Preparation and Performance of Carbon-Based Ce-Mn Catalysts for Efficient Degradation of Acetone at Low Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalysts Preparation
2.3. Catalysts Characterization
2.4. Catalytic Experiments
3. Results and Discussion
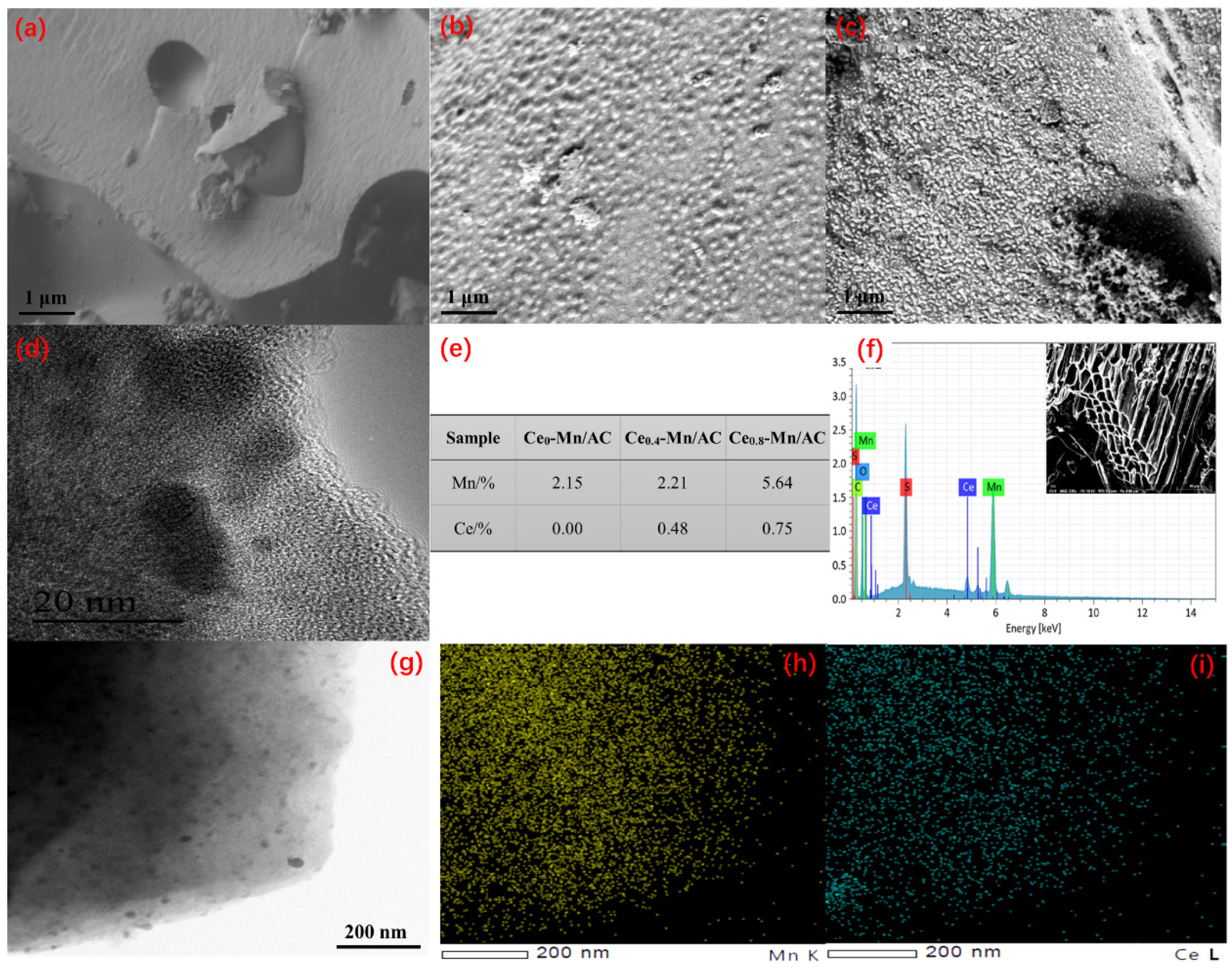
3.1. Catalyst Characterization
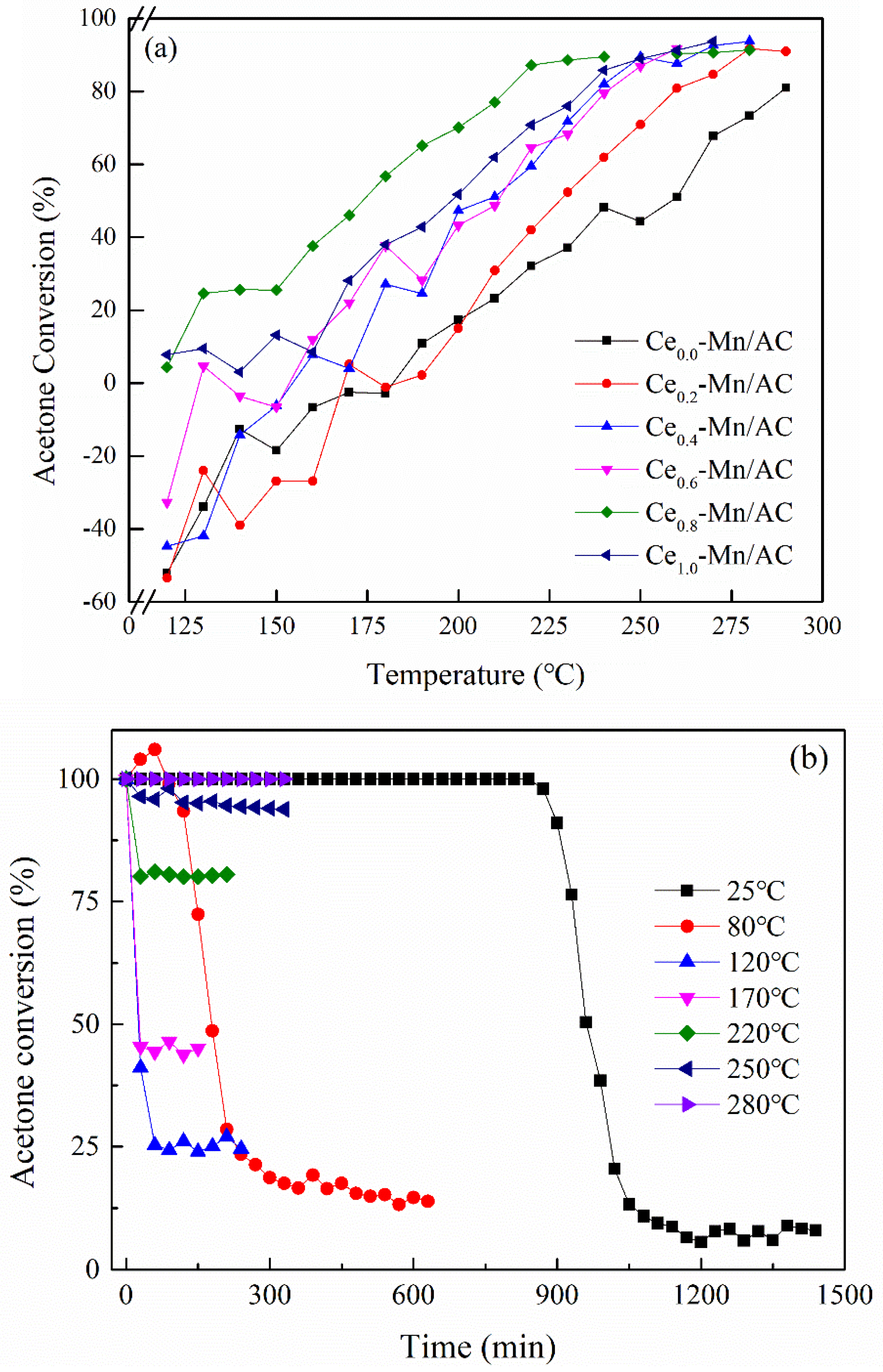
3.2. Catalytic Performance of Catalysts for Acetone
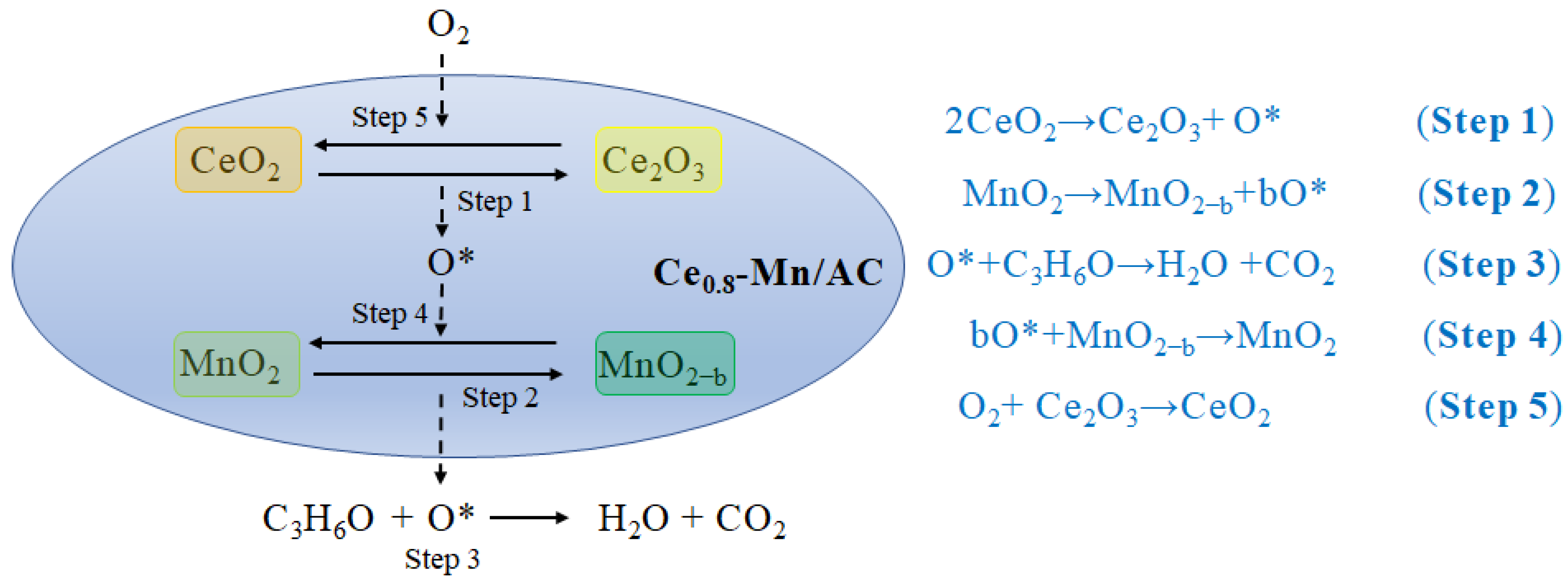
3.3. Mechanism of Catalytic Oxidation of the Ce0.8-Mn/AC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cleaner Production Center of Ministry of Environmental Protection; China Fermentation Industry Association Research. Report on Cleaner Production of Citric Acid Industry in China; China Environmental Science Press: Beijing, China, 2013; ISBN 9787511114488. [Google Scholar]
- Yao, X.; Wang, K.; Zhang, S.; Liang, S.; Li, K.; Wang, C.; Zhang, T.; Li, H.; Wang, J.; Dong, L.; et al. Degradation of the mixture of ethyl formate, propionic aldehyde, and acetone by Aeromonas salmonicida: A novel microorganism screened from biomass generated in the citric acid fermentation industry. Chemosphere 2020, 258, 127320. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Liu, Y.; Li, T.; Zhang, T.; Li, H.; Wang, W.; Shen, X.; Qian, F.; Yao, Z. Adsorption behavior of multicomponent volatile organic compounds on a citric acid residue waste-based activated carbon: Experiment and molecular simulation. J. Hazard. Mater. 2020, 392, 122323. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Chatterjee, P.; Chakraborty, T. Photo-oxidation of acetone to formic acid in synthetic air and its atmospheric implication. J. Phys. Chem. A 2015, 119, 8146–8155. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Xu, Y.; Jia, L. Effects of inorganic seeds on secondary organic aerosol formation from photochemical oxidation of acetone in a chamber. Atmos. Environ. 2017, 170, 205–215. [Google Scholar] [CrossRef]
- Zhang, C.; He, H. A comparative study of TiO2 supported noble metal catalysts for the oxidation of formaldehyde at room temperature. Catal. Today 2007, 126, 345–350. [Google Scholar] [CrossRef]
