The Nutrition Transition and the Double Burden of Malnutrition in Sub-Saharan African Countries: How Do These Countries Compare with the Recommended LANCET COMMISSION Global Diet?
Abstract
1. Introduction
2. Materials and Methods
2.1. Food Intake Data and Methodology
2.2. Data on Burden of Malnutrition, NCD Health, and Development Indicators
2.3. Associations between Food Clusters and Health and Development Indicators
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lottering, S.; Mafongoya, P.; Lottering, R. Drought and its impacts on small-scale farmers in sub-Saharan Africa: A Review. S. Afr. Geogr. J. 2021, 103, 319–341. [Google Scholar] [CrossRef]
- Aucoin, C. Less Armed Conflict But More Political Violence in Africa. Inst. Secur. Stud. 2017. Available online: https://issafrica.org/iss-today/less-armed-conflict-but-more-political-violence-in-Africa (accessed on 24 October 2020).
- Bello-Schünemann, J.; Cilliers, J.; Donnenfeld, Z.; Aucoin, C.; Porter, A. Policy Brief. African Futures: Key Trends to 2035. Inst. Secur. Stud. 2017. Available online: https://www.africaportal.org/publications/african-futures-key-trends-2035/ (accessed on 24 October 2020).
- Laar, A.K.; Addo, P.; Aryeetey, R.; Agyemang, C.; Zotor, F.; Asiki, G.; Rampalli, K.K.; Amevinya, G.S.; Tandoh, A.; Nanema, S.; et al. Perspective: Food Environment Research Priorities for Africa—Lessons from the Africa Food. Environment Research Network. Adv. Nutr. 2022, 13, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Ng, S.W. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes. Rev. 2021, 23, e13366. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ruiz, A.; Madrigal, C.; Soto-Méndez, M.J.; Gil, A. Challenges and perspectives of the double burden of malnutrition in Latin America. Clin. Investig. Arterioscler. 2022, 34 (Suppl. S1), S3–S16. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M. Nutrition Transition and the Global Diabetes Epidemic. Curr. Diabetes Rep. 2015, 15, 64. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Louzada, M.L.C.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-Processed Foods: What They Are and How to Identify Them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Baker, P.; Machado, P.; Santos, T.; Sievert, K.; Backholer, K.; Hadjikakou, M.; Russell, C.; Huse, O.; Bell, C.; Scrinis, G.; et al. Ultra-processed foods and the nutrition transition: Global, regional and national trends, food systems transformations and political economy drivers. Obes. Rev. 2020, 21, e13126. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.-C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA Food Classification and the Trouble with Ultra-Processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef]
- Pagliai, G.; Dinu, M.; Madarena, M.P.; Bonaccio, M.; Iacoviello, L.; Sofi, F. Consumption of ultra-processed foods and health status: A systematic review and meta-analysis. Br. J. Nutr. 2020, 125, 308–318. [Google Scholar] [CrossRef]
- Elizabeth, L.; Machado, P.; Zinöcker, M.; Baker, P.; Lawrence, M. Ultra-Processed Foods and Health Outcomes: A Narrative Review. Nutrients 2020, 12, 1955. [Google Scholar] [CrossRef]
- Cowan, E.; D’Ambruoso, L.; van der Merwe, M.; Witter, S.; Byass, P.; Ameh, S.; Wagner, R.G.; Twine, R. Understanding non-communicable diseases: Combining health surveillance with local knowledge to improve rural primary health care in South Africa. Glob. Heal. Action 2020, 14, 1852781. [Google Scholar] [CrossRef]
- Jayasinghe, S.; Byrne, N.M.; Patterson, K.A.; Ahuja, K.D.; Hills, A.P. The current global state of movement and physical activity—The health and economic costs of the inactive phenotype. Prog. Cardiovasc. Dis. 2020, 64, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, T.A.; Bitton, A.; Anand, S.; Abrahams-Gessel, S.; Murphy, A. Growing Epidemic of Coronary Heart Disease in Low- and Middle-Income Countries. Curr. Probl. Cardiol. 2010, 35, 72–115. [Google Scholar] [CrossRef] [PubMed]
- Saghir, J.; Santoro, J. Urbanization in Sub-Saharan Africa. Meeting Challenges by Bridging Stakeholders. Center for Strategic and International Studies. 2018. Available online: https://csis-website-prod.s3.amazonaws.com/s3fs-public/publication/180411_Saghir_UrbanizationAfrica_Web.pdf (accessed on 24 October 2022).
- Dia, K.B.; Beaudelaire, D.W. Climate Variability and Urbanization in Sub-Saharan Africa: Mitigating the Effects on Economic Growth. African Economic Research Consortium. Nairobi. 2021. Available online: http://3.65.68.50/bitstream/handle/123456789/2819/Working%20Paper%20Series%20CC-010.pdf?sequence=1&isAllowed=y (accessed on 24 October 2022).
- World Health Organisation. WHO Global Strategy on Diet Physical Activity and Health. 2004. Available online: https://www.who.int/dietphysicalactivity/strategy/eb11344/strategy_english_web.pdf (accessed on 24 October 2022).
