Screening and Optimization of Conditions for the Adsorption of Cd2+ in Serpentine by Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Experimental Method
2.2.1. Equilibrium Adsorption Experiment
2.2.2. Single-Factor Experiment
2.2.3. Box–Behnken Center Combination Experiment Design
2.3. Data Analysis
3. Results and Discussion
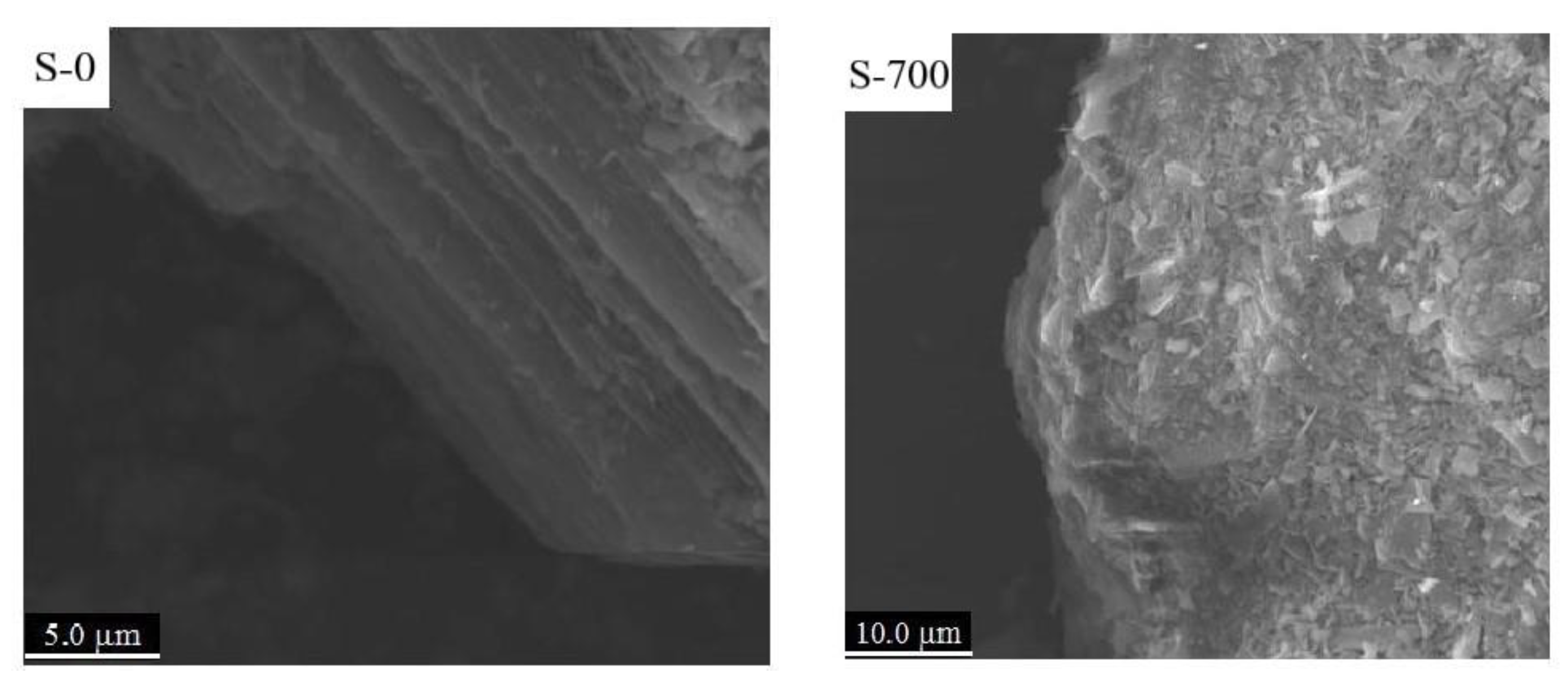
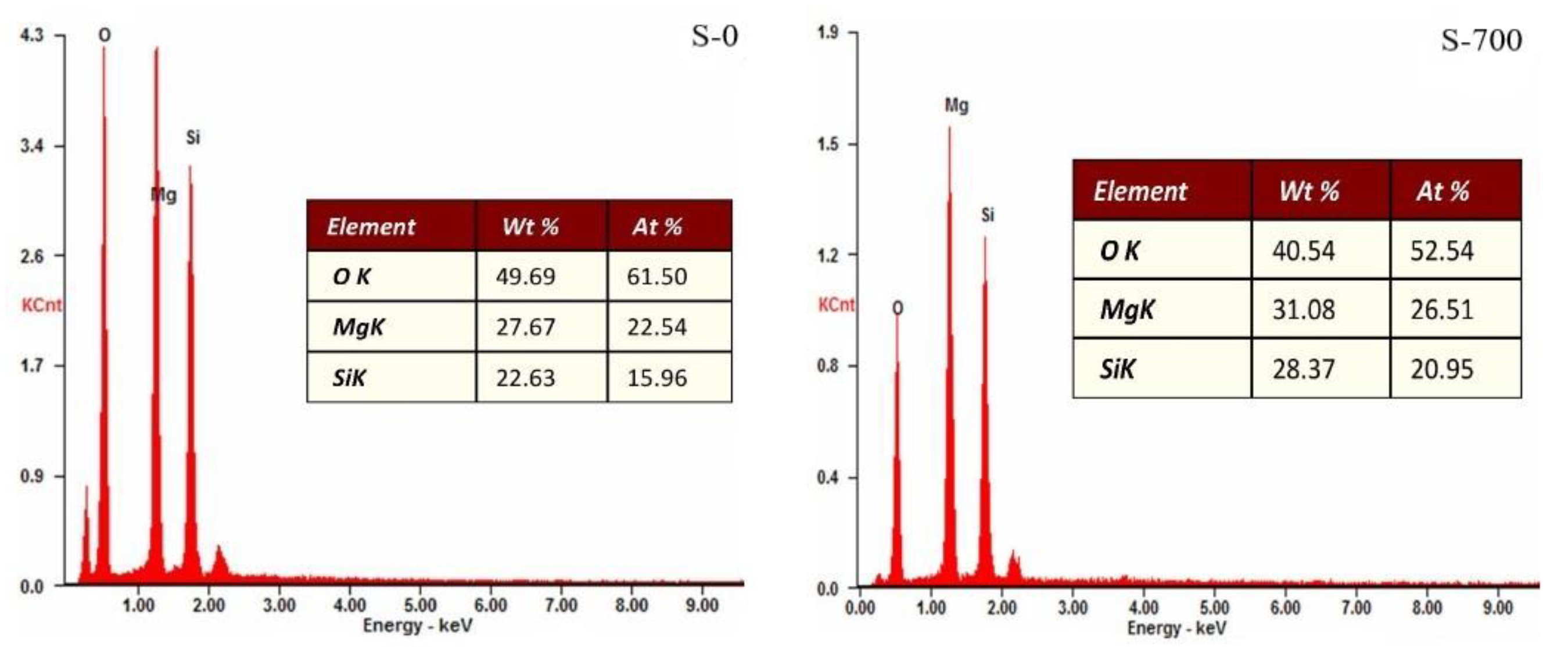
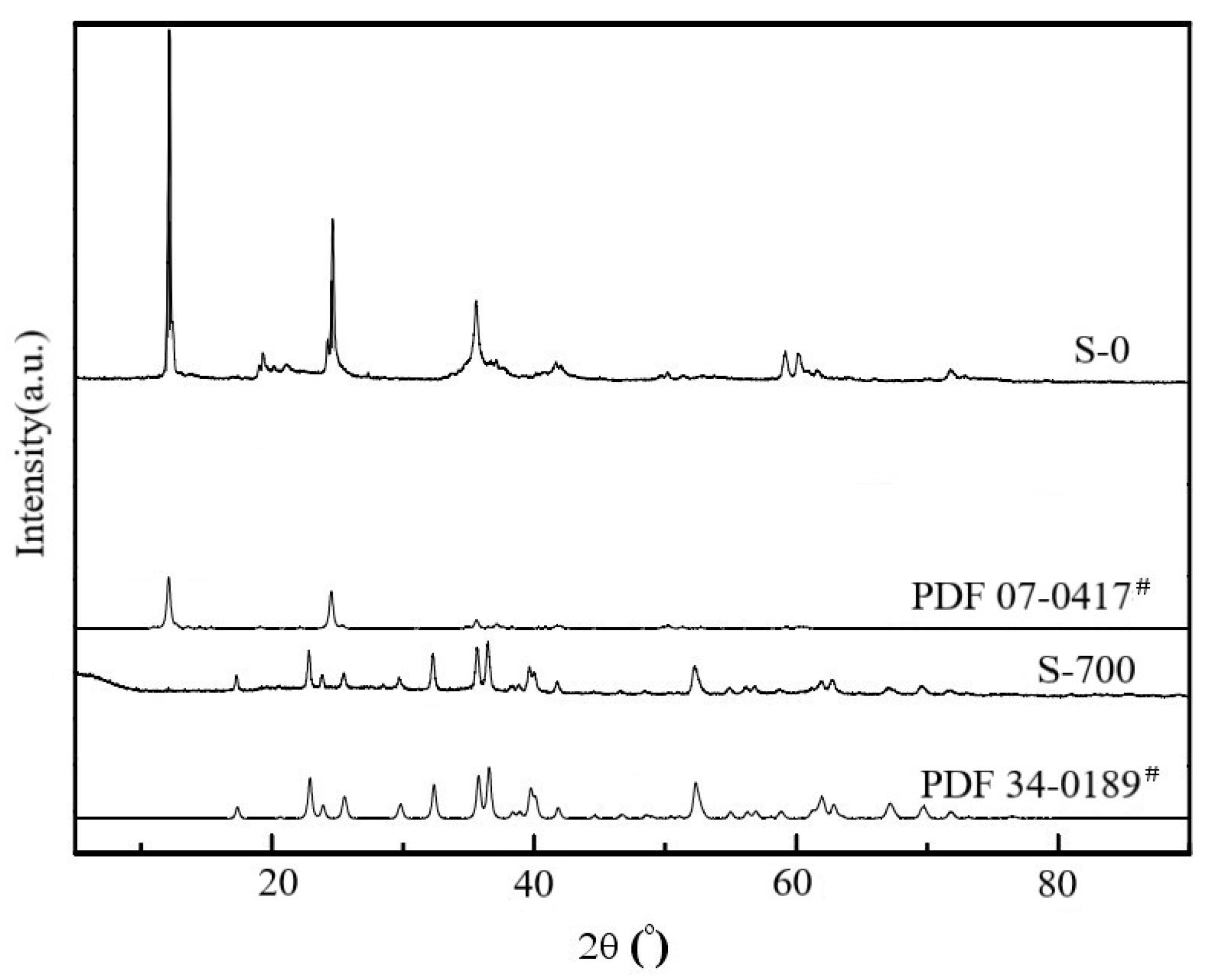
3.1. Serpentine Sample Characterization
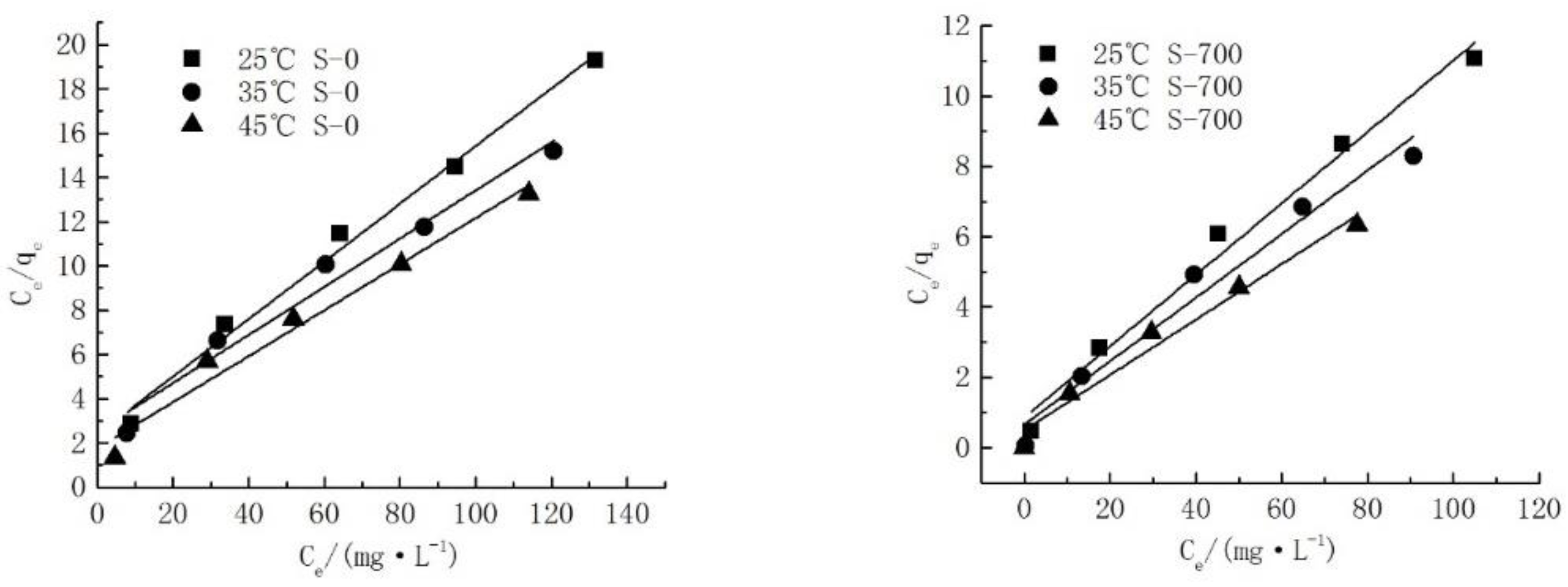
3.2. Study on the Adsorption Isotherms of Cd2+ on Serpentine
3.3. Single Factor Experiment Results
3.3.1. Effect of the AS on Adsorption Effect
3.3.2. Effect of the pH on Adsorption Effect
3.3.3. Effect of the C on Adsorption Effect
3.3.4. Effect of the T on Adsorption Effect
3.4. Experiment Design and Results of RSM
3.4.1. Experiment Design of RSM
− 0.005BC − 0.02BD − 0.005CD + 0.5765A2 + 0.009B2 − 0.0748C2 − 0.0573D2
0.2025BC − 0.7675BD − 0.2025CD − 5.47A2 + 0.2983B2 − 1.05C2 − 3.57D2
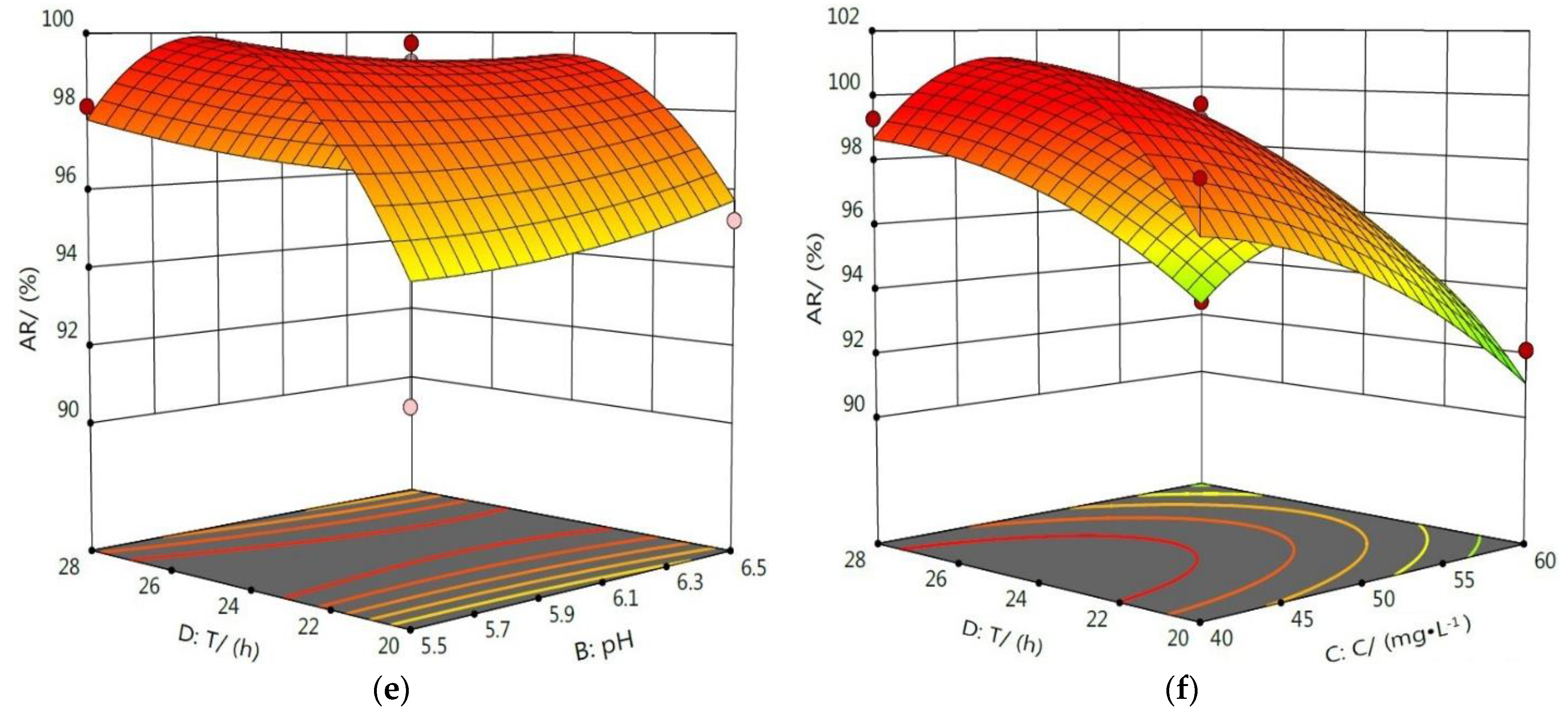
3.4.2. Response Surface Analysis of the Interaction Influence of Various Factors
3.5. Determination of Optimal Adsorption Conditions and Verification Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, Z.T.; Chen, Z.; Xu, A.; Gao, Q.; Song, K.Y.; Liu, J.H.; Hu, H.Y. Wastewater treatment and reuse situations and influential factors in major Asian countries. J. Environ. Manag. 2021, 282, 111976. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.T.; Chen, Z.; Wu, Y.H.; Xu, A.; Liu, J.H.; Hu, H.Y. Identification of development potentials and routes of wastewater treatment and reuse for Asian countries by key influential factors and prediction models. Resour. Conserv. Recycl. 2021, 168, 105259. [Google Scholar] [CrossRef]
- Han, G.H.; Wang, J.W.; Sun, H.; Liu, B.B.; Huang, Y.F. A critical review on the removal and recovery of hazardous Cd from Cd-containing secondary resources in Cu-Pb-Zn smelting processes. Metals 2022, 12, 1846. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Evaluation of the usefulness of sorbents in the remediation of soil exposed to the pressure of cadmium and cobalt. Materials 2022, 15, 5738. [Google Scholar] [CrossRef] [PubMed]