- Montecchio, F.; Babler, M.U.; Engvall, K. Development of an irradiation and kinetic model for UV processes in volatile organic compounds abatement applications. Chem. Eng. J. 2018, 348, 569–582. [Google Scholar] [CrossRef]
- Leoni, B.; Bettinetti, R.; Galassi, S. Sub-lethal effects of acetone on Daphnia magna. Ecotoxicology 2008, 17, 199–205. [Google Scholar] [CrossRef]
- Xie, R.; Ji, J.; Huang, H.; Lei, D.; Fang, R.; Shu, Y.; Zhan, Y.; Guo, K.; Leung, D. Heterogeneous activation of peroxymonosulfate over monodispersed Co3O4/activated carbon for efficient degradation of gaseous toluene. Chem. Eng. J. 2018, 341, 383–391. [Google Scholar] [CrossRef]
- Fu, D.; Feng, X.; Zhang, P. Absorption performance of (CO2 + N2) gas mixtures in amino acid ionic liquids promoted N-methyldiethanolamine aqueous solutions. J. Chem. Thermodyn. 2017, 113, 250–256. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Shen, B.; Smith, R.; Guo, H. Role of impurity components and pollutant removal processes in catalytic oxidation of o-xylene from simulated coal-fired flue gas. Sci. Total Environ. 2021, 764, 142805. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Pan, G.; Chong, S.; Chang, M. Removal of VOCs from gas streams with double perovskite-type catalysts. J. Environ. Sci. 2018, 69, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Ok, Y.S.; Tsang, D.C.W.; Song, J.; Jung, S.C.; Park, Y.K. Recent advances in volatile organic compounds abatement by catalysis and catalytic hybrid processes: A critical review. Sci. Total Environ. 2020, 719, 137405. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Yuan, C.; Hung, C.; Shen, H. Photoeletrocatalytic degradation of gaseous acetone using electrical glassfiber filter coated with nano-sized TiO2 photoelectrocatalyst. J. Taiwan Inst. Chem. Eng. 2017, 77, 187–195. [Google Scholar] [CrossRef]
- Banu, I.; Manta, C.M.; Bercaru, G.; Bozga, G. Combustion kinetics of cyclooctane and its binary mixture with o-xylene over a Pt/γ-alumina catalyst. Chem. Eng. Res. Des. 2015, 102, 399–406. [Google Scholar] [CrossRef]
- Santos, V.P.; Pereira, M.F.R.; Orfao, J.J.M.; Figueiredo, J.L. The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds. Appl. Catal. B 2010, 99, 353–363. [Google Scholar] [CrossRef]
- Piumetti, M.; Fino, D.; Russo, N. Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl. Catal. B 2015, 163, 277–287. [Google Scholar] [CrossRef]