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, K.; Bai, Y.; Headey, D.; Masters, W.A. Affordability of the EAT–Lancet reference diet: A global analysis. Lancet Glob. Health 2020, 8, e59–e66. [Google Scholar] [CrossRef] [PubMed]
- Hivonen, K.; Bai, Y.; Headey, D.; Masters, W.A. Cost and affordability of the EAT-Lancet diet in 159 countries. Lancet 2019. Available online: https://ssrn.com/abstract=3405576 (accessed on 24 October 2022).
- Drewnowski, A. Analysing the affordability of the EAT–Lancet diet. Lancet 2020, 8. Available online: www.thelancet.com/lancetgh (accessed on 24 October 2022).
- Zagmutt, F.J.; Pouzou, J.G.; Costard, S. The EAT-Lancet Commission: A flawed approach? Int. J. Environ. Res. Public Health 2020, 19, 1140–1141. [Google Scholar] [CrossRef]
- Vanham, D.; Mekonnen, M.M.; Hoekstra, A.Y. Treenuts and groundnuts in the EAT-Lancet reference diet: Concerns regarding sustainable water use. Glob. Food Secur. 2020, 24, 100357. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Data Structure, Concepts and Definitions Common to FAOSTAT and Country STAT Framework. 2013. Available online: https://www.fao.org/3/br301e/br301e.pdf (accessed on 24 October 2022).
- Jacobs, K.; Sumner, D.A. The Food Balance Sheets of the Food and Agriculture Organization: A Review of Potential Ways to Broaden the Appropriate Uses of the Data. Sponsored by FAO, March 2002. Available online: http://fpmu.gov.bd/agridrupal/sites/default/files/FBS_Review_of_Potential_Ways_to_Broaden_the_Uses_of_Data.pdf (accessed on 24 October 2022).
- European Commission. Knowledge for Policy. Dataset 1 January 2016. Food Balance Sheets. Available online: https://knowledge4policy.ec.europa.eu/dataset/beofao-fao-fbs_en (accessed on 24 October 2022).
- O’Rourke, N.; Hatcher, L. A Step-by-Step Approach to Using SAS for Factor Analysis and Structural Equation Modeling, 2nd ed.; Support.sas.com/bookstore; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Lee, J.-W.; Hwang, J.; Cho, H.-S. Dietary patterns of children and adolescents analyzed from 2001 Korea National Health and Nutrition Survey. Nutr. Res. Pract. 2007, 1, 84–88. [Google Scholar] [CrossRef][Green Version]
- Williams, B.; Onsman, A.; Brown, T. Exploratory factor analysis: A five-step guide for novices. J. Emerg. Prim. Health Care 2010, 8, 3. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical grouping to optimize and objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- UNICEF/WHO/World Bank Joint Child Malnutrition Estimates Expanded Database: Stunting (Survey Estimates), May 2022, New York. Available online: https://data.unicef.org/resources/dataset/malnutrition-data/ (accessed on 24 October 2022).
- UNICEF/WHO/World Bank Joint Child Malnutrition Estimates Expanded Database: Overweight (Survey Estimates), April 2021, New York. Available online: https://data.unicef.org/resources/dataset/malnutrition-data/ (accessed on 24 October 2022).
- United Nations Children’s Fund, Division of Data, Analysis, Planning and Monitoring (2022). Global UNICEF Global Databases: Overlapping Stunting, Wasting and Overweight (Survey Estimates), May 2022, New York. Available online: https://data.unicef.org/resources/dataset/malnutrition-data/ (accessed on 24 October 2022).
- Global Nutrition Report: Country Nutrition Profiles. Bristol, UK: Development Initiatives. Available online: https://globalnutritionreport.org/resources/nutrition-profiles/ (accessed on 24 October 2022).
- WHO. Department of Nutrition and Food Safety World Health Organization Geneva, Switzerland. March 2021. WHO Methods and Data Sources for Mean Haemoglobin and Anaemia Estimates in Women of Reproductive Age and Pre-School Age Children 2000–2019. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-anaemia-in-women-of-reproductive-age-(-) (accessed on 24 October 2022).
- WHO. The Global Health Observatory. Prevalence of Hypertension among Adults Aged 30–79 Years. Geneva: WHO. 2019. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-hypertension-among-adults-aged-30–79-years (accessed on 24 October 2022).
- NCD Risk Factor Collaboration. Worldwide Trends in Diabetes Since 1980: A Pooled Analysis of 751 Population-Based Studies with 4.4 Million Participants. Lancet 2016, 387, 1513–1530. Available online: https://ncdrisc.org/data-downloads-diabetes.html (accessed on 24 October 2022). [CrossRef]
- Barbagallo, C.M.; Taddei, C. Repositioning of the global epicentre of non-optimal cholesterol. Nature 2020, 582, 73–77. [Google Scholar] [CrossRef]
- World Bank. World Development Indicators: World Bank Data. Available online: https://data.worldbank.org/indicator (accessed on 24 October 2022).
