Multimorbidity Patterns and Their Association with Social Determinants, Mental and Physical Health during the COVID-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Measures
2.3. Data Analysis
3. Results
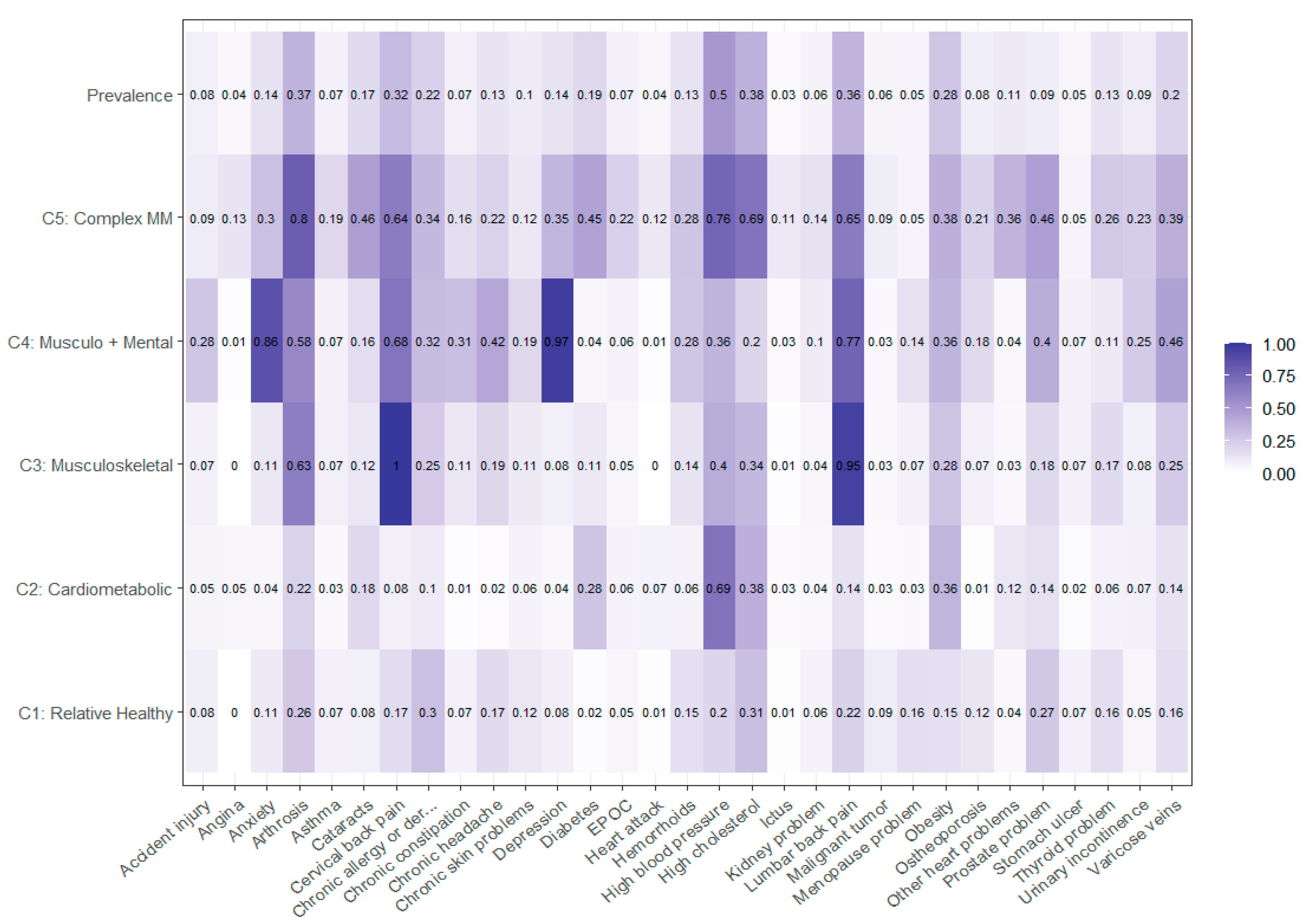
3.1. Model Selection of Multimorbidity Patterns
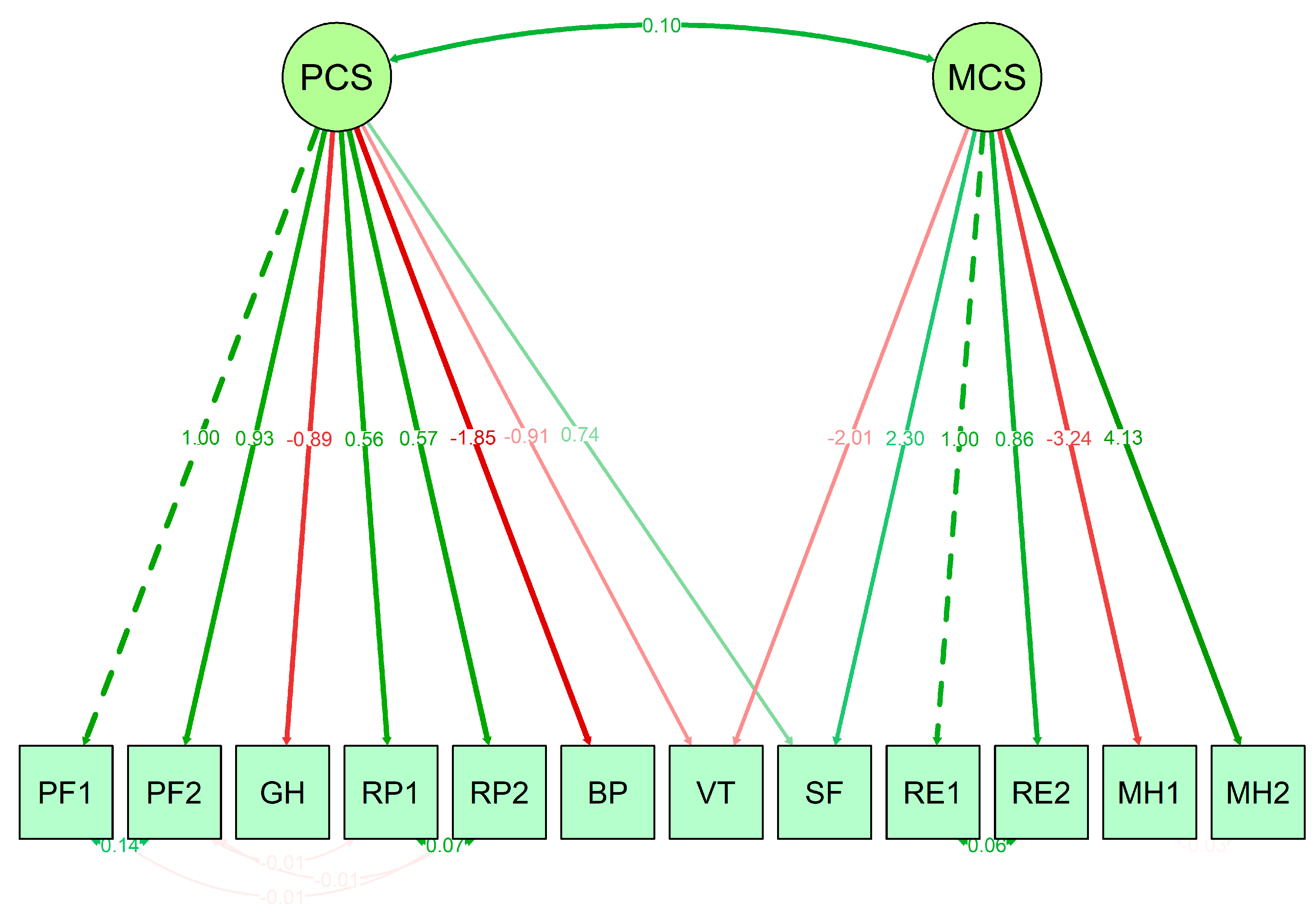
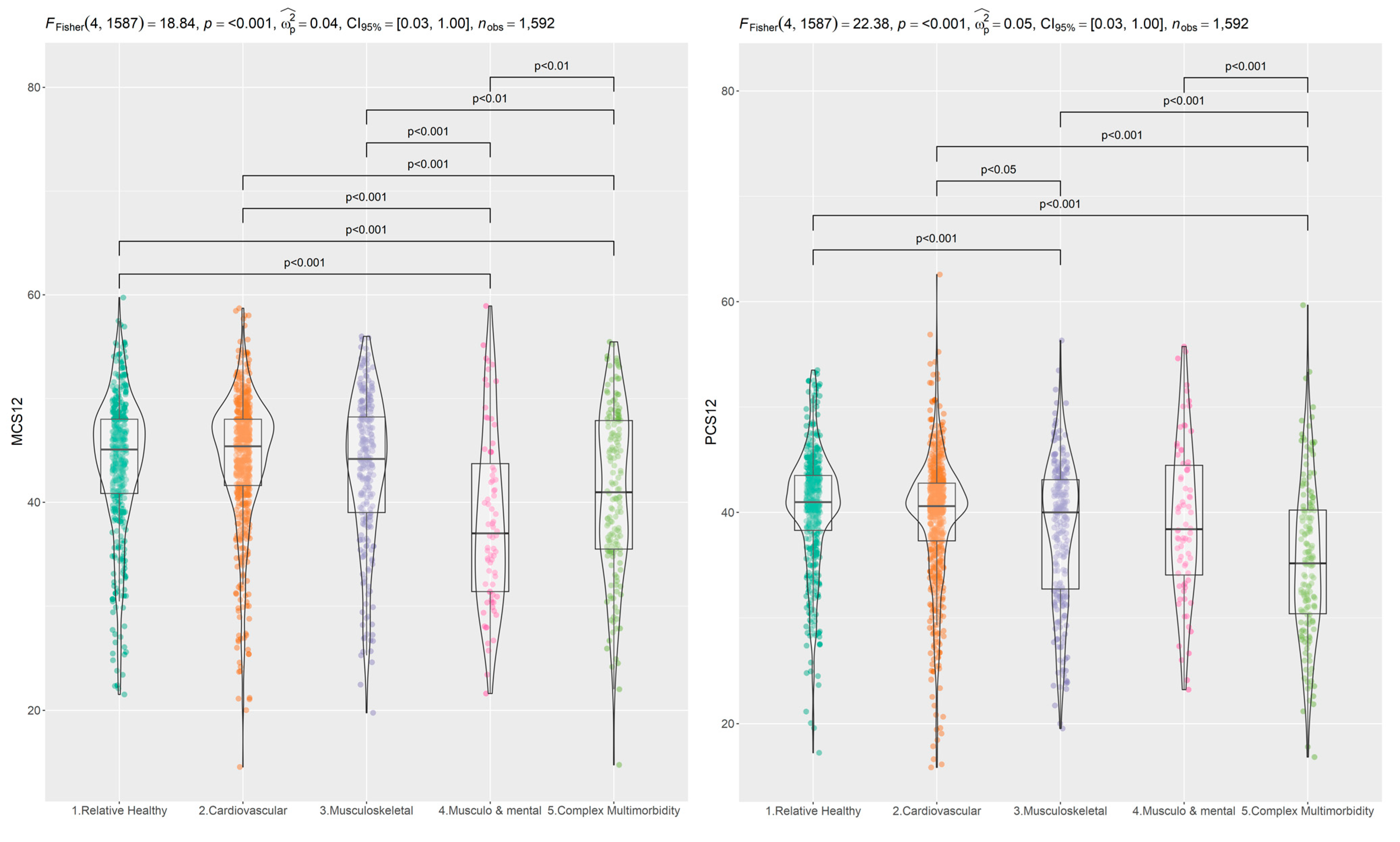
3.2. SF-12 Scale Properties and Relations
3.3. Social Determinants and Multimorbidity Patterns
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The Socio-Economic Implications of the Coronavirus Pandemic (COVID-19): A Review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Bambra, C.; Riordan, R.; Ford, J.; Matthews, F. The COVID-19 Pandemic and Health Inequalities. J. Epidemiol. Community Health (1978) 2020, 74, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Liu, J.; Liu, Q.; Kang, L.; Liu, R.; Jing, W.; Wu, Y.; Liu, M. Global Percentage of Asymptomatic SARS-CoV-2 Infections Among the Tested Population and Individuals With Confirmed COVID-19 Diagnosis: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e2137257. [Google Scholar] [CrossRef] [PubMed]
- Mohamadian, M.; Chiti, H.; Shoghli, A.; Biglari, S.; Parsamanesh, N.; Esmaeilzadeh, A. COVID-19: Virology, Biology and Novel Laboratory Diagnosis. J. Gene Med. 2021, 23, e3303. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhou, F.; Hou, W.; Silver, Z.; Wong, C.Y.; Chang, O.; Huang, E.; Zuo, Q.K. The Prevalence of Depression, Anxiety, and Sleep Disturbances in COVID-19 Patients: A Meta-Analysis. Ann. N. Y. Acad. Sci. 2021, 1486, 90–111. [Google Scholar] [CrossRef]
- Nagarajan, R.; Krishnamoorthy, Y.; Basavarachar, V.; Dakshinamoorthy, R. Prevalence of Post-Traumatic Stress Disorder among Survivors of Severe COVID-19 Infections: A Systematic Review and Meta-Analysis. J. Affect Disord. 2022, 299, 52–59. [Google Scholar] [CrossRef]
- Muñoz-Muñoz, M.; Carretero-Bravo, J.; Pérez-Muñoz, C.; Díaz-Rodríguez, M. Analysis of the Psychosocial Impact of the COVID-19 Pandemic on the Nursing Staff of the Intensive Care Units (ICU) in Spain. Healthcare 2022, 10, 796. [Google Scholar] [CrossRef]
- Vai, B.; Mazza, M.G.; Delli Colli, C.; Foiselle, M.; Allen, B.; Benedetti, F.; Borsini, A.; Casanova Dias, M.; Tamouza, R.; Leboyer, M.; et al. Mental Disorders and Risk of COVID-19-Related Mortality, Hospitalisation, and Intensive Care Unit Admission: A Systematic Review and Meta-Analysis. Lancet Psychiatry 2021, 8, 797–812. [Google Scholar] [CrossRef]
- GBD 2016 DALYs and HALE Collaborators. Global, Regional, and National Disability-Adjusted Life-Years (DALYs) for 333 Diseases and Injuries and Healthy Life Expectancy (HALE) for 195 Countries and Territories, 1990-2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1260–1344. [Google Scholar] [CrossRef]