- Ghavami, M.; Soltan, J. Kinetics and mechanism of catalytic ozonation of acetone in air over MnOx/Al2O3 catalyst. React. Kinet. Mech. Catal. 2021, 133, 953–970. [Google Scholar] [CrossRef]
- Lin, H.; Liu, Y.; Deng, J.; Zhang, K.; Zhang, X.; Xie, S.; Zhao, X.; Yang, J.; Han, Z.; Dai, H. Au-Pd/mesoporous Fe2O3: Highly active photocatalysts for the visible-light-driven degradation of acetone. J. Environ. Sci. 2018, 70, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Somekawa, S. Treatment of volatile organic compounds with a Pt/Co3O4-CeO2 catalyst. Chem. Eng. Technol. 2019, 42, 257–260. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, L.; Liu, Y.; Yang, T.; Deng, J.; Dai, H. Concurrent catalytic removal of typical volatile organic compound mixtures over Au-Pd/alpha-MnO2 nanotubes. J. Environ. Sci. 2018, 64, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Changsuphan, A.; Oanh, N.T.K. Catalytic oxidation of volatile organic compounds by 13X zeolite coated with nZnO in presence of UV and ozone at high bed temperature. Water Air Soil Pollut. 2018, 229, 201. [Google Scholar] [CrossRef]
- He, C.; Jiang, Z.; Ma, M.; Zhang, X.; Douthwaite, M.; Shi, J.; Hao, Z. Understanding the promotional effect of Mn2O3 on micro-/mesoporous hybrid silica nanocubic-supported Pt catalysts for the low-temperature destruction of methyl ethyl ketone: An experimental and theoretical study. ACS Catal. 2018, 8, 4213–4229. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Yang, P.; Cheng, Z.; Li, J.; Zuo, S. Ce-modified mesoporous γ-Al2O3 supported Pd-Pt nanoparticle catalysts and their structure-function relationship in complete benzene oxidation. Chem. Eng. J. 2019, 356, 255–261. [Google Scholar] [CrossRef]
- Liu, P.; He, H.; Wei, G.; Liang, X.; Qi, F.; Tan, F.; Tan, W.; Zhu, J.; Zhu, R. Effect of Mn substitution on the promoted formaldehyde oxidation over spinel ferrite: Catalyst characterization, performance and reaction mechanism. Appl. Catal. B 2016, 182, 476–484. [Google Scholar] [CrossRef]
- Hu, C. Catalytic combustion kinetics of acetone and toluene over Cu0.13Ce0.87Oy catalyst. Chem. Eng. J. 2011, 168, 1185–1192. [Google Scholar] [CrossRef]