- World Bank. Fertility Rate, Total (Births per Woman). Available online: https://data.worldbank.org/indicator/SP.DYN.TFRT.IN?view=chart (accessed on 24 October 2022).
- Kuhnlein, H.V. Receveur 0. Dietary change and traditional food systems of Indigenous Peoples. Annu. Rev. Nutr. 1996, 16, 417–442. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.C.; Kremer, P.J.; Magarey, A.M.; Swinburn, B.A. Contribution of “noncore” foods and beverages to the energy intake and weight status of Australian children. Eur. J. Clin. Nutr. 2005, 59, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Plantmaps. Africa Köppen Climate Classification Map. Available online: https://www.plantmaps.com/koppen-climate-classification-map-africa.php (accessed on 24 October 2022).
- World Health Organisation. Promoting Fruit and Vegetable Consumption around the World. Information Sheet. Available online: https://www.who.int/dietphysicalactivity/fruit/en/ (accessed on 24 October 2022).
- GBD Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Food and Nutrition Board Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Available online: https://www.nap.edu/read/11537/chapter/1#iii (accessed on 15 January 2022).
- Hisali, E.; Birungi, P.; Buyinza, F. Adaptation to climate change in Uganda: Evidence from micro level data. Glob. Environ. Chang. 2011, 21, 1245–1261. [Google Scholar] [CrossRef]
- Shiferaw, B.; Tesfaye, K.; Kassie, M.; Abate, T.; Prasanna, B.M.; Menkir, A. Managing vulnerability to drought and enhancing livelihood resilience in sub-Saharan Africa: Technological, institutional and policy options. Weather. Clim. Extrem. 2014, 3, 67–79. [Google Scholar] [CrossRef]
- Kotir, J.H. Climate change and variability in sub-Saharan Africa: A review of current and future trends and impacts on agriculture and food security. Environ. Dev. Sustain. 2011, 13, 587–605. [Google Scholar] [CrossRef]
- Bryan, E.; Ringler, C.; Okoba, B.; Roncoli, C.; Silvestri, S.; Herrero, M. Adapting agriculture to climate change in Kenya: Household strategies and determinants. J. Environ. Manag. 2013, 114, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Masipa, T.S. The impact of climate change on food security in South Africa: Current realities and challenges ahead. Jàmbá J. Disaster Risk Stud. 2017, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dumenu, W.K.; Obeng, E.A. Climate change and rural communities in Ghana: Social vulnerability, impacts, adaptations and policy implications. Environ. Sci. Policy 2016, 55, 208–217. [Google Scholar] [CrossRef]
- Behnassi, M.; Haiba, M.E. Implications of the Russia–Ukraine War for Global Food Security. Nat. Hum. Behav. 2022, 6, 754–755. Available online: https://www.nature.com/articles/s41562-022-01391-x (accessed on 24 October 2022). [CrossRef] [PubMed]
- United Nations, Department of Economic and Social Affairs, Population Division (2015). World Urbanization Prospects: The 2014 Revision, (ST/ESA/SER.A/366). Available online: https://population.un.org/wup/publications/files/wup2014-report.pdf (accessed on 24 October 2022).
- Steyn, N.P.; Bradshaw, D.; Norman, R.; Joubert, J.D.; Schneider, M.; Steyn, K. Dietary changes and the health transition in South Africa: Implications for health policy. In The Double Burden of Malnutrition. Case Studies from Six Developing Countries; FAO Food and Nutrition Paper: Québec, Canada, 2006; Volume 84, pp. 259–304. ISBN 92-5-105489-4. [Google Scholar]
- Holmes, M.D.; Dalal, S.; Sewram, V.; Diamond, M.B.; Adebamowo, S.N.; O Ajayi, I.; Adebamowo, C.; Chiwanga, F.S.; Njelekela, M.; Laurence, C.; et al. Consumption of processed food dietary patterns in four African populations. Public Heal. Nutr. 2018, 21, 1529–1537. [Google Scholar] [CrossRef]
- Harvard School of Public Health. The Nutrition Source: Legumes and Pulses. Available online: https://www.hsph.harvard.edu/nutritionsource/legumes-pulses/ (accessed on 24 October 2022).