- Mashhadikhan, S.; Amooghin, A.; Sanaeepur, H.; Shirazi, M. A critical review on cadmium recovery from wastewater towards environmental sustainability. Desalination 2022, 535, 115815. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.P.; Kang, X.R.; Yu, J.P.; Gao, S.; Zhang, J.; Wang, H.; Pan, H.; Yang, Q.G.; Zhuge, Y.P.; et al. Effective utilization of weak alkaline soils with Cd-contamination by wheat and rape intercropping. Ecotoxicol. Environ. Saf. 2022, 248, 114335. [Google Scholar] [CrossRef]
- Zhang, H.; Mindy, R. Cadmium exposure in living organisms: A short review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Y.C.; Xu, L.F.; Zhang, Y.M.; Li, H.X. Research and development on industrial heavy metal wastewater treatment technology. 2020 6th International Conference on Energy, Environ. Mater. Sci. 2020, 585, 012051. [Google Scholar] [CrossRef]
- Zou, Z.H.; Hen, S.F.; Han, C.Y.; Zhang, L.Y.; Luo, Y.M. Progress of research on treatment of heavy metal wastewater by adsorption. Environ. Protect. Sci. 2010, 36, 22–24. (In Chinese) [Google Scholar]
- Li, H.B.; Wang, L.; Wei, Y.F.; Yan, W.; Feng, J.T. Preparation of templated materials and their application to typical pollutants in wastewater: A review. Front. Chem. 2022, 10, 882876. [Google Scholar] [CrossRef]
- Zhang, X.G.; Zhang, L. Adsorption of phenol by modified activated carbons. Environ. Develop. 2011, 23, 182–183. (In Chinese) [Google Scholar]
- Hang, X.S.; Wang, H.Y.; Zhou, J.M. Soil heavy-metal distribution and transference to soybeans surrounding an electroplating factory. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2010, 60, 144–151. [Google Scholar] [CrossRef]