- Rezlescu, N.; Rezlescu, E.; Popa, P.D.; Doroftei, C.L.; Ignat, M. Some nanograined ferrites and perovskites for catalytic combustion of acetone at low temperature. Ceram. Int. 2015, 41, 4430–4437. [Google Scholar] [CrossRef]
- Li, G.; Hu, R.C.; Hao, Y.H.; Yang, T.L.; Li, L.L.; Luo, Z.H.; Xie, Y.J.; Zhao, N.; Liu, C.; Sun, C.; et al. CO2 and air pollutant emissions from bio-coal briquettes. Environ. Technol. Innov. 2023, 29, 102975. [Google Scholar] [CrossRef]
- Guo, S.J.; Sun, S.H. FePt nanoparticles assembled on graphene as enhanced catalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 2492–2495. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Tripathi, K.; Pant, K.K. Investigating the role of oxygen vacancies and basic site density in tuning methanol selectivity over Cu/CeO2 catalyst during CO2 hydrogenation. Fuel 2021, 303, 121289. [Google Scholar] [CrossRef]
- Li, G.; Hu, R.; Wang, N.; Yang, T.; Xu, F.; Li, J.; Wu, J.; Huang, Z.; Pan, M.; Lyu, T. Cultivation of microalgae in adjusted wastewater to enhance biofuel production and reduce environmental impact: Pyrolysis performances and life cycle assessment. J. Clean. Prod. 2022, 355, 131768. [Google Scholar] [CrossRef]
- Li, G.; Yang, T.L.; Xiao, W.B.; Wu, J.H.; Xu, F.Z.; Li, L.L.; Gao, F.; Huang, Z.G. Sustainable environmental assessment of waste-to-energy practices: Co-pyrolysis of food waste and discarded meal boxes. Foods 2022, 11, 3840. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhou, S.; Wang, H.; Cao, Z.; Liu, H. The application of an activated carbon supported Cu-Ce/Ac oxide anode on the electrocatalytic degradation of phenol. Int. J. Electrochem. Sci. 2017, 12, 9640–9651. [Google Scholar] [CrossRef]
- Qin, H.; Xiao, R.; Chen, J. Catalytic wet peroxide oxidation of benzoic acid over Fe/AC catalysts: Effect of nitrogen and sulfur co-doped activated carbon. Sci. Total Environ. 2018, 626, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, Y.; Cui, L.; An, D.; Feng, Y. Removal of elemental mercury from coal pyrolysis gas using Fe–Ce oxides supported on lignite semi-coke modified by the hydrothermal impregnation method. Energy Fuels 2018, 32, 12861–12870. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, L.; Qu, H.; Zhong, Q.; Liu, Z. The effect of CeO2 dispersity and active oxygen species on the SCR reaction over Fe-ZSM-5@Ce/meso-SiO2. Catal. Lett. 2019, 150, 514–523. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, Y.; Yang, D.; Dai, J.; Liu, Z.; Chen, Y.; Huang, J.; Li, Q. Biogenic Pt/CaCO3 nanocomposite as a robust catalyst toward benzene oxidation. ACS Appl. Mater. Interfaces 2020, 12, 2469–2480. [Google Scholar] [CrossRef]