- Afshin, A.; Micha, R.; Khatibzadeh, S.; Mozaffarian, D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 278–288. [Google Scholar] [CrossRef]
- Ebello, C.J.; Greenway, F.L.; Finley, J.W. A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities. Obes. Rev. 2014, 15, 392–407. [Google Scholar] [CrossRef]
- Wiesinger, J.A.; Cichy, K.A.; Hooper, S.D.; Hart, J.J.; Glahn, R.P. Processing white or yellow dry beans (Phaseolus vulgaris L.) into a heat treated flour enhances the iron bioavailability of bean-based pastas. J. Funct. Foods 2020, 71, 104018. [Google Scholar] [CrossRef]
- Rekhy, R.; McConchie, R. Promoting consumption of fruit and vegetables for better health. Have campaigns delivered on the goals? Appetite 2014, 79, 113–123. [Google Scholar] [CrossRef]
- WHO. Sugars Intake for Adults and Children: Guideline; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Hu, F.B.; Malik, V.S. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: Epidemiologic evidence. Physiol. Behav. 2010, 100, 47–54. [Google Scholar] [CrossRef]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.-P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type-2 diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Audain, K.; Levy, L.; Ellahi, B. Sugar-sweetened beverage consumption in the early years and implications for type-2 diabetes: A sub-Saharan Africa context. Proc. Nutr. Soc. 2019, 78, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Weeratunga, P.; Jayasinghe, S.; Perera, Y.; Jayasena, G.; Jayasinghe, S. Per capita sugar consumption and prevalence of diabetes mellitus—Global and regional associations. BMC Public Heal. 2014, 14, 186. [Google Scholar] [CrossRef] [PubMed]
- Steyn, N.P.; Temple, N.J. Evidence to support a food-based dietary guideline on sugar consumption in South Africa. BMC Public Heal. 2012, 12, 502. [Google Scholar] [CrossRef]
