Invasive Crayfish Faxonius limosus: Meat Safety, Nutritional Quality and Sensory Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Spiny-Cheek Crayfish
2.2. Microbial Safety
2.3. Chemical Composition
2.4. Fatty Acid Composition
2.5. Amino Acid Composition
2.6. Macro and Micro Elements and Heavy Metals
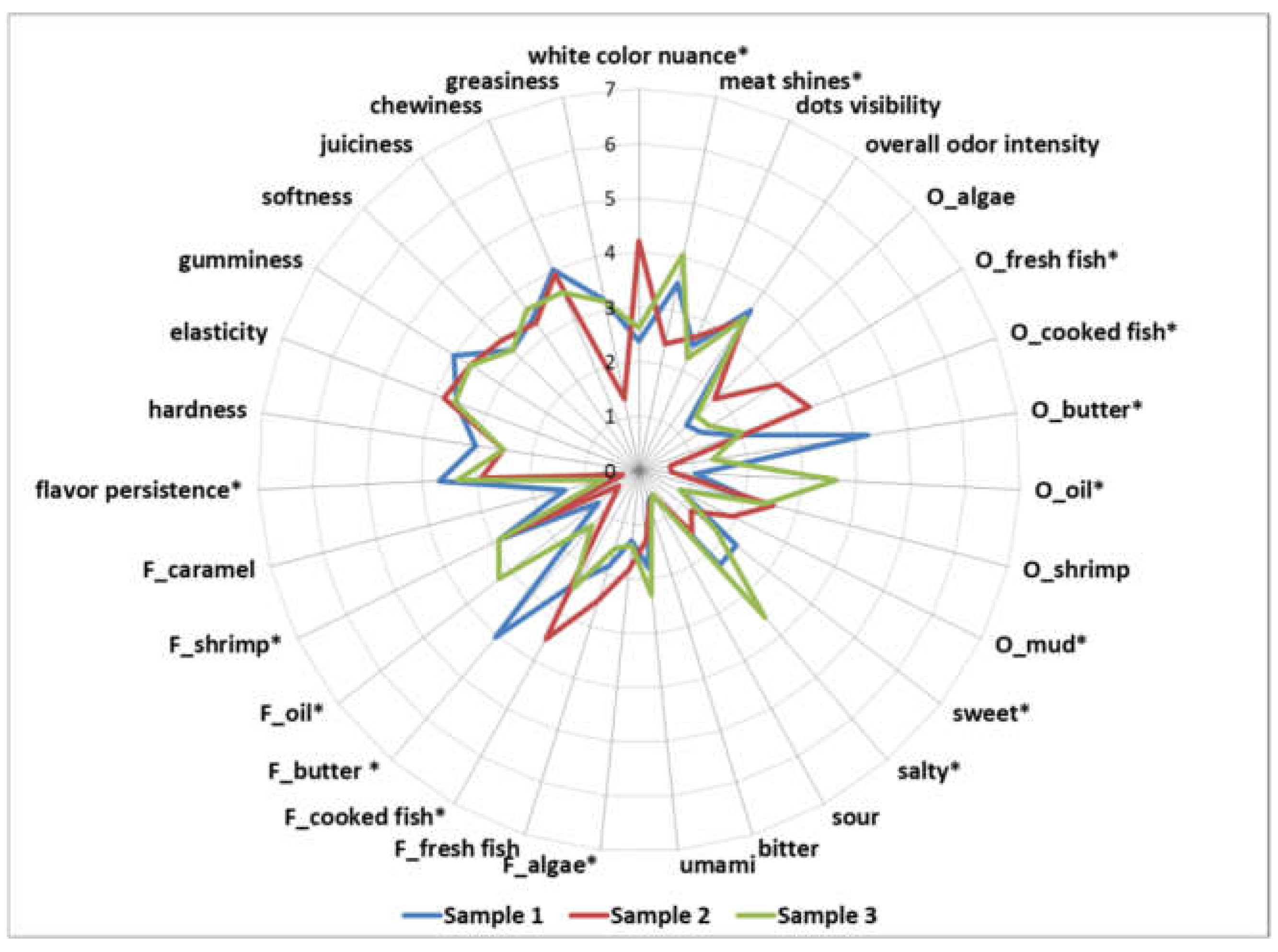
2.7. Sensory Analysis
2.8. Statistical Analysis
2.9. Ethical Statement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Regulation (EU) No. 2014/1143 of the European Parliament and of the Council on the Prevention and Management of the Introduction and Spread of Invasive Alien Species. Available online: http://data.europa.eu/eli/reg/2014/1143/oj (accessed on 22 October 2014).