- Jafari, A.J.; Kalantary, R.R.; Esrafili, A.; Arfaeinia, H. Synthesis of silica-functionalized graphene oxide/ZnO coated on fiberglass and its application in photocatalytic removal of gaseous benzene. Process Saf. Environ. Prot. 2018, 116, 377–387. [Google Scholar] [CrossRef]
- Dong, Z. Study on Denitrification Performance and Synergistic Catalytic Mechanism of Nano-Mn-Ce Oxide; Chongqing University: Chongqing, China, 2016. [Google Scholar]
- Yorgun, S.; Yıldız, D. Preparation and characterization of activated carbons from Paulownia wood by chemical activation with H3PO4. J. Taiwan Inst. Chem. Eng. 2015, 53, 122–131. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Gotti, E.; Toscano, L.; Ragaini, V. Preparation of Pd/C catalysts via ultrasound: A study of the metal distribution. Ultrason. Sonochem. 1997, 4, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ni, J. Different effects of surface heterogeneous atoms of porous and non-porous carbonaceous materials on adsorption of 1,1,2,2-tetrachloroethane in aqueous environment. Chemosphere 2017, 175, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hao, Y.; Yang, T.; Xiao, W.; Pan, M.; Huo, S.; Lyu, T. Enhancing bioenergy production from the raw and defatted microalgal biomass using wastewater as the cultivation medium. Bioengineering 2022, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, Q.; Zhao, Q.; Ren, S.; Kong, M.; Yao, L.; Meng, F. Promotional effect of Ce on the SCR of NO with NH3 at low temperature over MnOx supported by nitric acid-modified activated carbon. Res. Chem. Intermed 2017, 44, 1729–1744. [Google Scholar] [CrossRef]
- Li, Z.; Guo, X.; Tao, F.; Zhou, R. New insights into the effect of morphology on catalytic properties of MnOx–CeO2 mixed oxides for chlorobenzene degradation. RSC Adv. 2018, 8, 25283–25291. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Guo, Y.; Chen, X.; Li, C.; Lu, S.; Liew, K. CO Oxidation over nanostructured ceria supported bimetallic Cu–Mn oxides catalysts: Effect of Cu/Mn ratio and calcination temperature. Catal. Lett. 2017, 148, 181–193. [Google Scholar] [CrossRef]