- Brown, I.J.; Stamler, J.; Van Horn, L.; Robertson, C.E.; Chan, Q.; Dyer, A.R.; Huang, C.-C.; Bodriguez, B.L.; Zhao, L.; Daviglus, M.L.; et al. Sugar-sweetened beverage, sugar intake of individuals, and their blood pressure: International study of macro/micronutrients and blood pressure. Hypertension 2011, 57, 695–701. [Google Scholar] [CrossRef]
- Manyema, M.; Veerman, J.L.; Chola, L.; Labadarios, D.; Hofman, K. Decreasing the burden of type 2 diabetes in South Africa: The impact of taxing sugar-sweetened beverages. PLoS ONE 2015, 10, 0143050. [Google Scholar] [CrossRef]
- WHO. Diet, nutrition and the prevention of chronic diseases. In Report of a Joint WHO/FAO Expert Consultation; Series 916; WHO Technical Report: Geneva, Switzerland, 2003. [Google Scholar]
- Valsta, M.; Tapanainen, H.; Männistö, S. Meat fats in nutrition. Meat Sci. 2005, 70, 525–530. [Google Scholar] [CrossRef]
- Ekmekcioglu, C.; Wallner, P.; Kundi, M.; Weisz, U.; Haas, W.; Hutter, H.-P. Red meat, diseases, and healthy alternatives: A critical review. Crit. Rev. Food Sci. Nutr. 2017, 58, 247–261. [Google Scholar] [CrossRef]
- De Carvalho, A.M.; Selem, S.S.; Miranda, A.M.; Marchioni, D.M. Excessive red and processed meat intake: Relations with health and environment in Brazil. Br. J. Nutr. 2016, 115, 2011–2016. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs Evaluate Consumption of Red Meat and Processed Meat; Press Release No. 240; IARC: Lyon, France, 2018. [Google Scholar]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Steyn, N.P.; Nel, J.H.; Malczyk, S.; Drummond, L.; Senekal, M. Provincial Dietary Intake Study (PDIS): Energy and Macronutrient Intakes of Children in a Representative/Random Sample of 1–<10-Year-Old Children in Two Economically Active and Urbanized Provinces in South Africa. Int. J. Environ. Res. Public Health 2020, 17, 1717. [Google Scholar] [CrossRef]