- Regulation (EU) No. 2016/1141 of the European Parliament and of the Council on the Adopting a List of Invasive Alien Species of Union Concern. Available online: http://data.europa.eu/eli/reg_impl/2016/1141/oj (accessed on 13 July 2016).
- Regulation (EU) No. 2017/1263 of the European Parliament and of the Council to Updating the List of Invasive Alien Species of Union Concern. Available online: http://data.europa.eu/eli/reg_impl/2017/1263/oj (accessed on 12 July 2017).
- Regulation (EU) No. 2018/968 of the European Parliament and of the Council on the Risk Assessments in Relation to Invasive Alien Species. Available online: http://data.europa.eu/eli/reg_del/2018/968/oj (accessed on 30 April 2018).
- Regulation (EU) No. 2019/1262 of the European Parliament and of the Council to Updating the List of Invasive Alien Species of Union Concern. Available online: http://data.europa.eu/eli/reg_impl/2019/1262/oj (accessed on 25 July 2019).
- Lipták, B.; Vitázková, B.; Stloukal., E. First Record of the Spinycheek Crayfish (Orconectes limosus) in the Serbo-Romanian Tamiš River. Freshw. Crayfish 2013, 19, 229–232. [Google Scholar]
- Lipták, B.; Vitázková, B. A review of the current distribution and dispersal trends of two invasive crayfish species in the Danube Basin. Water Res. Manag. 2014, 4, 15–22. [Google Scholar]
- Karaman, I.; Machino, Y. Occurrence of the spiny-cheek crayfish (Orconectes limosus) and the Chinese mitten crab (Eriocheir sinensis) in Serbia. Crayfish News. 2004, 26, 19–20. [Google Scholar]
- Zorić, K.; Atanacković, A.; Ilić, M.; Csányi, B.; Paunović, M. The Spiny-cheek Crayfish Faxonius limosus (Rafinesque, 1817) (Decapoda: Cambaridae) Invades New Areas in Serbian Inland Waters. Acta Zool. Bulg. 2020, 72, 623–627. [Google Scholar]
- Todorov, M.; Trichkova, T.; Hubenov, Z.; Jurajda, P. Faxonius limosus (Rafinesque, 1817) (Decapoda: Cambaridae), a new invasive alien species of European Union concern in Bulgaria. Acta Zool. Bulg. 2020, 72, 113–121. [Google Scholar]
- Hudina, S.; Faller, M.; Lucić, A.; Klobučar, G.; Maguire, I. Distribution and dispersal of two invasive crayfish species in the Drava River basin, Croatia. Knowl. Manag. Aquat. Ecosyst. 2009, 9, 394–395. [Google Scholar] [CrossRef]
- Puky, M.; Schád, P. Orconectes limosus colonizes new areas fast along the Danube in Hungary. Bull. Fr. Pêche Piscic. 2006, 380–381, 919–926. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List Categories and Criteria Version 3.1. 2001. Available online: http://www.iucnredlist.org/technical-documents/categories-and-criteria/2001-categories-criteria#categories (accessed on 15 January 2016).
- Kus Veenvliet, J. Invasive Alien Species and Their Management Ljubljana, a Training Course Manual. EuroNatur Foundation and Institute Symbiosis, 2021. Available online: https://savaparks.eu/sava-ties-7448 (accessed on 15 January 2016).
- Buřič, M.; Kouba, A.; Kozák, P. Reproductive plasticity in freshwater invader: From long-term sperm storage to parthenogenesis. PLoS ONE 2013, 8, e77597. [Google Scholar] [CrossRef][Green Version]
- EC. Proposal for a Regulation of the European Parliament and of the Council on the Prevention and Management of the Introduction and Spread of Invasive Alien Species. 2013. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52013PC0620 (accessed on 9 September 2013).