- Jampaiah, D.; Tur, K.M.; Venkataswamy, P.; Ippolito, S.J.; Sabri, Y.M.; Tardio, J.; Bhargava, S.K.; Reddy, B.M. Catalytic oxidation and adsorption of elemental mercury over nanostructured CeO2–MnOx catalyst. RSC Adv. 2015, 5, 30331–30341. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Q.; Han, J.; Lu, S.; Su, Y.; Song, C.; Ji, N.; Lu, X.; Ma, D.; Cheung, O. The effect of cerium incorporation on the catalytic performance of cobalt and manganese containing layer double oxides for acetone oxidation. J. Chem. Technol. Biotechnol. 2018, 94, 3753–3762. [Google Scholar] [CrossRef]
- Li, G.; Hao, Y.; Yang, T.; Wu, J.; Xu, F.; Li, L.; Wang, B.; Li, M.; Zhao, N.; Wang, N.; et al. Air pollutant emissions from sludge-bituminous briquettes as a potential household energy source. Case Stud. Therm. Eng. 2022, 37, 102251. [Google Scholar] [CrossRef]
- He, J.; Reddy, G.K.; Thiel, S.W.; Smirniotis, P.G.; Pinto, N.G. Ceria-modified manganese oxide/titania materials for removal of elemental and oxidized mercury from flue gas. J. Phys. Chem. C 2011, 115, 24300–24309. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Li, J.; Zhu, J.; Ma, L. Novel V2O5–CeO2/TiO2 catalyst with low vanadium loading for the selective catalytic reduction of NOx by NH3. Appl. Catal. B 2014, 158, 11–19. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, C.; Bian, L. Highly dispersed Pd species supported on CeO2 catalyst for lean methane combustion: The effect of the occurrence state of surface Pd species on the catalytic activity. Catalysts 2021, 11, 772. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, F.; Jiang, S.; Qiu, K.; Kuang, M.; Whiddon, R.; Cen, K. Ceria substrate–oxide composites as catalyst for highly efficient catalytic oxidation of NO by O2. Fuel 2016, 166, 352–360. [Google Scholar] [CrossRef]
- Feng, Z. Preparation of Cerium Oxide Based Catalysts by Flame Combustion Method and CO Oxidation Performance; University of Chinese Academy of Sciences: Beijing, China, 2019. [Google Scholar]