- Elagizi, A.; Lavie, C.J.; O’Keefe, E.; Marshall, K.; O’Keefe, J.H.; Milani, R.V. An Update on Omega-3 Polyunsaturated Fatty Acids and Cardiovascular Health. Nutrients 2021, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Steyn, N.P.; Mchiza, Z.; Hill, J.; Davids, Y.D.; Venter, I.; Hinrichsen, E.; Opperman, M.; Rumbelow, J.; Jacobs, P.T. Nutritional contribution of street foods to the diet of people in developing countries: A systematic review. Public Heal. Nutr. 2013, 17, 1363–1374. Available online: http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=8920820 (accessed on 24 October 2022). [CrossRef] [PubMed]
- Steyn, N.P.; Mchiza, Z.J. Obesity and the nutrition transition in Sub-Saharan Africa. Ann. N. Y. Acad. Sci. 2014, 1311, 88–101. [Google Scholar] [CrossRef]
- Dunbar, B.; Bosire, R.; Deckelbaum, R. Omega 3 and omega 6 fatty acids in human and animal health: An African perspective. Mol. Cell. Endocrinol. 2014, 398, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, G.; Sela, Y. The ideal diet for humans to sustainably feed the growing population—Review, Meta-Analyses, and Policies for Change [version 1; peer review: Awaiting peer review]. F1000Research 2021, 10, 1135. [Google Scholar] [CrossRef]
- Springmann, M.; Mason-D’Croz, D.; Robinson, S.; Garnett, T.; Godfray, H.C.J.; Gollin, D.; Rayner, M.; Ballon, P.; Scarborough, P. Global and regional health effects of future food production under climate change: A modelling study. Lancet 2016, 387, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
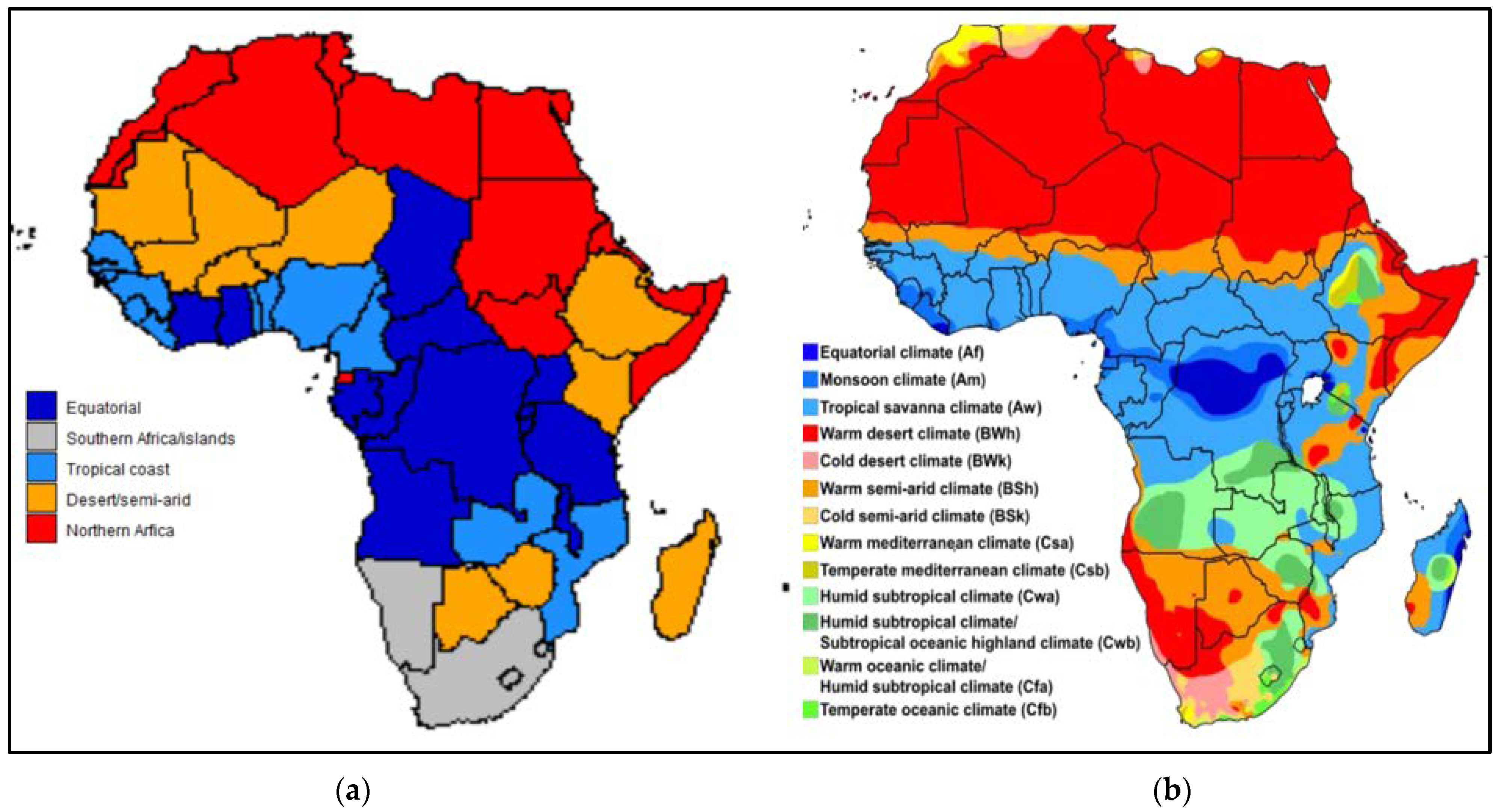

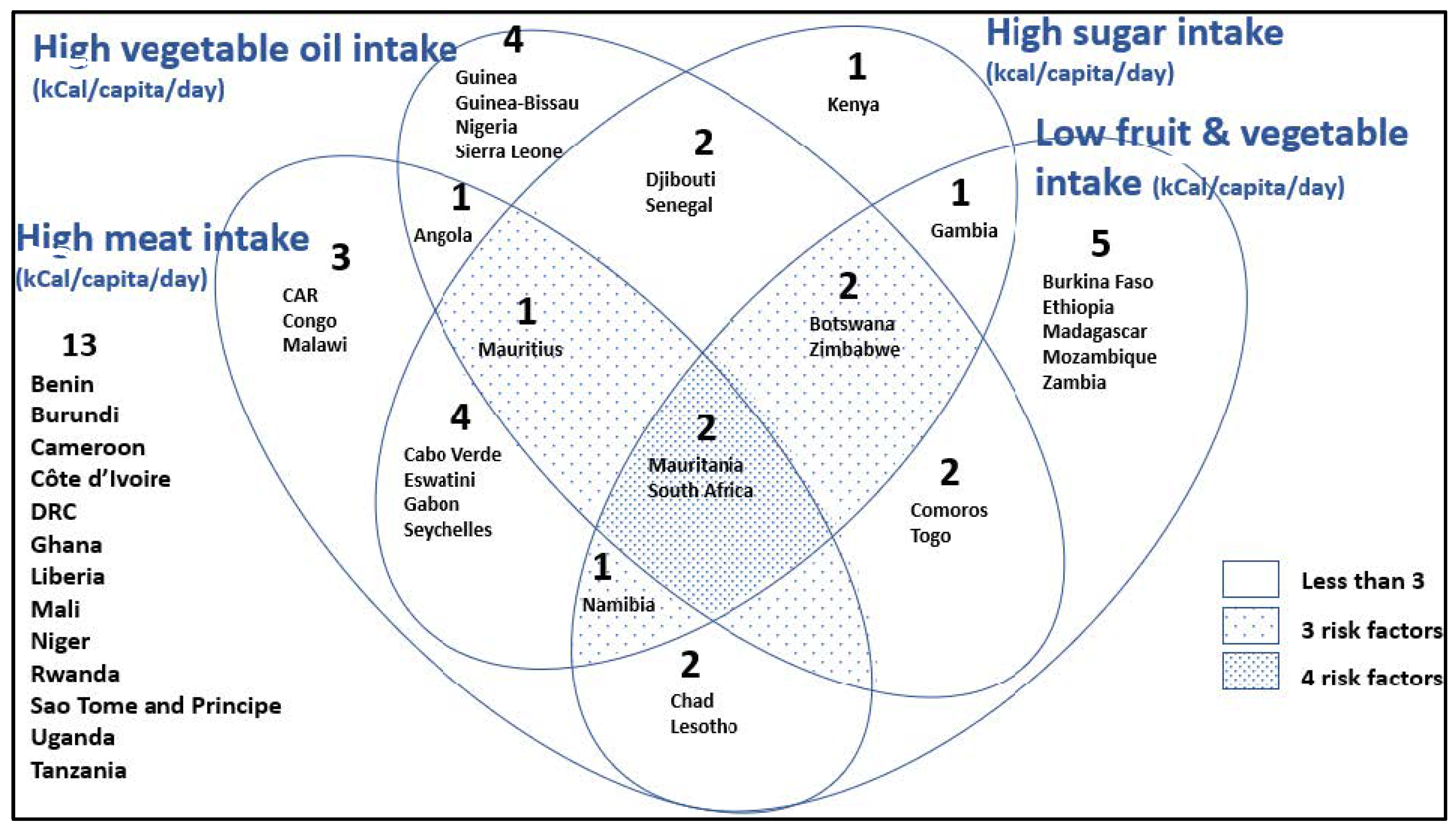
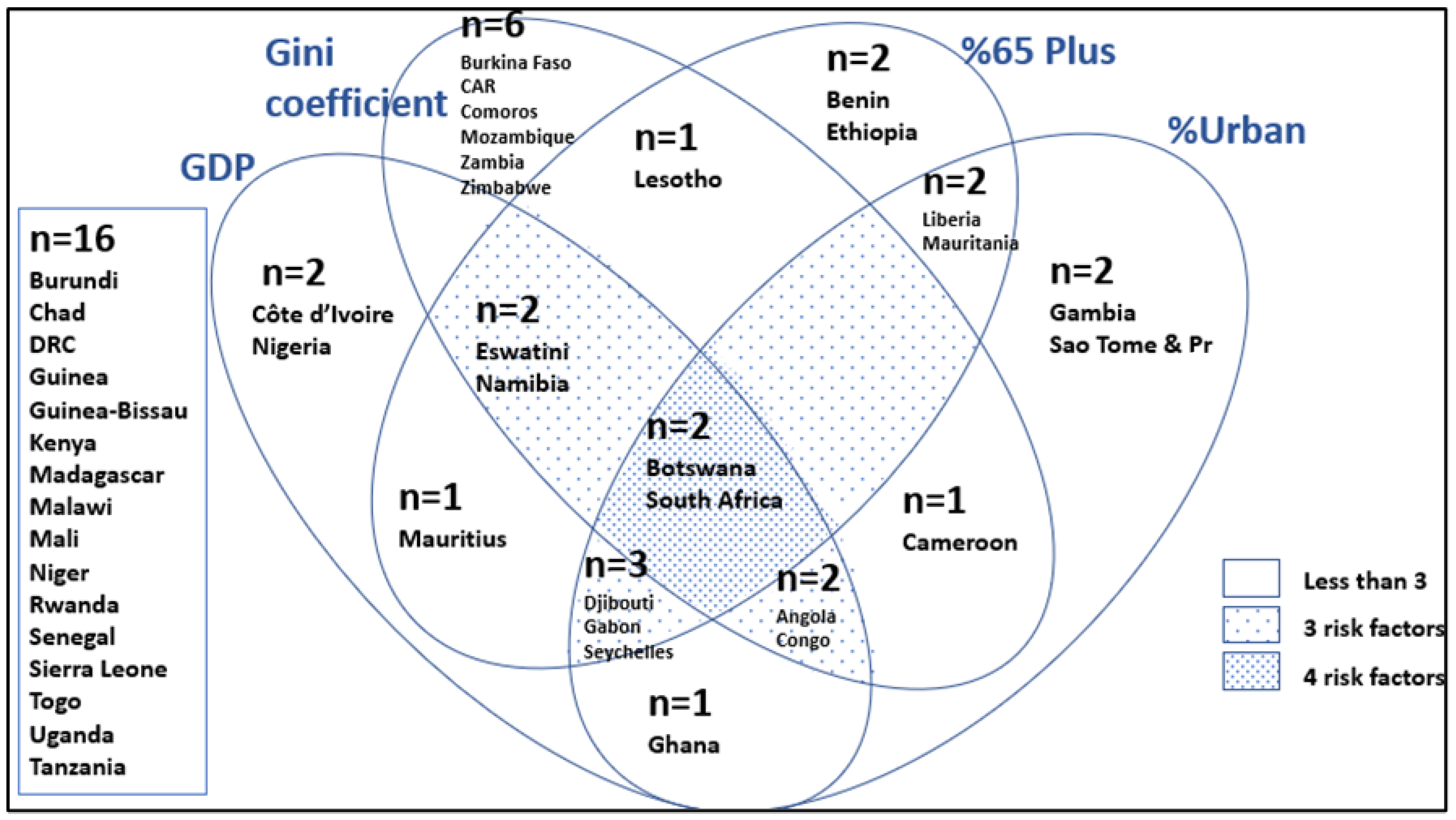
| Code | Description |
|---|---|
| 2905 | Cereals excluding beer (wheat, rice, barley, maize, rye, oats, millet, sorghum, other) |
| 2907 | Starchy roots (cassava, potatoes, sweet potatoes, yams, other) |
| 2909 | Sugar and sweeteners (sugar, sweeteners, honey) |