- Novikau, R.; Lujaniene, G. Adsorption behavior of pollutants: Heavy metals, radionuclides, organic pollutants, on clays and their minerals (raw, modified and treated): A review. J. Environ. Manag. 2022, 309, 114685. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.Y. Adsorption Mechanism of Heavy Metals on Serpentine and Its Effects on Heavy Metals Formations in Contaminated Soil; Shenyang Agricultural University: Shenyang, China, 2017. (In Chinese) [Google Scholar]
- Mokhtar, A.; Abdelkarim, S.; Hachemaoui, M.; Adjdir, M.; Zahraoui, M.; Boukoussa, B. Layered silicate magadiite and its composites for pollutants removal and antimicrobial properties: A review. Appl. Clay Sci. 2020, 198, 105823. [Google Scholar] [CrossRef]
- Cheng, D.B.; Cai, C.F. Application of two methods of estimating soil hydraulic properties on purple soil. J. Soil Water Conserv. 2007, 6, 143–146+158. (In Chinese) [Google Scholar]
- Cao, C.Y.; Liang, C.H.; Yin, Y.; Du, L.Y. Thermal activation of serpentine for adsorption of cadmium. J. Hazard. Mater. 2017, 329, 222–229. [Google Scholar] [CrossRef]
- Qu, J.H.; Dong, M.; Bi, F.X.; Tao, Y.; Wang, L.; Jiang, Z.; Zhang, G.S.; Zhang, B.; Zhang, Y. Microwave-assisted one-pot synthesis of β-cyclodextrin modified biochar for stabilization of Cd and Pb in soil. J. Clean Prod. 2022, 346, 131165. [Google Scholar] [CrossRef]
- Qu, J.H.; Xu, Y.; Zhang, X.B.; Sun, M.Z.; Tao, Y.; Zhang, X.M.; Zhang, G.S.; Ge, C.J.; Zhang, Y. Ball milling-assisted preparation of N-doped biochar loaded with ferrous sulfide as persulfate activator for phenol degradation: Multiple active sites-triggered radical/non-radical mechanism. Appl. Catal. B Environ. 2022, 316, 121639. [Google Scholar] [CrossRef]
- Lai, H.Z.; Wang, S.Q.; Yu, N. Mineralogical characteristics of the thermal treatment products of antigorite from Xiuyan, Liaoning. Acta Mineral. Sin. 2003, 2, 124–128. (In Chinese) [Google Scholar]
- Cao, C.Y.; Yu, B.; Wang, M.; Zhao, Y.Y.; Wan, X.; Zhao, S. Immobilization of cadmium in simulated contaminated soils using thermal-activated serpentine. Soil Sci. Plant Nutr. 2020, 66, 499–505. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V. Optimization of treatment of grey wastewater using electro-fenton technique—Modeling and validation. Process Saf. Environ. Protect. 2015, 95, 60–68. [Google Scholar] [CrossRef]