- FAO; WHO; UNU. World Health Organization Food and Agriculture of the United Nation, World Health Organization, United Nations University, Preparation and use of Food-Based Dietary Guidelines; Report of a Joint, FAO/WHO Consultation; WHO Technical Report Series 880; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- El-Sherif, S.A.; Abd El-Ghafour, S. Investigation of Quality Properties and Nutritional Values of Four Fish Species from Lake Qaroun, Egypt. Int. J. Chem Tech Res. 2016, 9, 16–26. [Google Scholar]
- Harlioglu, M.; Köprücü, K.; Yilmaz, O. The effects of dietary n−3 series fatty acid on the fatty acid composition, cholesterol and fat-soluble vitamins of pleopodal eggs and stage 1 juveniles in a freshwater crayfish. Astacus leptodactylus (Eschscholtz). Aquacultures 2012, 356, 310–316. [Google Scholar] [CrossRef]
- Nahid, F.; Zaglol, I.; Fayza, E. Study on Chemical Quality and Nutrition Value of Fresh Water Cray Fish (Procambarus Clarkii). J. Arab. Aquac. Soc. 2009, 4, 1–18. [Google Scholar]
- Iwar, M.; Amu, C.M. Nutrient and anti-nutrient composition of processed crayfish (Atya gabonensis) from River Benue, Nigeria. Sustain. Agri Food Environ. Res. 2021, 9, 205–215. [Google Scholar] [CrossRef]
- Stanek, M.; Borejszo, Z.; Dąbrowski, J.; Janicki, B. Fat and cholesterol content and fatty acid profiles in edible tissues of spiny-cheek crayfish, Orconectes limosus (Raf.) from Lake Gopło (Poland) December. Arch. Pol. Fish. 2011, 19, 241–248. [Google Scholar] [CrossRef]
- Śmietana, N.; Panicz, R.; Sobczak, M.; Śmietana, P.; Nędzarek, A. Spiny-Cheek Crayfish, Faxonius limosus (Rafinesque, 1817), as an Alternative Food Source. Animals 2021, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Śmietana, P. Uwarunkowania Rozmieszczenia i Mechanizmy Konkurencji Mi˛edzygatunkowej Raka Szlachetnego (Astacus astacus L.) i Raka Pregowatego (Orconectes limosus Raf.); Wydawnictwo Naukowe Uniwersytetu Szczecinskiego: Polska, Poland, 2013; pp. 5–266. [Google Scholar]
- Ben Yahkoub, Y.; Fekhaoui, M.; El Qoraychy, I.; Yahyaoui, A. Current state of knowledge on Louisiana crawfish (Procambarus clarkii Girard, 1852) in Morocco. AACL Bioflux. 2019, 12, 618–628. [Google Scholar]
- Longshaw, M.; Stebbing, P. Laboratory methods for crayfish. In Biology and Ecology of Crayfish; Longshaw, M., Stebbing, P., Eds.; CRC Press: New York, NY, USA, 2016; pp. 325–336. [Google Scholar]
- Larson, E.R.; Olden, J.D. Field sampling techniques for crayfish. In Biology and Ecology of Crayfish; Longshaw, M., Stebbing, P., Eds.; CRC Press: New York, NY, USA, 2016; pp. 287–324. [Google Scholar]
- ISO 4833-2:2013 (E); Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 2: Colony Count at 30 Degrees C by the Surface Plating Technique. International Organization for Standardization: London, UK, 2018. Available online: https://www.iso.org/standard/59509.html (accessed on 4 September 2013).
- ISO 6579-1:2017(E); Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. International Organization for Standardization: London, UK, 2018. Available online: https://www.iso.org/standard/56712.html (accessed on 23 February 2017).
- ISO 16649-2:2001(E); Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 degrees C Using 5-bromo-4-chloro-3-indolyl beta-D-glucuronide. International Organizationfor Standardization: London, UK, 2017. Available online: https://www.iso.org/standard/29824.html/ (accessed on 3 April 2001).
- ISO 6888-1:2021(E); Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and other species)—Part 1: Method Using Baird-Parker Agar Medium. International Organization for Standardization: London, UK, 2017. Available online: https://www.iso.org/standard/76672.html/ (accessed on 30 September 2021).
- ISO 11290-1:2017(E); Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method. International Organization for Standardization: London, UK, 2017. Available online: https://www.sis.se/api/document/preview/921869/ (accessed on 31 July 2017).
- ISO 1442:1997; Meat and Meat Products-Determination of Moisture Content (Reference Method). ISO: Geneva, Switzerland, 1997. Available online: https://www.iso.org/standard/6037.html (accessed on 26 February 1997).