| 2911 + 2912 | Pulses (beans, peas, other) and tree nuts |
| 2913 * | Oil crops (soyabeans, groundnuts, sunflower seed, rape and mustard seed, cotton seed, coconuts, sesame seed, palm kernels, olives, other) |
| 2914 | Vegetable oils (soyabean oil, groundnut oil, sunflower seed oil, rape and mustard seed oil, cottonseed oil, palm kernel oil, palm oil, coconut oil, sesame seed oil, olive oil, maize germ oil, other) |
| 2918 + 2019 | Vegetables (tomatoes, onions, other), fruit excluding wine (oranges, lemons, grapefruit, other citrus, bananas, plantain, apples, pineapples, dates, grapes, other) |
| 2922 + 2923 * | Stimulants (coffee, cacao, tea) and spices (pepper, pimento, cloves, other spices) |
| 2924 | Alcoholic beverages (wine, beer, fermented beverages, alcoholic beverages) |
| 2943 | Meat (bovine, mutton/goat, pork, poultry, other) |
| 2945 * | Offal |
| 2946 | Animal fats (butter/ghee, cream, animal fat, fish body oil, fish liver oil) |
| 2949 | Eggs |
| 2948 | Milk excluding butter |
| 2960 | Fish, seafood (freshwater fish, demersal fish, pelagic fish, marine fish, crustaceans, cephalopods, molluscs, other) |
| Factor 1 | Factor 2 | Factor 3 | ||||
|---|---|---|---|---|---|---|
| Food Item | Loading | Food Item | Loading | Food Item | Loading | |
| Factor loadings > 0.3 | Meat Animal fats Alcoholic beverages Fish and seafood Sugar/sweeteners Milk Eggs | 0.74 0.69 0.68 0.51 0.50 0.43 * 0.44 * | Cereals Milk Animal fats Sugar/sweeteners | 0.71 0.64 0.44 * 0.42 * | Vegetable oils Eggs Sugar/sweeteners Fish and seafood | 0.76 0.53 0.43 * 0.32 * |
| Factor loadings <−0.4 | - | - | Fruit and vegetables Starchy roots | −0.48 −0.83 | Pulses and tree nuts | −0.53 |
| % Variance explained (Total = 54.4%) | 20.7% | 20.6% | 13.1% | |||
| Type of diet | Westernised | Traditional/Westernised | Traditional/Westernised | |||