- Pathirana, T.I.; Jackson, C.A. Socioeconomic Status and Multimorbidity: A Systematic Review and Meta-Analysis. Aust. N. Z. J. Public Health 2018, 42, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Griffith, L.E.; Gruneir, A.; Kanters, D.; Markle-Reid, M.; Ploeg, J. Functional Limitations in People with Multimorbidity and the Association with Mental Health Conditions: Baseline Data from the Canadian Longitudinal Study on Aging (CLSA). PLoS ONE 2021, 16, e0255907. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, M. Era of Geriatric Medical Challenges: Multimorbidity among Older Patients. Geriatr. Gerontol Int. 2019, 19, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Pearson-Stuttard, J.; Ezzati, M.; Gregg, E.W. Multimorbidity-a Defining Challenge for Health Systems. Lancet Public Health 2019, 4, e599–e600. [Google Scholar] [CrossRef] [PubMed]
- Hernández, B.; Voll, S.; Lewis, N.A.; McCrory, C.; White, A.; Stirland, L.; Kenny, R.A.; Reilly, R.; Hutton, C.P.; Griffith, L.E.; et al. Comparisons of Disease Cluster Patterns, Prevalence and Health Factors in the USA, Canada, England and Ireland. BMC Public Health 2021, 21, 1674. [Google Scholar] [CrossRef]
- Larsen, F.B.; Pedersen, M.H.; Friis, K.; Gluèmer, C.; Lasgaard, M. A Latent Class Analysis of Multimorbidity and the Relationship to Socio-Demographic Factors and Health-Related Quality of Life. A National Population-Based Study of 162,283 Danish Adults. PLoS ONE 2017, 12, e0169426. [Google Scholar] [CrossRef]
- Onder, G.; Palmer, K.; Navickas, R.; Jurevičiene, E.; Mammarella, F.; Strandzheva, M.; Mannucci, P.; Pecorelli, S.; Marengoni, A. Time to Face the Challenge of Multimorbidity. A European Perspective from the Joint Action on Chronic Diseases and Promoting Healthy Ageing across the Life Cycle (JA-CHRODIS). Eur. J. Intern. Med. 2015, 26, 157–159. [Google Scholar] [CrossRef]
- Gonzalez-Chica, D.A.; Hoon, E.; Stocks, N. Multimorbidity, Health-Related Quality of Life and Health Service Use among Individuals with Mental Health Problems: Urban-Rural Differences in South Australia. Aust. J. Rural Health 2020, 28, 110–119. [Google Scholar] [CrossRef]
- Subramaniam, M.; Zhang, Y.; Lau, J.H.; Vaingankar, J.A.; Abdin, E.; Chong, S.A.; Lee, E.S. Patterns of Physical Activity and Health-Related Quality of Life amongst Patients with Multimorbidity in a Multi-Ethnic Asian Population. BMC Public Health 2019, 19, 1612. [Google Scholar] [CrossRef]
- Filipčić, I.Š.; Bajić, Ž.; Filipčić, I. The Onset and Accumulation of Physical Multimorbidity in Severe and Common Mental Disorders. Curr. Opin. Psychiatry 2020, 33, 484–490. [Google Scholar] [CrossRef]
- Read, J.R.; Sharpe, L.; Modini, M.; Dear, B.F. Multimorbidity and Depression: A Systematic Review and Meta-Analysis. J. Affect Disord. 2017, 221, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Moffat, K.; Mercer, S.W. Challenges of Managing People with Multimorbidity in Today’s Healthcare Systems. BMC Fam. Pr. 2015, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Sevick, M.A.; Trauth, J.M.; Ling, B.S.; Anderson, R.T.; Piatt, G.A.; Kilbourne, A.M.; Goodman, R.M. Patients with Complex Chronic Diseases: Perspectives on Supporting Self-Management. J. Gen. Intern. Med. 2007, 22 (Suppl. 3), 438–444. [Google Scholar] [CrossRef] [PubMed]