- Song, L.F.; Yu, X.; Xu, B.; Pang, R.; Zhang, Z.Y. 3D slope reliability analysis based on the intelligent response surface methodology. Bull. Eng. Geol. Environ. 2021, 80, 735–749. [Google Scholar] [CrossRef]
- Xu, J.; Long, Y.Y.; Shen, D.S.; Feng, H.J.; Chen, T. Optimization of fenton treatment process for degradation of refractory organics in pre-coagulated leachate membrane concentrates. J. Hazard. Mater. 2016, 323, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.L.; Jiao, S.J.; Deng, X.L.; Feng, L.; Zhang, H.Q.; Chen, R. Optimization of preparation and application of PPFS by response surface methodology. Chin. J. Environ. Eng. 2012, 6, 9–14. (In Chinese) [Google Scholar] [CrossRef]
- Khataee, A.; Kasiri, M.; Alidokht, L.; Khataee, A.; Kasiri, M.; Alidokht, L. Application of response surface methodology in the optimization of photocatalytic removal of environmental pollutants using nanocatalysts. Environ. Technol. 2011, 32, 1669–1684. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Wu, Z.H.; Tian, X.; An, X.Y.; Zhang, Y.; Zhang, G.S.; Deng, F.X.; Meng, X.L.; Qu, J.H. Concurrent elimination and stepwise recovery of Pb (II) and bisphenol A from water using β–cyclodextrin modified magnetic cellulose: Adsorption performance and mechanism investigation. J. Hazard. Mater. 2022, 432, 128758. [Google Scholar] [CrossRef]
- Sun, P.C. Research on the Mechanism of Lead and Cadmium Solidification and Nitrogen Fertilizer Synergy by Three Kinds of Environmental Materials; China University of Mining & Technology: Beijing, China, 2016. (In Chinese) [Google Scholar]
- Liu, W.; Zhao, C.C.; Wang, S.T.; Niu, L.; Wang, Y.L.; Liang, S.X.; Cui, Z. Adsorption of cadmium ions from aqueous solutions using nano-montmorillonite: Kinetics, isotherm and mechanism evaluations. Res. Chem. Intermed. 2018, 44, 1441–1458. [Google Scholar] [CrossRef]
- Qu, J.H.; We, S.Q.; Liu, Y.; Zhang, X.M.; Jiang, Z.; Tao, Y.; Zhang, G.S.; Zhang, B.; Wang, L.; Zhang, Y. Effective lead passivation in soil by bone char/CMC-stabilized FeS composite loading with phosphate-solubilizing bacteria. J. Hazard. Mater. 2022, 423, 127043. [Google Scholar] [CrossRef]