- ISO 936:1998; Meat and Meat Products-Determination of Total Ash (Reference Method). ISO: Geneva, Switzerland, 1998. Available online: https://www.iso.org/standard/24783.html (accessed on 30 July 1998).
- ISO 937:1978; Meat and Meat Products-Determination of Nitrogen Content (Reference Method). ISO: Geneva, Switzerland, 1998. Available online: https://www.iso.org/standard/82663.html (accessed on 1 December 1978).
- ISO 1443:1973; Meat and Meat Products-Determination of Total Fat Content (Reference Method). ISO: Geneva, Switzerland, 1973. Available online: https://www.iso.org/standard/6038.html (accessed on 1 April 1973).
- ISO 23776:2021; Meat and Meat Products-Determination of total Phosphorous Content-Determination of Nitrogen Content (Reference Method). ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/76935.html (accessed on 29 July 2021).
- Omole, O.A.; Adepoju, O.B.; Moradeyo, O.T.; Okuneye, O.J.; Bello, A.A. Comparative Analysis of the Nutritive Value of Smoked, Dried Procambarus clarkii(Freshwater Crayfish) from Three Nigerian States. Asian J. Fish. Aquat. Res. 2021, 11, 39–45. [Google Scholar] [CrossRef]
- Regulation (EU) No. 1169/2011 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32011R1169/ (accessed on 25 October 2011).
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipide from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Spackman, D.H.; Stein, W.H.; Moose, S. Automatic recording apparatus for use in the chromatography of amino acids. Anal. Chem. 1958, 30, 1190–1206. [Google Scholar] [CrossRef]
- ISO 6869:2000; Animal Feeding Stuffs-Determination of the Contents of Calcium, Copper, Iron, Magnesium, Manganese, Potassium, Sodium and Zinc—Method Using Atomic Absorption Spectrometry. Institute for Standardization of Serbia: Belgrade, Serbia, 2000. Available online: https://iss.rs/en/project/show/iso:proj:33707 (accessed on 14 December 2000).
- ISO 14082:2008; Determination of Trace Elements-Determination of Lead, Cadmium, Zinc, Copper, Iron and Chromium by Atomic Absorption Spectrometry (AAS) after Dry Ashing. Available online: https://iss.rs/en/project/show/iss:proj:20335 (accessed on 9 September 2008).
- Száková, J.; Kolihová, D.; Miholová, D.; Mader, P. Single-purpose atomic absorption spectrometer AMA-254 for mercury determination and its performance in analysis of agricultural and environmental materials. Chem. Pap. 2004, 58, 311–315. [Google Scholar]
- Palczak, J.; Giboreau, A.; Rogeaux, M.; Delarue, J. How do pastry and culinary chefs design sensory complexity? Int. J. Gastron. Food Sci. 2020, 19, 100182. [Google Scholar] [CrossRef]
- Regulation (EC) No. 2073/2005 on Microbiological Criteria for Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R2073 (accessed on 15 November 2005).
- Regulation (EC) No. 1441/2007. Regulation (EC) No 1441/2007 Amending Regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuffs. Available online: https://eur-lex.europa.eu/eli/reg/2007/1441/oj (accessed on 5 December 2007).
- FDA. Fish and fisheries products, hazards and controls guidance. In Pathogen Growth and Toxin Formation (other than Clostridium botulinum) as a Result of Time/Temperature Abuse, 3rd ed.; Chapter 12; FDA: Washington, DC, USA, 2001. Available online: https://www.fda.gov/food/resources-you-food/seafood (accessed on 15 June 2021).