- Prados-Torres, A.; Calderón-Larrañaga, A.; Hancco-Saavedra, J.; Poblador-Plou, B.; van den Akker, M. Multimorbidity Patterns: A Systematic Review. J. Clin. Epidemiol. 2014, 67, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Busija, L.; Lim, K.; Szoeke, C.; Sanders, K.M.; McCabe, M.P. Do Replicable Profiles of Multimorbidity Exist? Systematic Review and Synthesis. Eur. J. Epidemiol. 2019, 34, 1025–1053. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.K.; Holden, L.; Sun, J. Identifying Comorbidity Patterns of Health Conditions via Cluster Analysis of Pairwise Concordance Statistics. Stat. Med. 2012, 31, 3393–3405. [Google Scholar] [CrossRef]
- Cornell, J.E.; Pugh, J.A.; Williams, J.W., Jr.; Kazis, L.; Lee, A.F.S.; Parchman, M.L.; Zeber, J.; Pederson, T.; Montgomery, K.A.; Hitchcock Noël, P. Multimorbidity Clusters: Clustering Binary Data From Multimorbidity Clusters: Clustering Binary Data From a Large Administrative Medical Database. Appl. Multivar. Res. 2009, 12, 163. [Google Scholar] [CrossRef]
- Kirchberger, I.; Meisinger, C.; Heier, M.; Zimmermann, A.K.; Thorand, B.; Autenrieth, C.S.; Peters, A.; Ladwig, K.H.; Döring, A. Patterns of Multimorbidity in the Aged Population. Results from the KORA-Age Study. PLoS ONE 2012, 7, e30556. [Google Scholar] [CrossRef] [PubMed]
- Olaya, B.; Moneta, M.V.; Caballero, F.F.; Tyrovolas, S.; Bayes, I.; Ayuso-Mateos, J.L.; Haro, J.M. Latent Class Analysis of Multimorbidity Patterns and Associated Outcomes in Spanish Older Adults: A Prospective Cohort Study. BMC Geriatr. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Harrison, S.L.; Fazio-Eynullayeva, E.; Lane, D.A.; Underhill, P.; Lip, G.Y.H. Comorbidities Associated with Mortality in 31,461 Adults with COVID-19 in the United States: A Federated Electronic Medical Record Analysis. PLoS Med. 2020, 17, e1003321. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Qian, L.; Hong, V.; Wei, R.; Nadjafi, R.F.; Fischer, H.; Li, Z.; Shaw, S.F.; Caparosa, S.L.; Nau, C.L.; et al. Obesity and Mortality Among Patients Diagnosed With COVID-19: Results From an Integrated Health Care Organization. Ann. Intern. Med. 2020, 173, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, G.; Grassi, G.; Borghi, C.; Ferri, C.; Salvetti, M.; Volpe Massimo, M. Age and Multimorbidity Predict Death Among COVID-19 Patients: Results of the SARS-RAS Study of the Italian Society of Hypertension. Hypertension 2020, 76, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Maddaloni, E.; D’Onofrio, L.; Alessandri, F.; Mignogna, C.; Leto, G.; Pascarella, G.; Mezzaroma, I.; Lichtner, M.; Pozzilli, P.; Agrò, F.E.; et al. Cardiometabolic Multimorbidity Is Associated with a Worse COVID-19 Prognosis than Individual Cardiometabolic Risk Factors: A Multicentre Retrospective Study (CoViDiab II). Cardiovasc. Diabetol. 2020, 19, 164. [Google Scholar] [CrossRef] [PubMed]