- Qu, J.H.; Tian, X.; Zhang, X.B.; Yao, J.Y.; Xue, J.Q.; Li, K.G.; Zhang, B.; Wang Zhang, Y. Free radicals-triggered reductive and oxidative degradation of highly chlorinated compounds via regulation of heat-activated persulfate by low-molecular-weight organic acids. Appl. Catal. B Environ. 2022, 310, 121359. [Google Scholar] [CrossRef]
- Qu, J.H.; Yuan, Y.H.; Zhang, X.M.; Wang, L.; Tao, Y.; Jiang, Z.; Yu, H.; Dong, M.; Zhang, Y. Stabilization of lead and cadmium in soil by sulfur-iron functionalized biochar: Performance, mechanisms and microbial community evolution. J. Hazard. Mater. 2022, 425, 127876. [Google Scholar] [CrossRef]
- Sdiri, A.; Higashi, T.; Chaabouni, R.; Jamoussi, F. Competitive removal of heavy metals from aqueous solutions by montmorillonitic and calcareous clays. Water Air Soil Pollut. 2012, 223, 1191–1204. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.W.; Liao, L.; Huang, Y.; Hou, J.N.; Yang, J.; Xie, Y.; Tang, R.K.; Li, D. Optimization of enzymatic hydrolysis condition of glucosinolates from rapeseed meal by response surface methododlogy and the preliminary research of its bacteriostasis. Sci. Technol. Food Ind. 2017, 38, 264–268+273. (In Chinese) [Google Scholar]
- Karimifard, S.; Moghaddam, M. Application of response surface methodology in physicochemical removal of dyes from wastewater: A critical review. Sci. Total Environ. 2018, 640, 772–797. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Makwana, A.; Ahammed, M. The use of response surface methodology for modelling and analysis of water and wastewater treatment processes: A review. Water Sci. Technol. 2014, 69, 464–478. [Google Scholar] [CrossRef]
- Da, T.X.; Chen, T.; Ma, Y.; Tong, Z.F. Application of response surface method in the separation of radioactive material: A review. Radiochim. Acta 2022, 110, 51–66. [Google Scholar] [CrossRef]