- Lalitha, K.V.; Surendran, P.K. Surendran. Microbiological changes in farm reared freshwater prawn (Macrobrachium rosenbergii de Man) in ice. Food Control 2006, 17, 802–807. [Google Scholar] [CrossRef]
- Leitao, M.F.F.; Rios, D.P.A. Microbiological and chemical changes in Freshwater Prawn (Macrobrachium rosenbergii) stored under refrigeration. Braz. J. Microbiol. 2000, 31, 178–183. [Google Scholar] [CrossRef]
- Śmietana, N.; Panicz, R.; Sobczak, M.; Nedzarek, A. Variability of elements and nutritional value of spiny-cheek crayfish (Faxonius limosus, Rafinesque, 1817). J. Food Compos. Anal. 2020, 94, 4. [Google Scholar] [CrossRef]
- El-Sherif, S.A.; El-Ghafour, G.A. Nutritive value of canned River Nile Crayfish (Procambarus clarkii) products. Egypt J. Aqua. Res. 2015, 41, 265–272. [Google Scholar] [CrossRef]
- Papachristou, E.; Tyrpenou, A.E.; Kastritsi-Katharios, I.; Kotzamanis, Y. Proximate Composition and Amino Acid Profile of the Edible Muscle of European Crayfish Astacus astacus L. Inhabiting the Orchomenos Region in Central Greece. Austin. J. Nutr. Food Sci. 2021, 9, 1152. [Google Scholar]
- El-Kholie, E.M.; Khader, S.A.; Abdelreheem, M.A.T. Chemical, physical, microbiological and quality attributes studies on River Nile crayfish. Afr. J. Biotech. 2012, 11, 11262–11270. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations: Fisheries and Aquaculture Department. In The State of World Aquaculture and Fisheries; FAO: Rome, Italy, 2007. [Google Scholar]
- Harlioglu, A.G.; Aydın, S.; Yilmaz, O. Fatty acid, cholesterol and fat-soluble vitamin composition of wild and captive freshwater crayfish (Astacus leptodactylus). Food Sci Technol Int. 2012, 18, 93–100. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Bichkaeva, F.A.; Baranova, N.F.; Dvoretsky, V.G. Fatty acid composition of the Barents Sea red king crab (Paralithodes camtschaticus) leg meat. J. Food Compos. Anal. 2021, 98, 103826. [Google Scholar] [CrossRef]
- Balzano, M.; Pacetti, D.; Lucci, P.; Fiorini, D.; Frega, N.G. Bioactive fatty acids in mantis shrimp, crab and caramote prawn: Their content and distribution among the main lipid classes. J. Food Compos. Anal. 2017, 59, 88–94. [Google Scholar] [CrossRef]
- Stanek, M.; Dabrowski, J.; Różański, S.; Janicki, B.; Długosz, J. Heavy Metals Bioaccumulation in Tissues of Spiny-Cheek Crayfish (Orconectes limosus) from Lake Gopło: Effect of Age and Sex. Bull Environ Contam Toxicol. 2017, 98, 740–746. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Protasowicki, M.; Własow, T.; Rajkowska, M.; Polna, M.; Bernad, A. Metal concentrations in selected organs of crayfish Orconectes limosus and Pacifastacus leniusculus from Mazurian Lakes. J. Elem. 2013, 18, 683–694. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2021/1323 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Cadmium in Certain Foodstuffs (Text with EEA Relevance). Available online: http://data.europa.eu/eli/reg/2021/1323/oj (accessed on 10 August 2021).