- Malta, D.C.; Gomes, C.S.; Barros, M.B.d.A.; Lima, M.G.; de Almeida, W.d.S.; de Sá, A.C.M.G.N.; Prates, E.J.S.; Machado, Í.E.; da Silva, D.R.P.; Werneck, A.d.O.; et al. Noncommunicable Diseases and Changes in Lifestyles during the COVID-19 Pandemic in Brazil. Rev. Bras. Epidemiol. 2021, 24, e210009. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, M.; Carretero-Bravo, J.; Pérez-Muñoz, C.; Deudero-Sánchez, M. Lockdown Due to COVID-19 in Spanish Children Up to 6 Years: Consequences on Diet, Lifestyle, Screen Viewing, and Sleep. Int. J. Public Health 2022, 67, 79. [Google Scholar] [CrossRef]
- Wister, A.; Li, L.; Best, J.R.; Cosco, T.D.; Kim, B. Multimorbidity, COVID-19 and Mental Health: Canadian Longitudinal Study on Aging (CLSA) Longitudinal Analyses. Clin. Gerontol. 2022. [Google Scholar] [CrossRef]
- Wister, A.; Li, L.; Cosco, T.D.; McMillan, J.; Griffith, L.E.; Costa, A.; Anderson, L.; Balion, C.; Kirkland, S.; Yukiko, A.; et al. Multimorbidity Resilience and COVID-19 Pandemic Self-Reported Impact and Worry among Older Adults: A Study Based on the Canadian Longitudinal Study on Aging (CLSA). BMC Geriatr. 2022, 22, 92. [Google Scholar] [CrossRef]
- Bono, F.; Matranga, D. Socioeconomic Inequality in Non-Communicable Diseases in Europe between 2004 and 2015: Evidence from the SHARE Survey. Eur. J. Public Health 2019, 29, 105–110. [Google Scholar] [CrossRef]
- Alvarez-Galvez, J. Multidimensionality of Health Inequalities: A Cross-Country Identification of Health Clusters through Multivariate Classification Techniques. Int. J. Environ. Res. Public Health 2018, 15, 1900. [Google Scholar] [CrossRef]
- Global Burden of Disease Study 2013 Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 301 Acute and Chronic Diseases and Injuries in 188 Countries, 1990-2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef] [PubMed]
- Northwood, M.; Ploeg, J.; Markle-Reid, M.; Sherifali, D. Integrative Review of the Social Determinants of Health in Older Adults with Multimorbidity. J. Adv. Nurs. 2018, 74, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Galán, I.; Rodríguez-Artalejo, F.; Zorrilla, B. Telephone versus Face-to-Face Household Interviews in the Assessment of Health Behaviors and Preventive Practices. Gac. Sanit 2004, 18, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Donovan, R.J.; Holman, C.D.A.J.; Corti, B.; Jalleh, G. Face-to-Face Household Interviews versus Telephone Interviews for Health Surveys. Aust. N. Z. J. Public Health 1997, 21, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Estadística Cádiz: Población Por Municipios y Sexo. Available online: https://www.ine.es/jaxiT3/Tabla.htm?t=2864&L=0 (accessed on 5 December 2022).