- Manuela, M.; Fátima, B.; Annick, B.; Hannes, W.; Simone, M.; Cristina, D. Valorization of apple tree wood residues by polyphenols extraction: Comparison between conventional and microwave-assisted extraction. Ind. Crops Prod. 2017, 104, 210–220. [Google Scholar] [CrossRef]
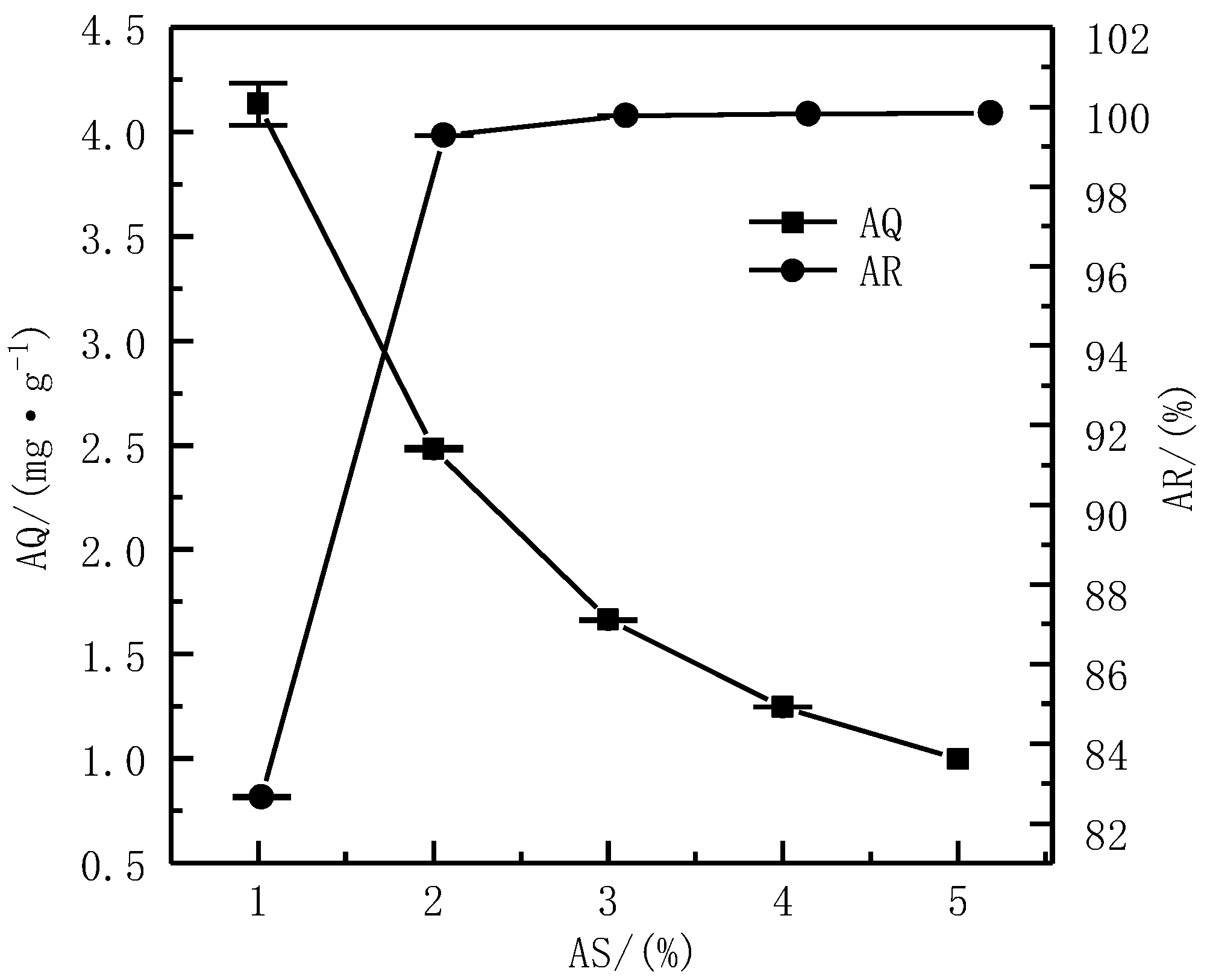
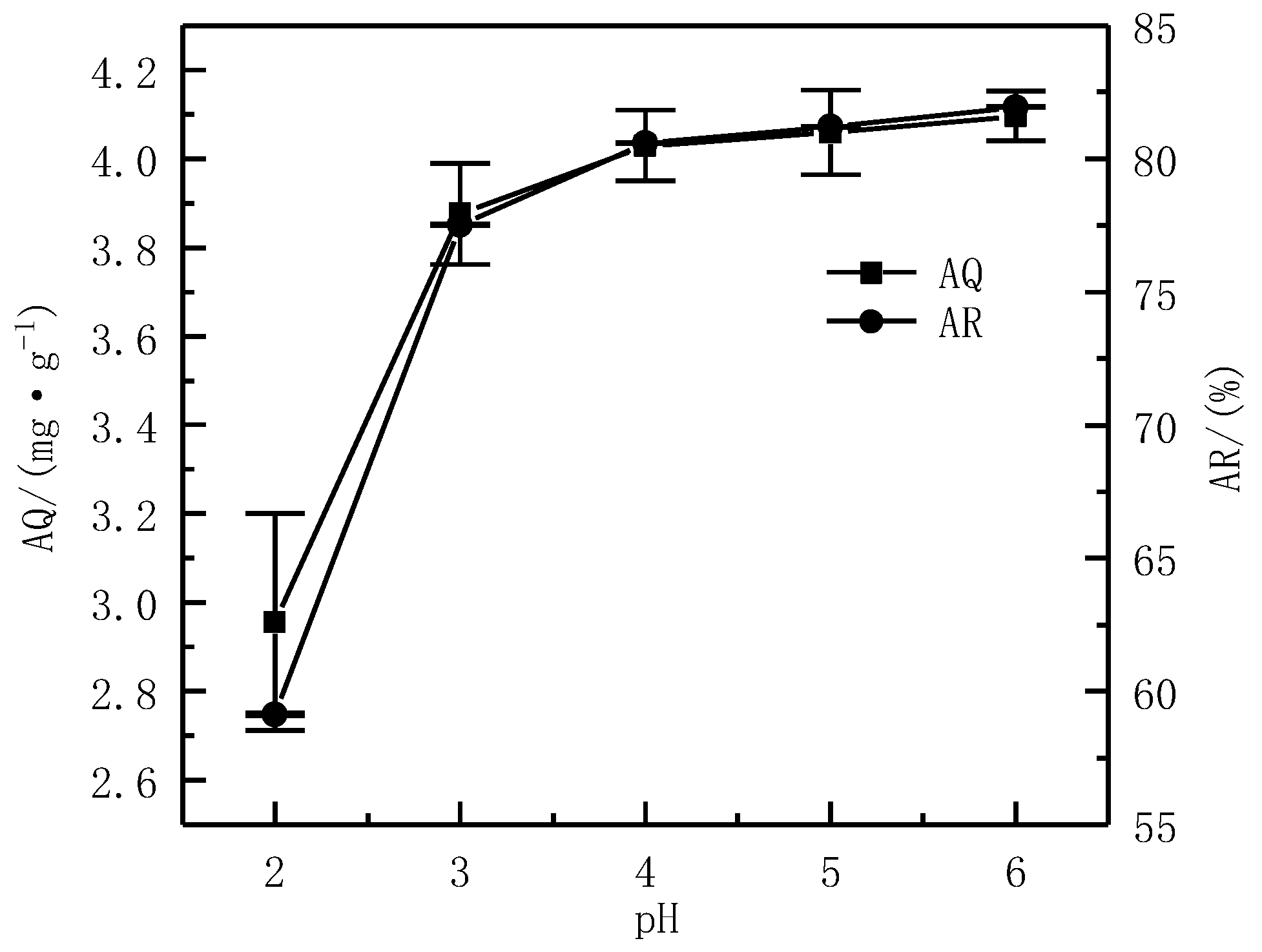

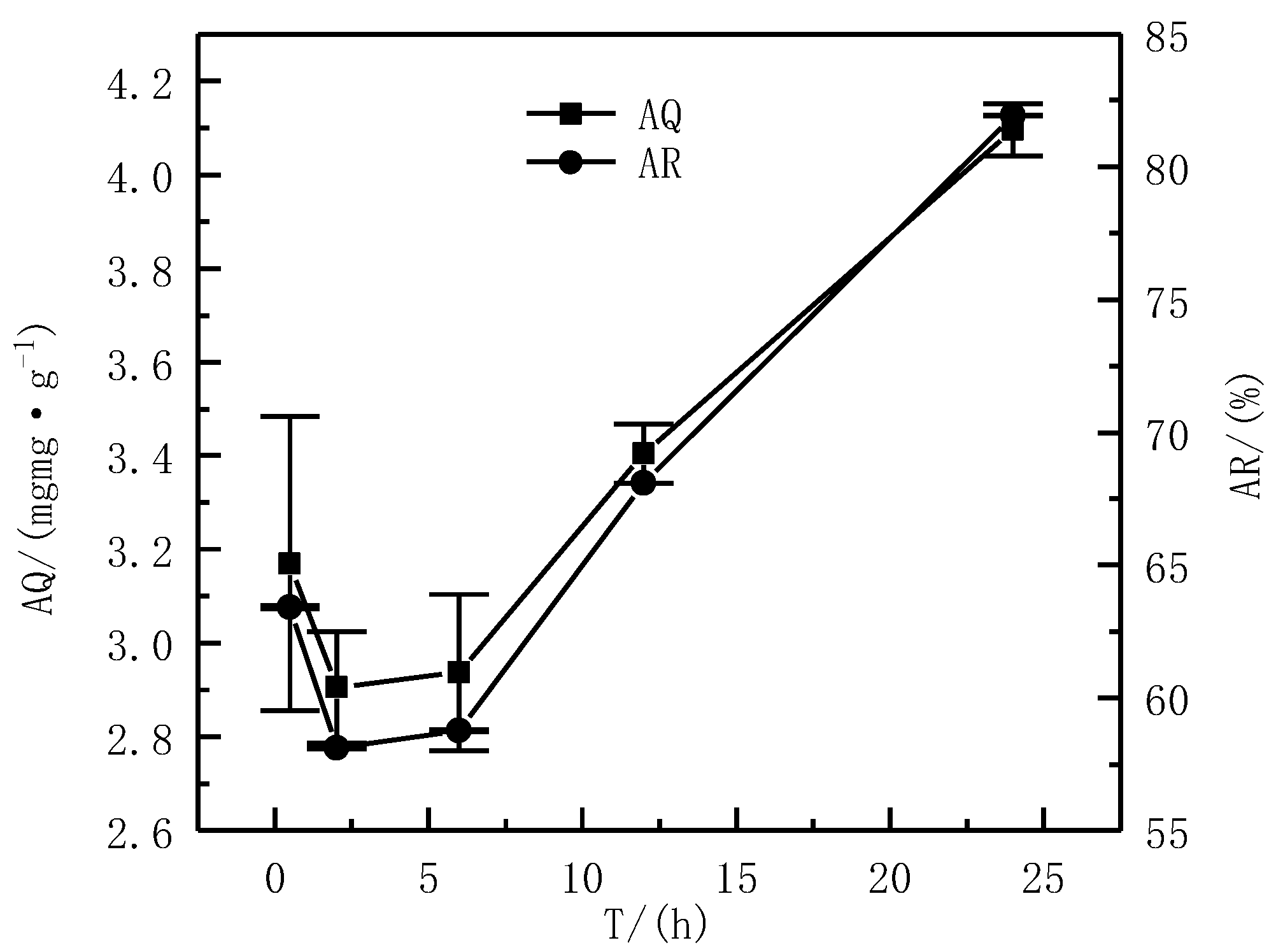
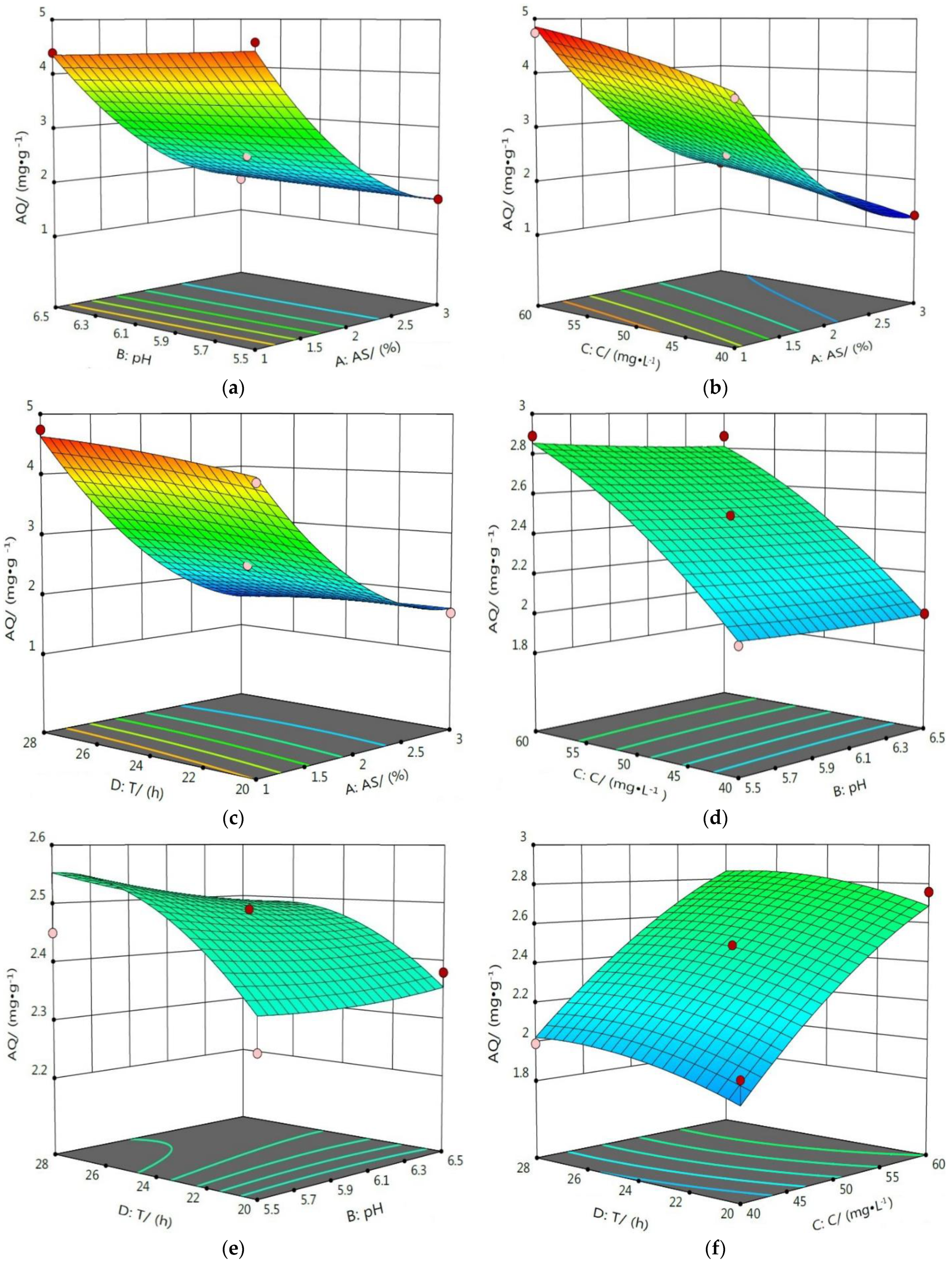

| Composition | SiO2 | MgO | CaO | Al2O3 | Fe2O3 |
|---|---|---|---|---|---|
| Content (%) | 52.93 | 46.52 | 0.85 | 0.34 | 0.36 |
| Factor | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A AS (%) | 1 | 2 | 3 |
| B pH | 5.5 | 6 | 6.5 |
| C C (mg·L−1) | 40 | 50 | 60 |
| D T (h) | 20 | 24 | 28 |
| S-T | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Aperture (nm) |
|---|---|---|---|
| S-0 | 6.1 | 0.017 | 11.01 |
| S-700 | 13.7 | 0.028 | 8.10 |
| S-T | Temperature | Langmuir | ||
|---|---|---|---|---|
| R2 | qm/(mg∙g−1) | KL | ||
| S-0 | 25 °C | 0.9883 | 7.6982 | 0.0536 |
| 35 °C | 0.9651 | 9.1971 | 0.0428 | |
| 45 °C | 0.9670 | 9.6246 | 0.0585 | |
| S-700 | 25 °C | 0.9816 | 9.8774 | 0.1185 |
| 35 °C | 0.9527 | 11.0693 | 0.1379 | |
| 45 °C | 0.9696 | 12.6904 | 0.1594 | |
| Experimental No. | A | B | C | D | Y1 AQ (mg·g−1) | Y2 AR (%) |
|---|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 0 | 4.65 ± 0.0803 | 92.99 ± 1.61 |
| 2 | 0 | −1 | −1 | 0 | 1.99 ± 0.0008 | 99.39 ± 0.04 |
| 3 | −1 | 0 | 0 | 1 | 4.33 ± 0.0243 | 86.56 ± 0.49 |
| 4 | 0 | 0 | 0 | 0 | 2.49 ± 0.0037 | 99.75 ± 0.15 |
| 5 | 0 | 0 | 0 | 0 | 2.48 ± 0.0055 | 99.21 ± 0.22 |
| 6 | −1 | 1 | 0 | 0 | 4.39 ± 0.0254 | 87.84 ± 0.51 |
| 7 | 0 | 0 | 1 | −1 | 2.76 ± 0.0295 | 92.10 ± 0.98 |
| 8 | 0 | 0 | −1 | 1 | 1.99 ± 0.0018 | 99.29 ± 0.09 |
| 9 | 0 | 1 | 0 | 1 | 2.46 ± 0.0172 | 98.58 ± 0.69 |
| 10 | 1 | 0 | 1 | 0 | 1.97 ± 0.0015 | 98.59 ± 0.07 |
| 11 | 0 | 1 | 1 | 0 | 2.87 ± 0.0033 | 95.78 ± 0.11 |
| 12 | 0 | 1 | −1 | 0 | 1.99 ± 0.0004 | 99.70 ± 0.02 |
| 13 | 1 | 1 | 0 | 0 | 1.65 ± 0.0031 | 99.17 ± 0.18 |
| 14 | 0 | −1 | 1 | 0 | 2.89 ± 0.0122 | 96.28 ± 0.41 |
| 15 | 1 | 0 | 0 | −1 | 1.66 ± 0.0006 | 99.41 ± 0.03 |