- He, C.; Yu, Y.; Yue, L.; Qiao, N.; Li, J.; Shen, Q.; Yu, W.; Chen, J.; Hao, Z. Low-temperature removal of toluene and propanal over highly active mesoporous CuCeOx catalysts synthesized via a simple self-precipitation protocol. Appl. Catal. B 2014, 147, 156–166. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Pan, M.; Hao, Y.; Hu, R.; Xiao, W.; Li, G.; Lyu, T. Valorisation of microalgae residues after lipid extraction: Pyrolysis characteristics for biofuel production. Biochem. Eng. J. 2022, 179, 108330. [Google Scholar] [CrossRef]
- Lv, K.; Xiang, Q.; Yu, J. Effect of calcination temperature on morphology and photocatalytic activity of anatase TiO2 nanosheets with exposed {001} facets. Appl. Catal. B 2011, 104, 275–281. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, W.; Zhang, T.; Zhang, Y.; Jiang, L.; Yin, S. Facile fabrication of shape-controlled CoxMnyObeta nanocatalysts for benzene oxidation at low temperatures. Chem. Comm. 2018, 54, 2154–2157. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, J.; Wang, X. Oxidation treatment of diesel soot particulate on CexZr1-xO2. J. Hazard. Mater. 2007, 140, 205–210. [Google Scholar] [CrossRef]
- Xie, Y.; Li, C.; Zhao, L.; Zhang, J.; Zeng, G.; Zhang, X.; Zhang, W.; Tao, S. Experimental study on Hg0 removal from flue gas over columnar MnOx-CeO2/activated coke. Appl. Surf. Sci. 2015, 333, 59–67. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Wang, S.; Zhao, M.; Gong, M.; Chen, Y. Activity and thermal stability of Pt/Ce0.64Mn0.16R0.2Ox (R=Al, Zr, La, or Y) for soot and NO oxidation. Fuel Process. Technol. 2015, 137, 38–47. [Google Scholar] [CrossRef]
- Lin, L.; Wang, C.; Bai, H. A comparative investigation on the low-temperature catalytic oxidation of acetone over porous aluminosilicate-supported cerium oxides. Chem. Eng. J. 2015, 264, 835–844. [Google Scholar] [CrossRef]
- Kam, S.K.; Kang, K.H.; Lee, M.G. Adsorption characteristics of acetone, benzene, and metylmercaptan in the fixed bed reactor packed with activated carbon prepared from waste citrus peel. Appl. Chem. Eng. 2018, 29, 28–36. [Google Scholar] [CrossRef]
- Chai, H.; Zhang, Z.; Zhou, Y.; Zhu, L.; Lv, H.; Wang, N. Roles of intrinsic Mn(3+) sites and lattice oxygen in mechanochemical debromination and mineralization of decabromodiphenyl ether with manganese dioxide. Chemosphere 2018, 207, 41–49. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, H.; He, C.; Shen, Z.; Wang, T. Synergistic effect and mechanism of non-thermal plasma catalysis system in volatile organic compounds removal: A review. Catal. Sci. Technol. 2018, 8, 936–954. [Google Scholar] [CrossRef]