- Instituto Nacional de Estadística Tasas de Actividad, Paro y Empleo Por Provincia y Sexo. Available online: https://www.ine.es/jaxiT3/Tabla.htm?t=3996 (accessed on 5 December 2022).
- Instituto Nacional de Estadística. Encuesta Europea de Salud En España 2020; Instituto Nacional de Estadística: Madrid, Spain, 2021. [Google Scholar]
- Ware, J.E.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Kosinski, M.A.; Keller, S.D. How to Score the SF-12 Physical and Mental Health Summary Scales; The Health Institute, New England Medical Centet: Boston, MA, USA, 1995. [Google Scholar]
- Weller, B.E.; Bowen, N.K.; Faubert, S.J. Latent Class Analysis: A Guide to Best Practice. J. Black Psychol. 2020, 46, 287–311. [Google Scholar] [CrossRef]
- Nylund, K.L.; Asparouhov, T.; Muthén, B.O. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Struct. Equ. Model. A Multidiscip. J. 2007, 14, 535–569. [Google Scholar] [CrossRef]
- Dziak, J.J.; Lanza, S.T.; Tan, X. Effect Size, Statistical Power and Sample Size Requirements for the Bootstrap Likelihood Ratio Test in Latent Class Analysis. Struct. Equ. Model. 2014, 21, 534. [Google Scholar] [CrossRef]
- Shou, J.; Ren, L.; Wang, H.; Yan, F.; Cao, X.; Wang, H.; Wang, Z.; Zhu, S.; Liu, Y. Reliability and Validity of 12-Item Short-Form Health Survey (SF-12) for the Health Status of Chinese Community Elderly Population in Xujiahui District of Shanghai. Aging Clin. Exp. Res. 2016, 28, 339–346. [Google Scholar] [CrossRef]
- Watkins, M.W. Exploratory Factor Analysis: A Guide to Best Practice. J. Black Psychol. 2018, 44, 219–246. [Google Scholar] [CrossRef]
- Rigdon, E.E. CFI versus RMSEA: A Comparison of Two Fit Indexes for Structural Equation Modeling. Struct. Equ. Model. 1996, 3, 369–379. [Google Scholar] [CrossRef]
- Asparouhov, T.; Muthén, B. Auxiliary Variables in Mixture Modeling: Three-Step Approaches Using Mplus. Struct. Equ. Model. A Multidiscip. J. 2014, 21, 329–341. [Google Scholar] [CrossRef]
- Patil, I. Visualizations with Statistical Details: The “ggstatsplot” Approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Ng, S.K.; Tawiah, R.; Sawyer, M.; Scuffham, P. Patterns of Multimorbid Health Conditions: A Systematic Review of Analytical Methods and Comparison Analysis. Int. J. Epidemiol. 2018, 47, 1687–1704. [Google Scholar] [CrossRef]
- Khorrami, Z.; Rezapour, M.; Etemad, K.; Yarahmadi, S.; Khodakarim, S.; Mahdavi Hezaveh, A.; Kameli, M.; Khanjani, N. The Patterns of Non-Communicable Disease Multimorbidity in Iran: A Multilevel Analysis. Sci. Rep. 2020, 10, 3034. [Google Scholar] [CrossRef]
- Zheng, D.D.; McCollister, K.E.; Christ, S.L.; Lam, B.L.; Feaster, D.J.; Lee, D.J. Chronic Condition Patterns in the US Population and Their Association with Health Related Quality of Life. Prev. Med. (Balt.) 2020, 136, 106102. [Google Scholar] [CrossRef]
- Afshar, S.; Roderick, P.J.; Kowal, P.; Dimitrov, B.D.; Hill, A.G. Multimorbidity and the Inequalities of Global Ageing: A Cross-Sectional Study of 28 Countries Using the World Health Surveys. BMC Public Health 2015, 15, 776. [Google Scholar] [CrossRef]
- Cohen, M.R. Technical Series on Safer Primary Care: Multimorbidity; World Health Organization: Geneva, Switzerland, 2016; Volume 47. [Google Scholar]
- Garin, N.; Koyanagi, A.; Chatterji, S.; Tyrovolas, S.; Olaya, B.; Leonardi, M.; Lara, E.; Koskinen, S.; Tobiasz-Adamczyk, B.; Ayuso-Mateos, J.L.; et al. Global Multimorbidity Patterns: A Cross-Sectional, Population-Based, Multi-Country Study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 205–214. [Google Scholar] [CrossRef]
- Loza, E.; Jover, J.A.; Rodriguez, L.; Carmona, L. Multimorbidity: Prevalence, Effect on Quality of Life and Daily Functioning, and Variation of This Effect When One Condition Is a Rheumatic Disease. Semin. Arthritis. Rheum. 2009, 38, 312–319. [Google Scholar] [CrossRef]
- Pati, S.; Swain, S.; Knottnerus, J.A.; Metsemakers, J.F.M.; van den Akker, M. Health Related Quality of Life in Multimorbidity: A Primary-Care Based Study from Odisha, India. Health Qual Life Outcomes 2019, 17, 116. [Google Scholar] [CrossRef]
