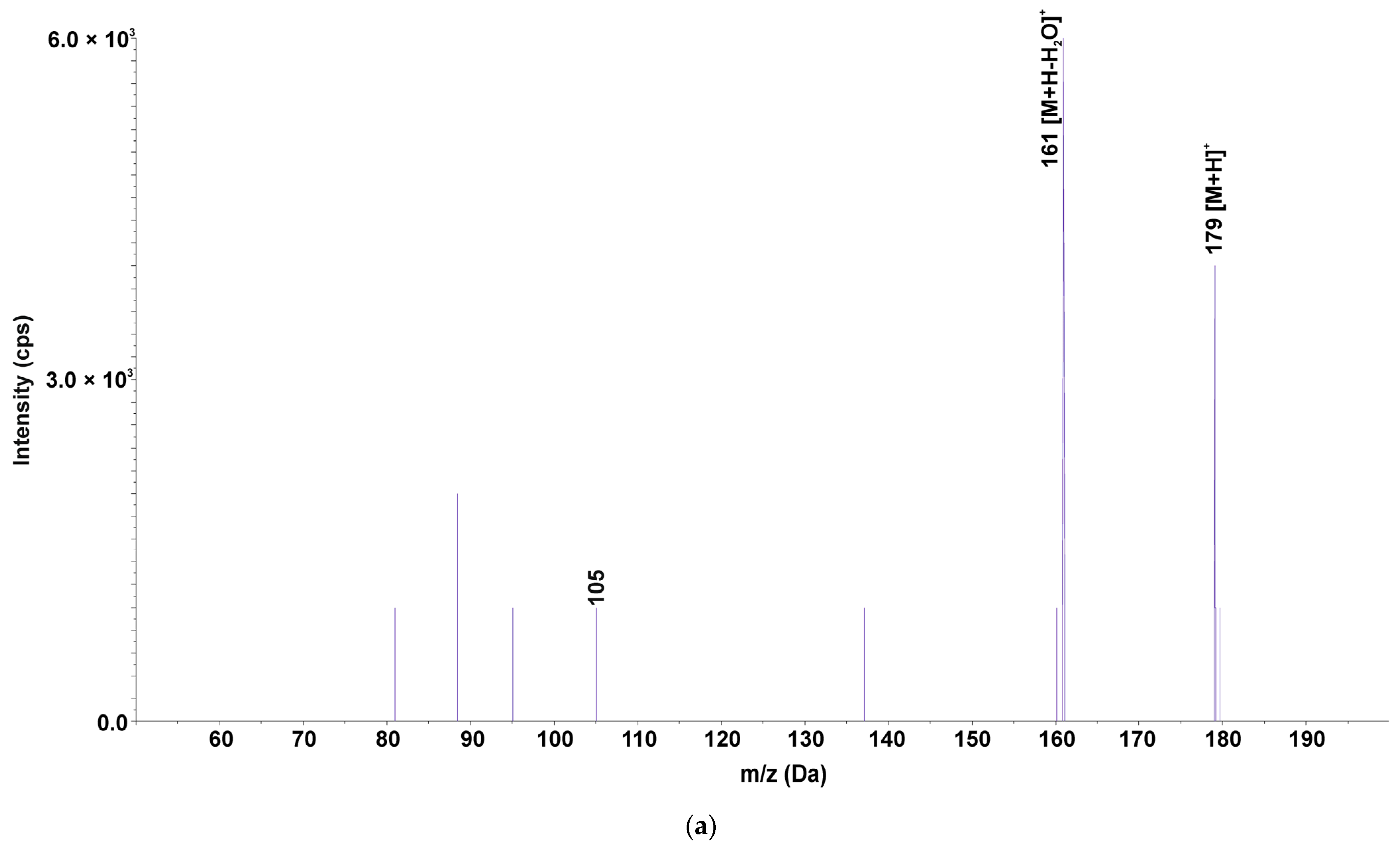
| SMX179 | 179.4 | - | - | - | - | - | - | - | - | - | - | + | - | + | + | + | + | + | + | - | + | - | + | - | + |
| SMX246 | 246.0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stando, K.; Czyż, A.; Gajda, M.; Felis, E.; Bajkacz, S. Study of the Phytoextraction and Phytodegradation of Sulfamethoxazole and Trimethoprim from Water by Limnobium laevigatum. Int. J. Environ. Res. Public Health 2022, 19, 16994. https://doi.org/10.3390/ijerph192416994
Stando K, Czyż A, Gajda M, Felis E, Bajkacz S. Study of the Phytoextraction and Phytodegradation of Sulfamethoxazole and Trimethoprim from Water by Limnobium laevigatum. International Journal of Environmental Research and Public Health. 2022; 19(24):16994. https://doi.org/10.3390/ijerph192416994
Chicago/Turabian StyleStando, Klaudia, Aleksandra Czyż, Magdalena Gajda, Ewa Felis, and Sylwia Bajkacz. 2022. "Study of the Phytoextraction and Phytodegradation of Sulfamethoxazole and Trimethoprim from Water by Limnobium laevigatum" International Journal of Environmental Research and Public Health 19, no. 24: 16994. https://doi.org/10.3390/ijerph192416994
APA StyleStando, K., Czyż, A., Gajda, M., Felis, E., & Bajkacz, S. (2022). Study of the Phytoextraction and Phytodegradation of Sulfamethoxazole and Trimethoprim from Water by Limnobium laevigatum. International Journal of Environmental Research and Public Health, 19(24), 16994. https://doi.org/10.3390/ijerph192416994