- Suárez-Serrano, A.; Alcaraz, C.; Ibáňez, C.; Trobajo, R.; Barata, C. Procambarus clarkii as a bioindicator of heavy metal pollution sources in the Lower Ebro River and Delta. Ecotoxicol. Environ. Safe. 2010, 73, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Harlıoğlu, A.G.; Yilmaz, Ö.; Erdoğdu, A.; Siltar, Y.B. Protein and amino acid composition of wild caught freshwater crayfish (Pontastacus leptodactylus) in the reproductive season. Nauplius 2021, 29, e2021044. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, X.; Long, X.; Zhu, W.; Ma, T.; Cheng, Y. Nutritional quality of different grades of adult male chinese mitten crab, Eriocheir sinensis. J. Food Sci. Technol. 2018, 55, 944–955. [Google Scholar] [CrossRef]
- Fan, Y.; Odabasi, A.; Sims, C.; Schneider, K.; Gao, Z.; Sarnoski, P. Utilization of descriptive sensory analysis and volatile analysis to determine quality indicators of aquacultured whiteleg shrimp (Litopanaeus vannemei) during refrigerated storage. J. Aquat. Food Prod. Technol. 2020, 29, 722–735. [Google Scholar] [CrossRef]
- Townsend, D.C. Utilization of Descriptive Analysis and Volatile Analysis to Determine Quality Changes in Bay Scallops (Argopecten irradians) during Storage. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2019. [Google Scholar]

| Microorganisms | Mean Average (cfu/g) | Range |
|---|---|---|
| Total bacterial count | 1000 ± 0.11 | 500–1500 |
| E.coli | <10 | <10 |
| L. monocytogenes | <100 | <100 |
| Salmonella spp. | not detected in 25 g | not detected in 25 g |
| Coagulase positive Staphylococcus | <100 | <100 |
| Nutrients (% of Wet Weight) | Quantity (Mean Average) | Range |
|---|---|---|
| Gross nutritive value (kJ/100 g) | 75.15 ± 0.73 | 74.34–76.11 |
| Gross nutritive value (Kcal/100 g) | 314.58 ± 0.70 | 311.18–318.60 |
| Moisture (%) | 80.15 ± 0.22 | 79.84–80.34 |
| Ash (%) | 1.37 ± 0.05 | 1.30–1.42 |
| Proteins (%) | 18.12 ± 0.21 | 17.90–18.40 |
| Carbohydrates (%) | 0.10 ± 0.01 | 0.09–0.11 |
| Fats (%) | 0.25 ± 0.02 | 0.23–0.27 |
| Saturated fats (%) | 0.07 | 0.05–0.08 |
| Monounsaturated fats (%) | 0.07 | 0.06–0.07 |
| Polyunsaturated fats (%) | 0.11 | 0.10–0.12 |
| Omega-3 fats (%) | 0.07 | 0.06–0.08 |
| Omega-6 fats (%) | 0.04 | 0.03–0.05 |
| Fatty Acid | % of Total Fatty Acids (Mean Average) | Range |
|---|---|---|
| 1 SFA | 27.34 ± 0.48 | 24.61 ± 0.25–28.66 ± 0.32 |
| Myristic acid (C14:0) | 1.09 ± 0.10 | 0.75–1.32 |
| Pentadecanoic acid (C15:0) | 0.70 ± 0.90 | 0.59–0.92 |
| Palmitic acid (C16:0) | 17.78 ± 0.15 | 16.60–18.26 |
| Heptadecanoic acid (C17:0) | 0.90 ± 0.07 | 0.65–1.06 |
| Stearic acid (C18:0) | 6.87 ± 0.11 | 6.02–7.10 |
| 2 MUFA | 26.90 ± 0.36 | 26.35 ± 0.20–27.34 ± 0.42 |
| Pentadecenoic acid (C15:1) | 0.33 ± 0.18 | 0.20–0.65 |
| Palmitoleic (C16:1) | 4.42 ± 0.22 | 3.59–5.03 |
| Heptadecenoic acid (C17:1) | 0.33 ± 0.02 | 0.19–0.42 |
| Oleic acid (C18:1 n-9) | 20.60 ± 0.16 | 20.16–20.99 |
| Eicosenoic acid (C20:1 n-9) | 1.22 ± 0.02 | 1.12–1.35 |
| PUFA | 45.76 ± 0.84 | 44.44 ± 0.55–46.50 ± 0.68 |
| 3 n-3 PUFA | 29.34 ± 0.39 | 27.75 ± 0.18–30.00 ± 0.25 |
| alfa-Linolenic aci(C18:3 n-3) (ALA) | 0.55 ± 0.15 | 0.31–0.70 |
| Eicosappentaenoic acid (C20:5 n-3) (EPA) | 24.94 ± 0.33 | 24.12–25.71 |
| Docosahexaenoic acid (C22:6 n-3) (DHA) | 3.85 ± 0.24 | 3.15–4.28 |