- Vilagut, G.; Valderas, J.M.; Ferrer, M.; Garin, O.; López-García, E.; Alonso, J. Interpretación de Los Cuestionarios de Salud SF-36 y SF-12 En España: Componentes Físico y Mental. Med. Clin. (Barc) 2008, 130, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Gandek, B.; Ware, J.E.; Aaronson, N.K.; Apolone, G.; Bjorner, J.B.; Brazier, J.E.; Bullinger, M.; Kaasa, S.; Leplege, A.; Prieto, L.; et al. Cross-Validation of Item Selection and Scoring for the SF-12 Health Survey in Nine Countries: Results from the IQOLA Project. J. Clin. Epidemiol. 1998, 51, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.R.; Halling, A.; Andersen-Ranberg, K. Disparities in Multimorbidity across Europe—Findings from the SHARE Survey. Eur. Geriatr. Med. 2017, 8, 16–21. [Google Scholar] [CrossRef]
- Walker, V.; Perret-Guillaume, C.; Kesse-Guyot, E.; Agrinier, N.; Hercberg, S.; Galan, P.; Assmann, K.E.; Briançon, S.; Rotonda, C. Effect of Multimorbidity on Health-Related Quality of Life in Adults Aged 55 Years or Older: Results from the SU.VI.MAX 2 Cohort. PLoS ONE 2016, 11, e0169282. [Google Scholar] [CrossRef]
- Kanesarajah, J.; Waller, M.; Whitty, J.A.; Mishra, G.D. Multimorbidity and Quality of Life at Mid-Life: A Systematic Review of General Population Studies. Maturitas 2018, 109, 53–62. [Google Scholar] [CrossRef]
- Xiong, J.; Lipsitz, O.; Nasri, F.; Lui, L.M.W.; Gill, H.; Phan, L.; Chen-Li, D.; Iacobucci, M.; Ho, R.; Majeed, A.; et al. Impact of COVID-19 Pandemic on Mental Health in the General Population: A Systematic Review. J. Affect Disord 2020, 277, 55–64. [Google Scholar] [CrossRef]
- Sonza, A.; da Cunha de Sá-Caputo, D.; Sartorio, A.; Tamini, S.; Seixas, A.; Sanudo, B.; Süßenbach, J.; Provenza, M.M.; Xavier, V.L.; Taiar, R.; et al. COVID-19 Lockdown and the Behavior Change on Physical Exercise, Pain and Psychological Well-Being: An International Multicentric Study. Int. J. Environ. Res. Public Health 2021, 18, 3810. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple Early Factors Anticipate Post-Acute COVID-19 Sequelae. Cell 2022, 185, 881–895. [Google Scholar] [CrossRef]
- Latre, M.L.; Andrés, E.M.; Cordero, A.; Pascual, I.; Vispe, C.; Laclaustra, M.; Luengo, E.; Casasnovas, J.A. Relación Entre El Síndrome Metabólico y La Mortalidad Por Cardiopatía Isquémica En España. Rev. Esp. Cardiol. 2009, 62, 1469–1472. [Google Scholar] [CrossRef]
- Jackson, C.A.; Dobson, A.J.; Tooth, L.R.; Mishra, G.D. Lifestyle and Socioeconomic Determinants of Multimorbidity Patterns among Mid-Aged Women: A Longitudinal Study. PLoS ONE 2016, 11, e0156804. [Google Scholar] [CrossRef]
- Bayes-Marin, I.; Sanchez-Niubo, A.; Egea-Cortés, L.; Nguyen, H.; Prina, M.; Fernández, D.; Haro, J.M.; Olaya, B. Multimorbidity Patterns in Low-Middle and High Income Regions: A Multiregion Latent Class Analysis Using ATHLOS Harmonised Cohorts. BMJ. Open 2020, 10, e034441. [Google Scholar] [CrossRef] [PubMed]
- Prenovost, K.M.; Fihn, S.D.; Maciejewski, M.L.; Nelson, K.; Vijan, S.; Rosland, A.M. Using Item Response Theory with Health System Data to Identify Latent Groups of Patients with Multiple Health Conditions. PLoS ONE 2018, 13, e0206915. [Google Scholar] [CrossRef] [PubMed]
- Aughterson, H.; Baxter, L.; Fancourt, D. Social Prescribing for Individuals with Mental Health Problems: A Qualitative Study of Barriers and Enablers Experienced by General Practitioners. BMC Fam Pr. 2020, 21, 194. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Gálvez, J.; Carretero-Bravo, J.; Suárez-Lledó, V.; Ortega-Martín, E.; Ramos-Fiol, B.; Lagares-Franco, C.; O’Ferrall-González, C.; Almenara-Barrios, J.; González-Caballero, J.L. Social Inequalities in Multimorbidity Patterns in Europe: A Multilevel Latent Class Analysis in the European Social Survey (ESS). SSM Popul Health 2022, 20, 101268. [Google Scholar] [CrossRef]
- Buja, A.; Rivera, M.; de Battisti, E.; Corti, M.C.; Avossa, F.; Schievano, E.; Rigon, S.; Baldo, V.; Boccuzzo, G.; Ebell, M.H. Multimorbidity and Hospital Admissions in High-Need, High-Cost Elderly Patients. J. Aging Health 2020, 32, 259–268. [Google Scholar] [CrossRef]
- Violán, C.; Bejarano-Rivera, N.; Foguet-Boreu, Q.; Roso Llorach, A.; Pons-Vigués, M.; Martin Mateo, M.; Pujol-Ribera, E. The Burden of Cardiovascular Morbidity in a European Mediterranean Population with Multimorbidity: A Cross-Sectional Study. BMC Fam Pr. 2016, 17, 150. [Google Scholar] [CrossRef]