| 16 | −1 | 0 | −1 | 0 | 3.74 ± 0.0466 | 93.53 ± 1.17 |
| 17 | 0 | 1 | 0 | −1 | 2.38 ± 0.0152 | 95.23 ± 0.61 |
| 18 | 0 | −1 | 0 | 1 | 2.45 ± 0.0085 | 98.14 ± 0.34 |
| 19 | 0 | 0 | −1 | −1 | 1.96 ± 0.0069 | 98.10 ± 0.35 |
| 20 | 1 | −1 | 0 | 0 | 1.66 ± 0.0003 | 99.75 ± 0.02 |
| 21 | 0 | −1 | 0 | −1 | 2.29 ± 0.0044 | 91.72 ± 0.18 |
| 22 | 1 | 0 | −1 | 0 | 1.33 ± 0 | 99.49 ± 0 |
| 23 | 0 | 0 | 0 | 0 | 2.48 ± 0.0121 | 99.14 ± 0.48 |
| 24 | 1 | 0 | 0 | 1 | 1.66 ± 0.0006 | 99.45 ± 0.04 |
| 25 | −1 | 0 | 0 | −1 | 4.02 ± 0.1325 | 80.46 ± 2.65 |
| 26 | 0 | 0 | 0 | 0 | 2.48 ± 0.0005 | 99.03 ± 0.02 |
| 27 | 0 | 0 | 1 | 1 | 2.77 ± 0.0301 | 92.48 ± 1.00 |
| 28 | −1 | 0 | 1 | 0 | 4.74 ± 0.1982 | 79.06 ± 3.30 |
| 29 | 0 | 0 | 0 | 0 | 2.48 ± 0.0032 | 99.27 ± 0.13 |
| Source of the Variance | Square-Sum | Degree of Freedom | Average Variance | F Value | p Value | Significancy |
|---|---|---|---|---|---|---|
| Model | 25.68 | 14 | 1.83 | 222.24 | <0.0001 | ** |
| A | 21.2 | 1 | 21.2 | 2568.79 | <0.0001 | ** |
| B | 0.0026 | 1 | 0.0026 | 0.3153 | 0.5833 | |
| C | 2.10 | 1 | 2.10 | 254.23 | <0.0001 | ** |
| D | 0.0285 | 1 | 0.0285 | 3.46 | 0.0842 | |
| AB | 0.0154 | 1 | 0.0154 | 1.86 | 0.1940 | |
| AC | 0.0319 | 1 | 0.0319 | 3.86 | 0.0696 | |
| AD | 0.0232 | 1 | 0.0232 | 2.81 | 0.1160 | |
| BC | 0.0001 | 1 | 0.0001 | 0.0137 | 0.9083 | |
| BD | 0.0015 | 1 | 0.0015 | 0.1782 | 0.6793 | |
| CD | 0.0000 | 1 | 0.0000 | 0.0047 | 0.9461 | |
| A2 | 1.91 | 1 | 1.91 | 230.86 | <0.0001 | ** |
| B2 | 0.0050 | 1 | 0.0050 | 0.6097 | 0.4479 | |
| C2 | 0.0212 | 1 | 0.0212 | 2.57 | 0.1309 | |
| D2 | 0.0536 | 1 | 0.0536 | 6.50 | 0.0232 | * |
| Residual | 0.1155 | 14 | 0.0083 | |||
| Lack of fit | 0.1153 | 10 | 0.0115 | 239.23 | <0.0001 | ** |
| Pure Error | 0.0002 | 4 | 0.0000 | |||
| Cor Total | 25.79 | 28 |
| Source of the Variance | Square-Sum | Degree of Freedom | Average Variance | F Value | p Value | Significancy |
|---|---|---|---|---|---|---|
| Model | 854.14 | 14 | 61.01 | 15.03 | <0.0001 | ** |
| A | 474.01 | 1 | 474.01 | 116.76 | <0.0001 | ** |