| 4 n-6 PUFA | 16.42 ± 0.14 | 14.44 ± 0.10–17.10 ± 0.08 |
| Linoleic acid (C18:2 n-6) (LA) | 5.29 ± 0.20 | 4.51–6.39 |
| Eicosadienoic acid (C20:2 n-6) | 1.95 ± 0.05 | 1.85–2.02 |
| Arachidonic acid (C20:4 n-6) (AA) | 9.18 ± 0.90 | 8.08–10.20 |
| n3/n6 ratio | 1.79 ± 0.01 | 1.92–1.75 |
| AI | 0.40 ± 0.01 | 0.39–0.40 |
| TI | 0.21 ± 0.01 | 0.20–0.21 |
| Minerals and Heavy Metals | Quantity (mg/100 g) | Range |
|---|---|---|
| Macrominerals | ||
| K | 440.33 ± 89.67 | 428.50–450.20 |
| Ca | 52.17 ± 6.24 | 51.50–53.01 |
| Mg | 25.60 ± 9.24 | 24.50–26.80 |
| Na | 99.67 ± 41.10 | 95.01–105.00 |
| P | 50.33 ± 35.20 | 46.50–55.00 |
| Microminerals | ||
| Fe | 0.98 ± 0.54 | 0.92–1.05 |
| Mn | 0.12 ± 0.04 | 0.11–0.13 |
| Cu | 2.09 ± 0.09 | 2.07–2.10 |
| Zn | 2.53 ± 0.33 | 2.49–2.59 |
| Heavy metals | ||
| Hg | 0.025 ± 0.04 | 0.019–0.029 |
| Pb | <0.1 | <0.1 |
| Cd | <0.02 | <0.02 |
| As | <0.1 | <0.1 |
| Amino Acids | Quantity (g/100 g) | Range |
|---|---|---|
| His | 0.59 ± 0.04 | 0.55–0.62 |
| Ile | 0.78 ± 0.10 | 0.72–0.80 |
| Leu | 1.37 ± 0.06 | 1.30–1.40 |
| Met | 0.46 ± 0.05 | 0.40–0.50 |
| Phe | 0.71 ± 0.07 | 0.65–0.78 |
| Thr | 0.80 ± 0.01 | 0.72–0.84 |
| Val | 0.74 ± 0.04 | 0.70–0.85 |
| Lys | 1.51 ± 0.03 | 1.49–1.54 |
| Total EAAs | 6.96 ± 0.40 | 6.53–7.33 |
| Glu | 2.46 ± 0.04 | 2.42–2.50 |
| Asp | 1.58 ± 0.02 | 1.50–1.65 |
| Pro | 0.94 ± 0.05 | 0.89–0.98 |
| Ala | 0.85 ± 0.06 | 0.78–0.90 |
| Arg | 1.98 ± 0.11 | 1.88–2.10 |
| Gly | 0.86 ± 0.08 | 0.82–0.90 |
| Ser | 0.66 ± 0.04 | 0.62–0.69 |
| Tyr | 0.82 ± 0.11 | 0.82–1.02 |
| Cys | 0.11 ± 0.05 | 0.10–0.15 |
| Total NEAAs | 10.26 ± 0.65 | 9.83–10.89 |
| Total AAs | 17.22 ± 1.05 | 16.36–18.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarević, J.; Čabarkapa, I.; Rakita, S.; Banjac, M.; Tomičić, Z.; Škrobot, D.; Radivojević, G.; Kalenjuk Pivarski, B.; Tešanović, D. Invasive Crayfish Faxonius limosus: Meat Safety, Nutritional Quality and Sensory Profile. Int. J. Environ. Res. Public Health 2022, 19, 16819. https://doi.org/10.3390/ijerph192416819
Lazarević J, Čabarkapa I, Rakita S, Banjac M, Tomičić Z, Škrobot D, Radivojević G, Kalenjuk Pivarski B, Tešanović D. Invasive Crayfish Faxonius limosus: Meat Safety, Nutritional Quality and Sensory Profile. International Journal of Environmental Research and Public Health. 2022; 19(24):16819. https://doi.org/10.3390/ijerph192416819
Chicago/Turabian StyleLazarević, Jasmina, Ivana Čabarkapa, Slađana Rakita, Maja Banjac, Zorica Tomičić, Dubravka Škrobot, Goran Radivojević, Bojana Kalenjuk Pivarski, and Dragan Tešanović. 2022. "Invasive Crayfish Faxonius limosus: Meat Safety, Nutritional Quality and Sensory Profile" International Journal of Environmental Research and Public Health 19, no. 24: 16819. https://doi.org/10.3390/ijerph192416819
APA StyleLazarević, J., Čabarkapa, I., Rakita, S., Banjac, M., Tomičić, Z., Škrobot, D., Radivojević, G., Kalenjuk Pivarski, B., & Tešanović, D. (2022). Invasive Crayfish Faxonius limosus: Meat Safety, Nutritional Quality and Sensory Profile. International Journal of Environmental Research and Public Health, 19(24), 16819. https://doi.org/10.3390/ijerph192416819