- Wallace, E.; Salisbury, C.; Guthrie, B.; Lewis, C.; Fahey, T.; Smith, S.M. Managing Patients with Multimorbidity in Primary Care. BMJ 2015, 350, h176. [Google Scholar] [CrossRef]
| Classes | CAIC | BIC | ABIC | Entropy | BLRT p-Value |
|---|---|---|---|---|---|
| 2 | 36,327.68 | 36,264.68 | 36,064.54 | 0.763 | 0.44 |
| 3 | 36,250.13 | 36,155.13 | 35,853.33 | 0.651 | 0.46 |
| 4 | 36,321.47 | 36,194.47 | 35,791.02 | 0.703 | 0.40 |
| 5 | 36,436.36 | 36,277.36 | 35,772.25 | 0.728 | 0.32 |
| 6 | 36,579.01 | 36,388.01 | 35,781.24 | 0.700 | 0.40 |
| 7 | 36,744.60 | 36,521.60 | 35,813.17 | 0.720 | 0.44 |
| 8 | 36,920.61 | 36,665.61 | 35,855.53 | 0.739 | 0.22 |
| C1—Relative Healthy | C2—Cardio-Metabolic | C3—Musculo-Skeletal | C4—Musculo-Skeletal and Mental | C5—Complex Multimorbidity | |
|---|---|---|---|---|---|
| MCS 1 | |||||
| NO COVID-19 | 44.171 | 44.526 | 43.358 | 38.238 | 41.017 |
| COVID-19 | 42.253 | 42.918 | 42.530 | 38.450 | 40.734 |
| t = −2.274 p = 0.024 * | t = −2.391 p = 0.017 * | t = −0.732 p = 0.466 | t = 0.100 p = 0.921 | t = −0.149 p = 0.883 | |
| PCS 2 | |||||
| NO COVID-19 | 40.806 | 39.618 | 37.694 | 39.094 | 35.466 |
| COVID-19 | 39.025 | 39.369 | 38.357 | 39.350 | 34.699 |
| t = −2.506 p = 0.013 * | t= −0.424 p = 0.672 | t = −0.613 p = 0.541 | t = −0.123 p = 0.901 | t = −0.466 p = 0.643 |
| C1—Relative Healthy (Ref.) | C2—Cardio- metabolic | C3—Musculo- skeletal | C4—Musculo- skeletal and Mental | C5—Complex Multimorbidity |
|---|---|---|---|---|
| Category | OR (95% IC) | OR (95% IC) | OR (95% IC) | OR (95% IC) |
| MCS12 (mental) | 0.98 (0.956, 1.005) | 0.949 (0.921, 0.977) *** | 0.949 (0.907, 0.992) * | 0.892 (0.861, 0.924) *** |
| PCS12 (physical) | 0.986 (0.964, 1.009) | 0.971 (0.948, 0.996) * | 0.908 (0.874, 0.942) *** | 0.911 (0.882, 0.941) *** |
| Gender | ||||
| Female (Ref.) | 1 | 1 | 1 | 1 |
| Male | 3.013 (2.193, 4.141) *** | 1.197 (0.793, 1.807) | 1.233 (0.611, 2.488) | 1.134 (0.644, 1.996) |
| Age | ||||
| 50–59 (Ref.) | 1 | 1 | 1 | 1 |
| 60–69 | 2.06 (1.439, 2.95) *** | 1.465 (0.963, 2.228) | 0.78 (0.385, 1.581) | 2.871 (1.474, 5.593) ** |
| >69 | 3.417 (2.184, 5.346) *** | 1.597 (0.922, 2.765) | 1.001 (0.419, 2.392) | 8.159 (3.875, 17.183) *** |
| Disability | ||||
| Yes (Ref.) | 1 | 1 | 1 | 1 |
| No | 0.943 (0.63, 1.413) | 2.086 (1.329, 3.275) ** | 1.016 (0.487, 2.118) | 1.967 (1.147, 3.371) * |
| Education | ||||
| No Education (Ref.) | 1 | 1 | 1 | 1 |
| Primary | 0.909 (0.571, 1.447) | 1.317 (0.723, 2.398) | 0.985 (0.424, 2.288) | 0.811 (0.447, 1.473) |
| Secondary | 0.843 (0.51, 1.392) | 1.047 (0.549, 2) | 1.089 (0.432, 2.742) | 0.358 (0.171, 0.746) ** |
| University | 0.831 (0.472, 1.462) | 0.827 (0.386, 1.77) | 1.332 (0.437, 4.063) | 0.318 (0.128, 0.791) * |
| Fruits and Vegetables | ||||
| One per week or less (Ref.) | 1 | 1 | 1 | 1 |
| 4 times per week | 0.965 (0.583, 1.596) | 1.073 (0.57, 2.02) | 1.461 (0.533, 4.004) | 0.985 (0.447, 2.171) |
| One per day | 0.891 (0.534, 1.487) | 1.174 (0.618, 2.233) | 0.803 (0.265, 2.439) | 0.859 (0.378, 1.953) |
| Two or more per day | 0.91 (0.552, 1.5) | 0.986 (0.525, 1.853) | 1.325 (0.479, 3.661) | 0.896 (0.403, 1.991) |
| Physical Activity | ||||
| Never (Ref.) | 1 | 1 | 1 | 1 |
| One per month or less | 1.127 (0.623, 2.039) | 1.215 (0.606, 2.436) | 0.773 (0.278, 2.147) | 0.794 (0.29, 2.174) |
| Several times per week | 0.912 (0.644, 1.292) | 0.804 (0.525, 1.232) | 0.353 (0.171, 0.729) ** | 0.768 (0.446, 1.323) |
| All days | 1.064 (0.747, 1.514) | 0.773 (0.496, 1.203) | 0.395 (0.194, 0.804) ** | 0.849 (0.489, 1.472) |
| Alcohol Consumption | ||||
| Never (Ref.) | 1 | 1 | 1 | 1 |
| One per month or less | 1.251 (0.711, 2.198) | 1.214 (0.627, 2.353) | 0.855 (0.313, 2.333) | 1.691 (0.78, 3.668) |
| One per week or less | 1.061 (0.74, 1.522) | 0.88 (0.565, 1.372) | 0.433 (0.2, 0.935) * | 0.799 (0.441, 1.448) |
| Several times per week | 1.031 (0.73, 1.458) | 0.773 (0.495, 1.206) | 0.383 (0.166, 0.885) * | 0.459 (0.248, 0.849) * |
| Tobacco Consumption | ||||
| Neither smokes nor has smoked (Ref.) | 1 | 1 | 1 | 1 |
| Used to smoke | 0.783 (0.578, 1.061) | 1.646 (1.111, 2.439) * | 0.844 (0.437, 1.629) | 1.327 (0.794, 2.219) |
| 1–10 cigarettes | 0.792 (0.5, 1.256) | 1.178 (0.657, 2.112) | 1.26 (0.549, 2.89) | 0.577 (0.216, 1.54) |