| B | 0.3234 | 1 | 0.3234 | 0.0797 | 0.7819 | |
| C | 103.31 | 1 | 103.31 | 25.45 | 0.0002 | ** |
| D | 25.46 | 1 | 25.46 | 6.27 | 0.0253 | * |
| AB | 5.22 | 1 | 5.22 | 1.29 | 0.2758 | |
| AC | 46.04 | 1 | 46.04 | 11.34 | 0.0046 | ** |
| AD | 9.18 | 1 | 9.18 | 2.26 | 0.1549 | |
| BC | 0.1640 | 1 | 0.1640 | 0.0404 | 0.8436 | |
| BD | 2.36 | 1 | 2.36 | 0.5804 | 0.4588 | |
| CD | 0.1640 | 1 | 0.1640 | 0.0404 | 0.8436 | |
| A2 | 150.36 | 1 | 150.36 | 37.04 | <0.0001 | ** |
| B2 | 0.0062 | 1 | 0.0062 | 0.0015 | 0.9695 | |
| C2 | 12.32 | 1 | 12.32 | 3.04 | 0.1034 | |
| D2 | 55.01 | 1 | 55.01 | 13.55 | 0.0025 | ** |
| Residual | 56.84 | 14 | 4.06 | |||
| Lack of fit | 56.53 | 10 | 5.65 | 73.41 | 0.0004 | ** |
| Pure Error | 0.3080 | 4 | 0.0770 | |||
| Cor Total | 910.98 | 28 |
| Experimental No. | AQ (mg·g−1) | AR (%) |
|---|---|---|
| 1 | 3.95 | 95.36 |
| 2 | 3.85 | 94.11 |
| 3 | 3.93 | 94.57 |
| Average | 3.91 | 94.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Du, L.; Jin, W. Screening and Optimization of Conditions for the Adsorption of Cd2+ in Serpentine by Using Response Surface Methodology. Int. J. Environ. Res. Public Health 2022, 19, 16848. https://doi.org/10.3390/ijerph192416848
Zhang X, Du L, Jin W. Screening and Optimization of Conditions for the Adsorption of Cd2+ in Serpentine by Using Response Surface Methodology. International Journal of Environmental Research and Public Health. 2022; 19(24):16848. https://doi.org/10.3390/ijerph192416848
Chicago/Turabian StyleZhang, Xufeng, Liyu Du, and Wenjuan Jin. 2022. "Screening and Optimization of Conditions for the Adsorption of Cd2+ in Serpentine by Using Response Surface Methodology" International Journal of Environmental Research and Public Health 19, no. 24: 16848. https://doi.org/10.3390/ijerph192416848
APA StyleZhang, X., Du, L., & Jin, W. (2022). Screening and Optimization of Conditions for the Adsorption of Cd2+ in Serpentine by Using Response Surface Methodology. International Journal of Environmental Research and Public Health, 19(24), 16848. https://doi.org/10.3390/ijerph192416848