- Almukhlifi, H.A.; Burns, R.C. The complete oxidation of isobutane over CeO2 and Au/CeO2, and the composite catalysts MOx/CeO2 and Au/MOx/CeO2 (Mn+ = Mn, Fe, Co and Ni): The effects of gold nanoparticles obtained from n-hexanethiolate-stabilized gold nanoparticles. J. Mol. Catal. A Chem. 2016, 415, 131–143. [Google Scholar] [CrossRef]

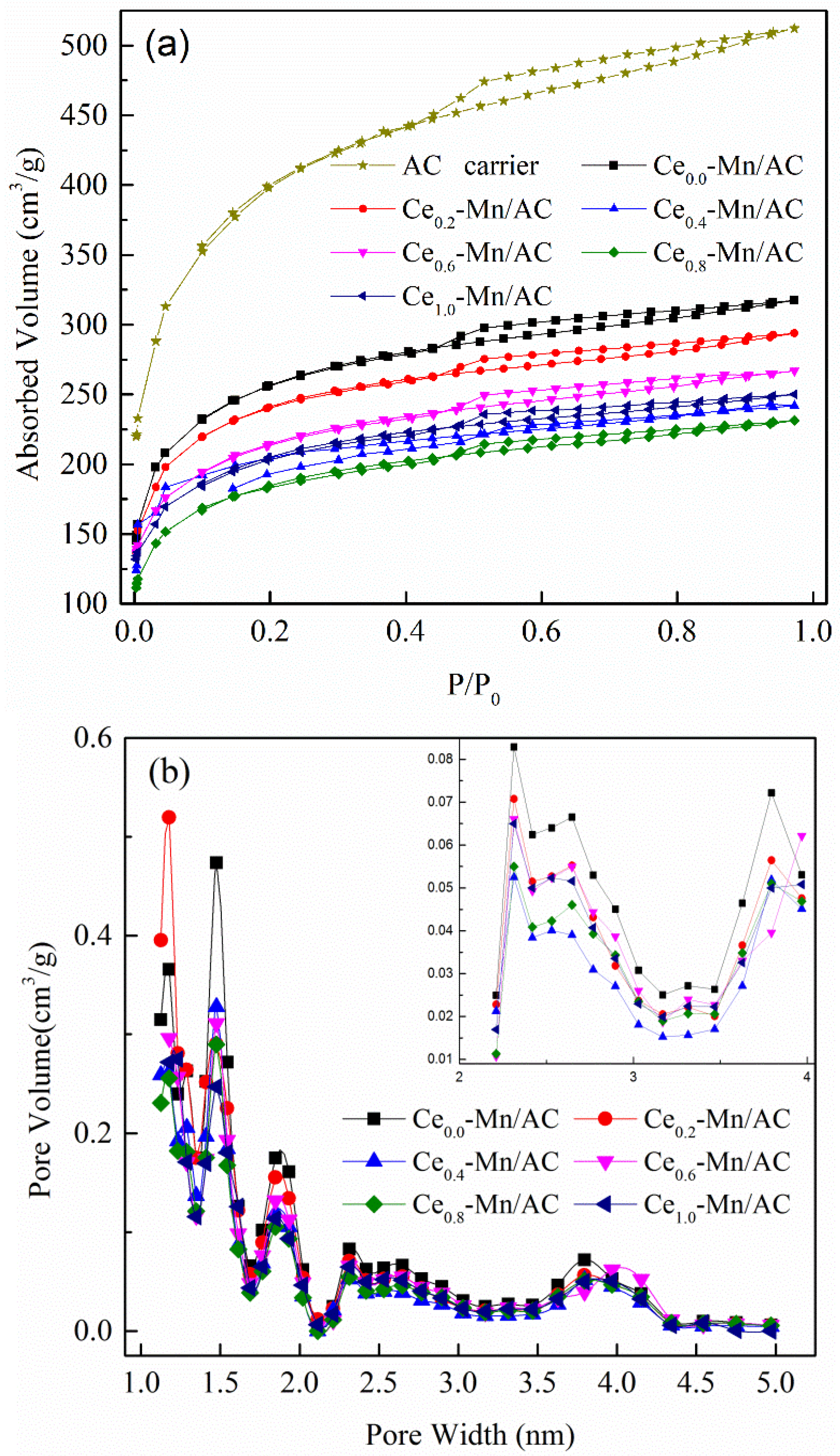
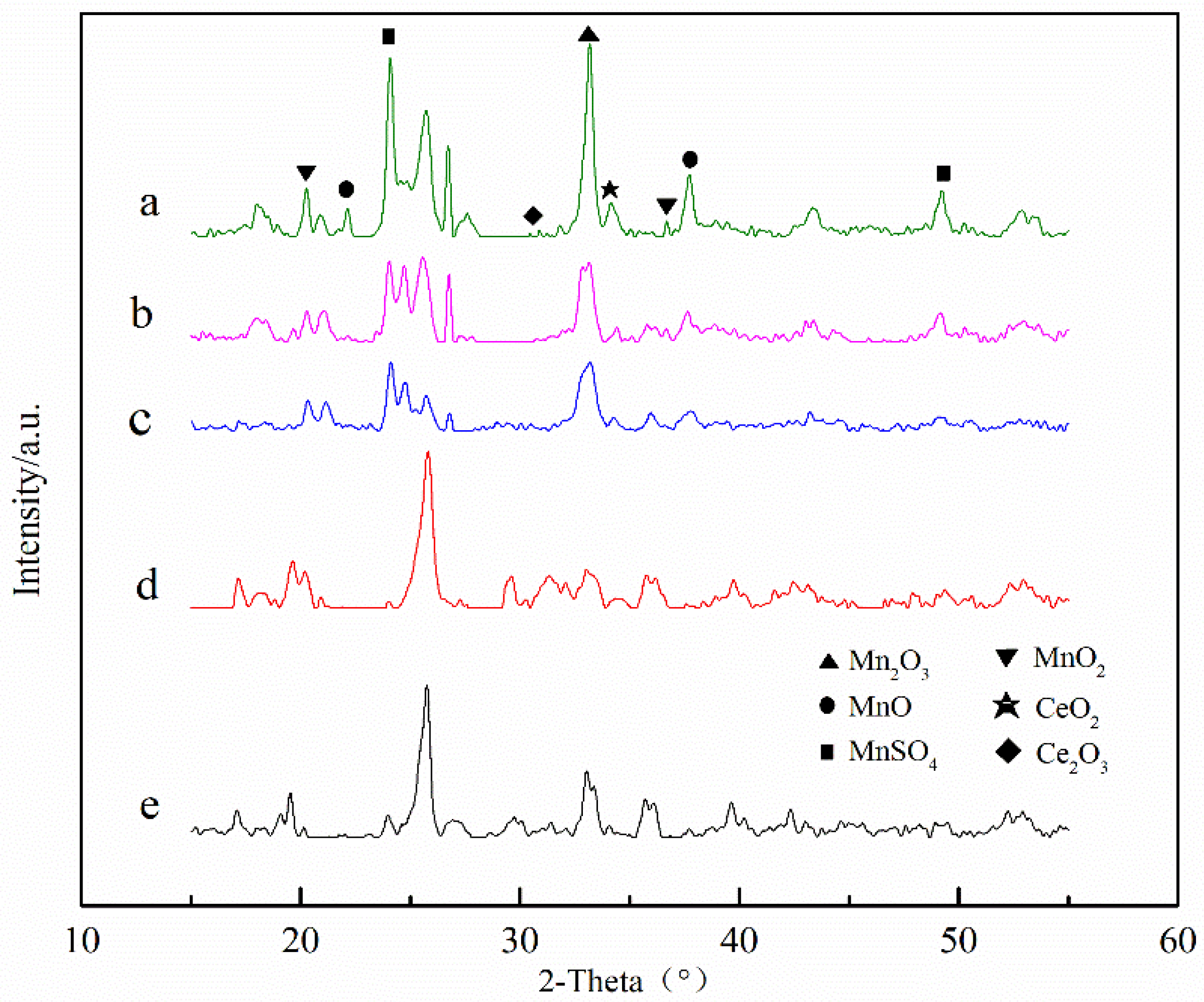
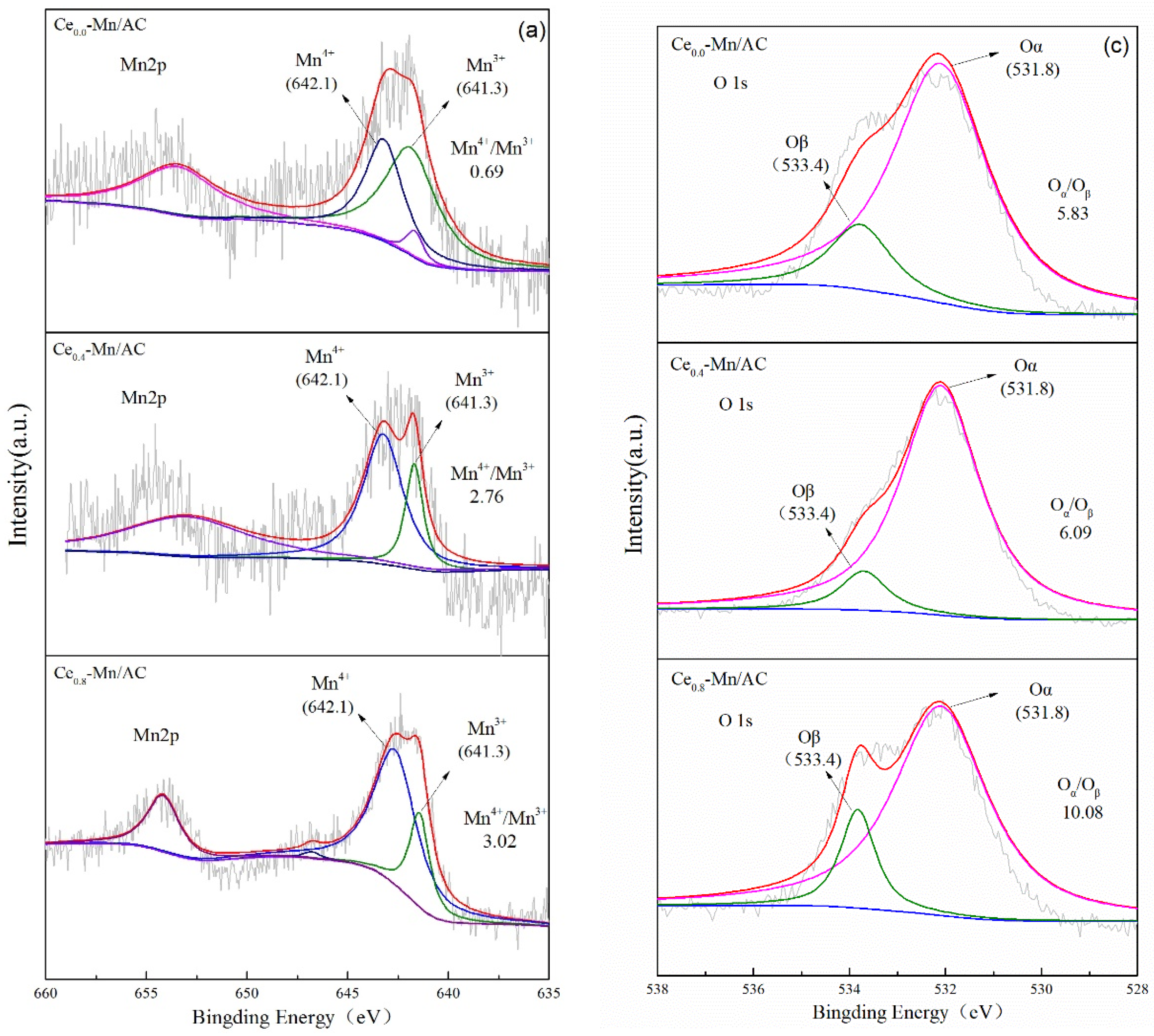
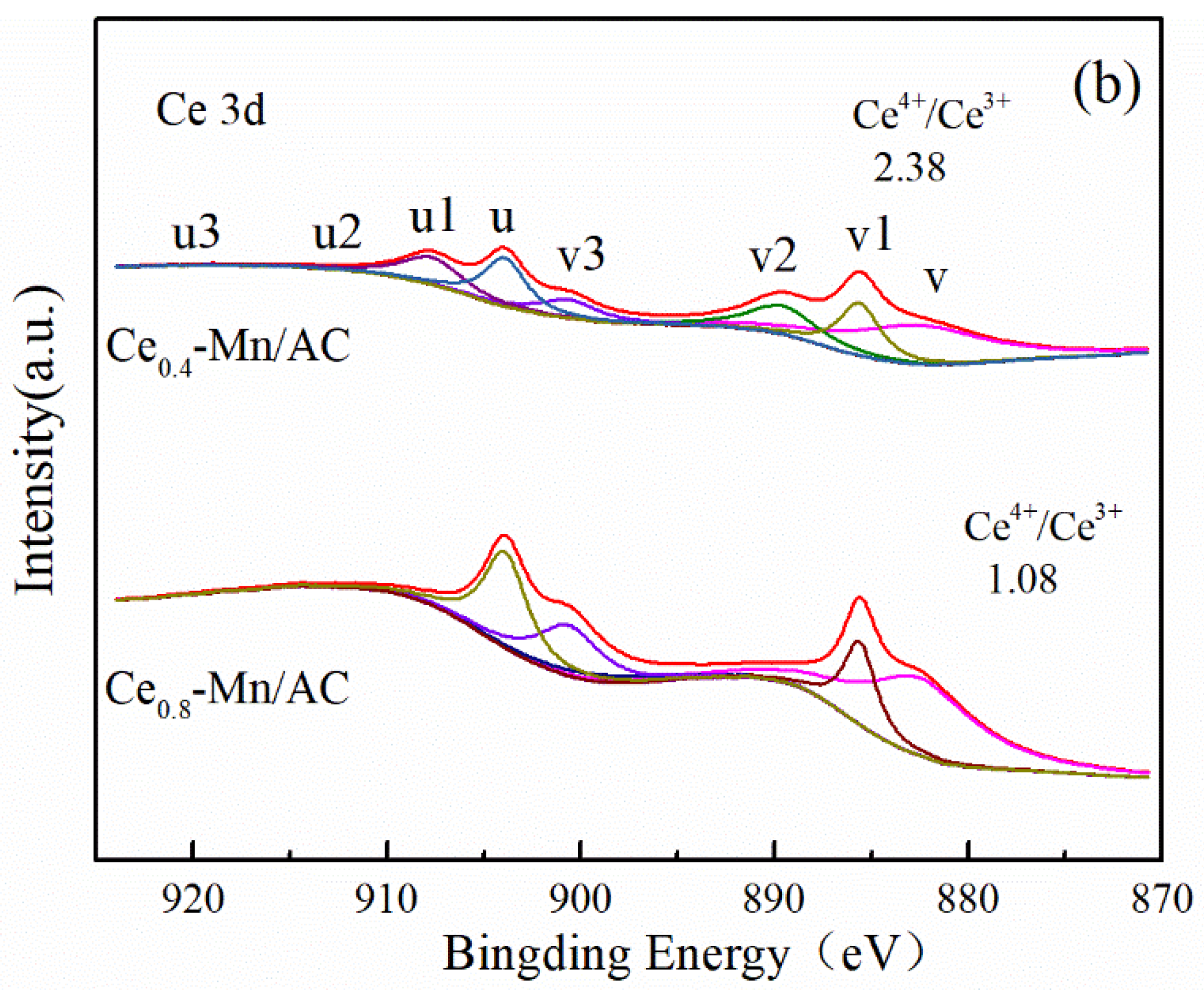

| Catalyst | SBET (m2/g) | Mesoporous Volume (cm3/g) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| Biomass-activated carbon | 1400.25 | 0.179 | 0.793 |
| Ce0.0-Mn/AC | 918.07 | 0.096 | 0.491 |
| Ce0.2-Mn/AC | 837.83 | 0.084 | 0.454 |
| Ce0.4-Mn/AC | 702.42 | 0.071 | 0.376 |
| Ce0.6-Mn/AC | 776.49 | 0.084 | 0.413 |
| Ce0.8-Mn/AC | 645.18 | 0.074 | 0.358 |
| Ce1.0-Mn/AC | 725.98 | 0.113 | 0.387 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Wang, Z.; Shi, Y.; Yao, X. Preparation and Performance of Carbon-Based Ce-Mn Catalysts for Efficient Degradation of Acetone at Low Temperatures. Int. J. Environ. Res. Public Health 2022, 19, 16879. https://doi.org/10.3390/ijerph192416879
Li T, Wang Z, Shi Y, Yao X. Preparation and Performance of Carbon-Based Ce-Mn Catalysts for Efficient Degradation of Acetone at Low Temperatures. International Journal of Environmental Research and Public Health. 2022; 19(24):16879. https://doi.org/10.3390/ijerph192416879
Chicago/Turabian StyleLi, Tong, Zhibo Wang, Yue Shi, and Xiaolong Yao. 2022. "Preparation and Performance of Carbon-Based Ce-Mn Catalysts for Efficient Degradation of Acetone at Low Temperatures" International Journal of Environmental Research and Public Health 19, no. 24: 16879. https://doi.org/10.3390/ijerph192416879
APA StyleLi, T., Wang, Z., Shi, Y., & Yao, X. (2022). Preparation and Performance of Carbon-Based Ce-Mn Catalysts for Efficient Degradation of Acetone at Low Temperatures. International Journal of Environmental Research and Public Health, 19(24), 16879. https://doi.org/10.3390/ijerph192416879