| >10 cigarettes | 1.084 (0.612, 1.922) | 2.119 (1.09, 4.12) * | 0.888 (0.291, 2.708) | 2.407 (0.976, 5.934) |
| Job Situation | ||||
| Active (Ref.) | 1 | 1 | 1 | 1 |
| Retiree | 0.957 (0.631, 1.452) | 0.682 (0.408, 1.141) | 1.114 (0.47, 2.642) | 1.832 (0.807, 4.158) |
| Unemployed | 0.923 (0.556, 1.532) | 0.759 (0.418, 1.378) | 0.4 (0.138, 1.16) | 1.028 (0.334, 3.165) |
| Domestic Work | 0.787 (0.488, 1.269) | 0.964 (0.555, 1.674) | 1.32 (0.556, 3.135) | 1.93 (0.808, 4.605) |
| Income | ||||
| <600 € (Ref.) | 1 | 1 | 1 | 1 |
| 601–900 € | 0.978 (0.534, 1.79) | 1.185 (0.566, 2.479) | 0.343 (0.135, 0.871) * | 0.901 (0.39, 2.079) |
| 901–1200 € | 0.701 (0.386, 1.271) | 1.531 (0.755, 3.104) | 0.374 (0.152, 0.921) * | 0.759 (0.326, 1.766) |
| 1201–1800 € | 0.769 (0.423, 1.4) | 0.847 (0.402, 1.782) | 0.285 (0.106, 0.766) * | 0.693 (0.283, 1.697) |
| >1800 € | 0.864 (0.455, 1.641) | 0.758 (0.332, 1.731) | 0.158 (0.045, 0.555) ** | 0.756 (0.271, 2.109) |
| COVID-19 | ||||
| Yes (Ref.) | 1 | 1 | 1 | 1 |
| No | 0.701 (0.498, 0.987) * | 0.778 (0.511, 1.186) | 0.844 (0.428, 1.666) | 0.771 (0.446, 1.331) |
| Primary Attention Visit | ||||
| No (Ref.) | 1 | 1 | 1 | 1 |
| Yes | 1.226 (0.892, 1.684) | 1.675 (1.073, 2.617) * | 2.086 (0.896, 4.857) | 2.005 (1.072, 3.751) * |
| Emergencies Visit | ||||
| No (Ref.) | 1 | 1 | 1 | 1 |
| Yes | 1.03 (0.761, 1.395) | 1.255 (0.874, 1.801) | 1.179 (0.665, 2.089) | 1.647 (1.053, 2.575) * |
| Hospital Admission | ||||
| No (Ref.) | 1 | 1 | 1 | 1 |
| Yes | 1.138 (0.701, 1.849) | 1.222 (0.696, 2.147) | 1.191 (0.508, 2.793) | 1.727 (0.924, 3.228) |
| Specialist Visit | ||||
| No (Ref.) | 1 | 1 | 1 | 1 |
| Yes | 0.584 (0.442, 0.772) *** | 0.814 (0.572, 1.159) | 1.333 (0.748, 2.377) | 1.092 (0.696, 1.712) |
| Administrative Regions | ||||
| Cadiz Bay (Ref.) | 1 | 1 | 1 | 1 |
| Jerez and Rural | 1.199 (0.792, 1.815) | 1.023 (0.598, 1.748) | 0.947 (0.392, 2.288) | 1.435 (0.693, 2.968) |
| Gibraltar Zone | 0.921 (0.552, 1.535) | 1.066 (0.558, 2.037) | 1.082 (0.379, 3.088) | 1.164 (0.471, 2.878) |
| Northwest Coast | 0.985 (0.582, 1.664) | 1.199 (0.631, 2.28) | 0.889 (0.298, 2.65) | 1.591 (0.667, 3.799) |
| La Janda | 1.651 (0.96, 2.837) | 1.067 (0.531, 2.144) | 1.041 (0.345, 3.143) | 1.223 (0.487, 3.07) |
| Cadiz Mountains | 1.153 (0.681, 1.95) | 1.22 (0.634, 2.346) | 1.134 (0.395, 3.258) | 1.192 (0.497, 2.859) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carretero-Bravo, J.; Ramos-Fiol, B.; Ortega-Martín, E.; Suárez-Lledó, V.; Salazar, A.; O’Ferrall-González, C.; Dueñas, M.; Peralta-Sáez, J.L.; González-Caballero, J.L.; Cordoba-Doña, J.A.; et al. Multimorbidity Patterns and Their Association with Social Determinants, Mental and Physical Health during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 16839. https://doi.org/10.3390/ijerph192416839
Carretero-Bravo J, Ramos-Fiol B, Ortega-Martín E, Suárez-Lledó V, Salazar A, O’Ferrall-González C, Dueñas M, Peralta-Sáez JL, González-Caballero JL, Cordoba-Doña JA, et al. Multimorbidity Patterns and Their Association with Social Determinants, Mental and Physical Health during the COVID-19 Pandemic. International Journal of Environmental Research and Public Health. 2022; 19(24):16839. https://doi.org/10.3390/ijerph192416839
Chicago/Turabian StyleCarretero-Bravo, Jesús, Begoña Ramos-Fiol, Esther Ortega-Martín, Víctor Suárez-Lledó, Alejandro Salazar, Cristina O’Ferrall-González, María Dueñas, Juan Luis Peralta-Sáez, Juan Luis González-Caballero, Juan Antonio Cordoba-Doña, and et al. 2022. "Multimorbidity Patterns and Their Association with Social Determinants, Mental and Physical Health during the COVID-19 Pandemic" International Journal of Environmental Research and Public Health 19, no. 24: 16839. https://doi.org/10.3390/ijerph192416839
APA StyleCarretero-Bravo, J., Ramos-Fiol, B., Ortega-Martín, E., Suárez-Lledó, V., Salazar, A., O’Ferrall-González, C., Dueñas, M., Peralta-Sáez, J. L., González-Caballero, J. L., Cordoba-Doña, J. A., Lagares-Franco, C., Martínez-Nieto, J. M., Almenara-Barrios, J., & Álvarez-Gálvez, J. (2022). Multimorbidity Patterns and Their Association with Social Determinants, Mental and Physical Health during the COVID-19 Pandemic. International Journal of Environmental Research and Public Health, 19(24), 16839. https://doi.org/10.3390/ijerph192416839