| Cluster 1 Desert/Semi-Arid N = 12 | Cluster 2 Tropical Coastal N = 12 | Cluster 3 Equatorial N = 14 | Cluster 4 Southern African/Island N = 5 | |
|---|---|---|---|---|
| Countries | Botswana, Burkina Faso #, Djibouti, Ethiopia #, Gambia #, Kenya #, Lesotho *, Madagascar #, Mali #, Mauritania, Niger, Zimbabwe # | Benin, Cameroon, Comoros, Guinea, Guinea-Bissau, Liberia, Mozambique#, Nigeria #, Senegal #, Sierra Leone, Togo, Zambia * | Angola, Burundi, Central African Republic (CAR), Chad #, Republic of the Congo, Democratic Republic of the Congo (DRC) &, Gabon, Ghana, Ivory Coast, Malawi, Rwanda, São Tomé and Príncipe, Uganda, Tanzania # | Cabo Verde, Eswatini, Mauritius, Namibia, South Africa (Seychelles excluded as an outlier in the analysis) |
| Description | Warm desert and warm semi-arid climate. | Mostly coastal countries with tropical savanna and tropical monsoon climate. | Tropical savanna and subtropical climate areas in the equatorial region. | Mostly southern African countries and well-developed islands, with a spread of climate regions. |
| Exceptions which refer to countries above [33] | * Lesotho has cold semi-arid and tropical/sub-tropical regions. # Includes additional tropical/sub-tropical regions. | * Zambia has a humid subtropical climate. # Includes additional semi-arid regions. | # Include additional semi-arid/desert regions. & Tropical rainforest. | South Africa: 5 major climate groups. Namibia: desert/semi-arid. Mauritius: Tropical. Eswatini: subtropical/semi-arid. Cabo Verde: desert. |
| Desert/Semi-Arid (Cluster 1) N = 12 | Tropical Coastal (Cluster 2) N = 12 | Equatorial (Cluster 3) N = 14 | Southern African and Islands (Cluster 4) N = 5 | Kruskal–Wallis p-Value | UK | USA | |
|---|---|---|---|---|---|---|---|
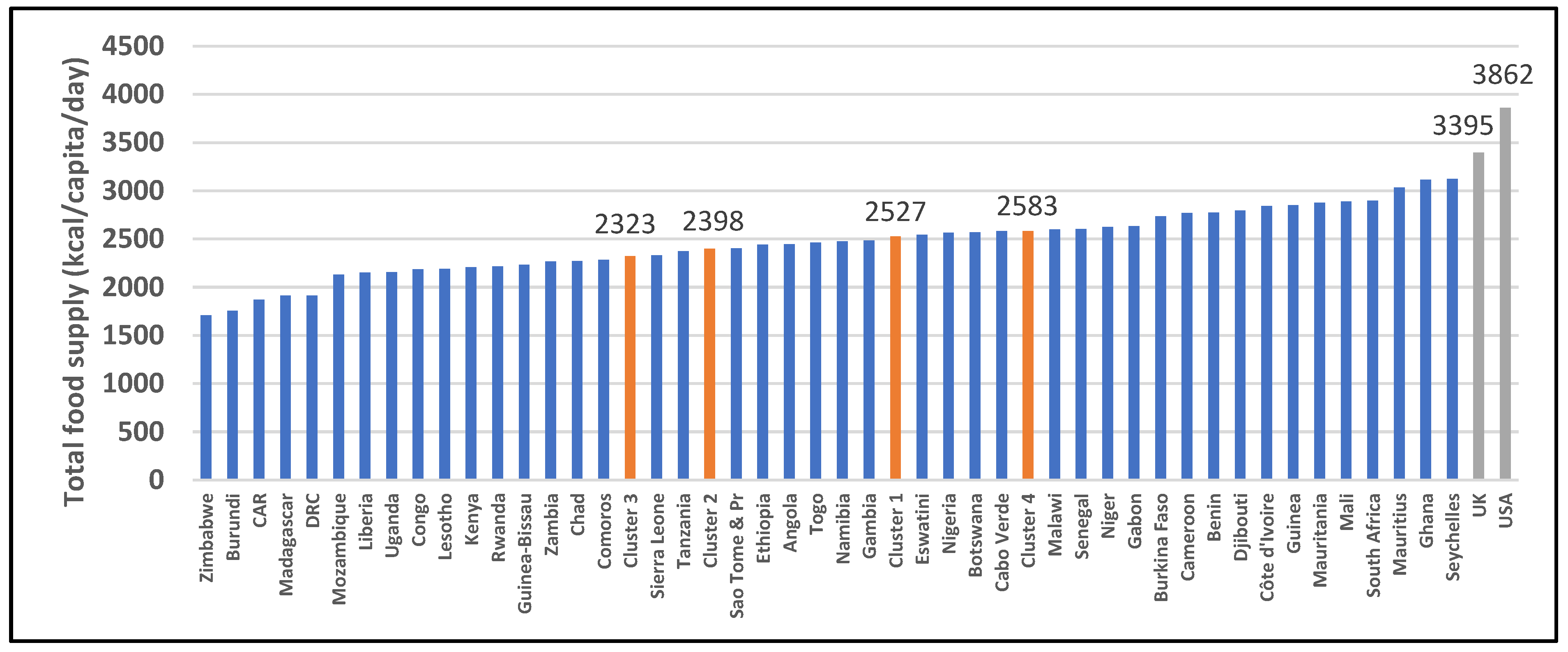
| 2901: All food items (kcal/capita/day) | 2527 (2198–2764) | 2398 (2249–2686) | 2323 (2156–2600) | 2583 (2544–2898) | 0.211 | 3395 | 3862 |
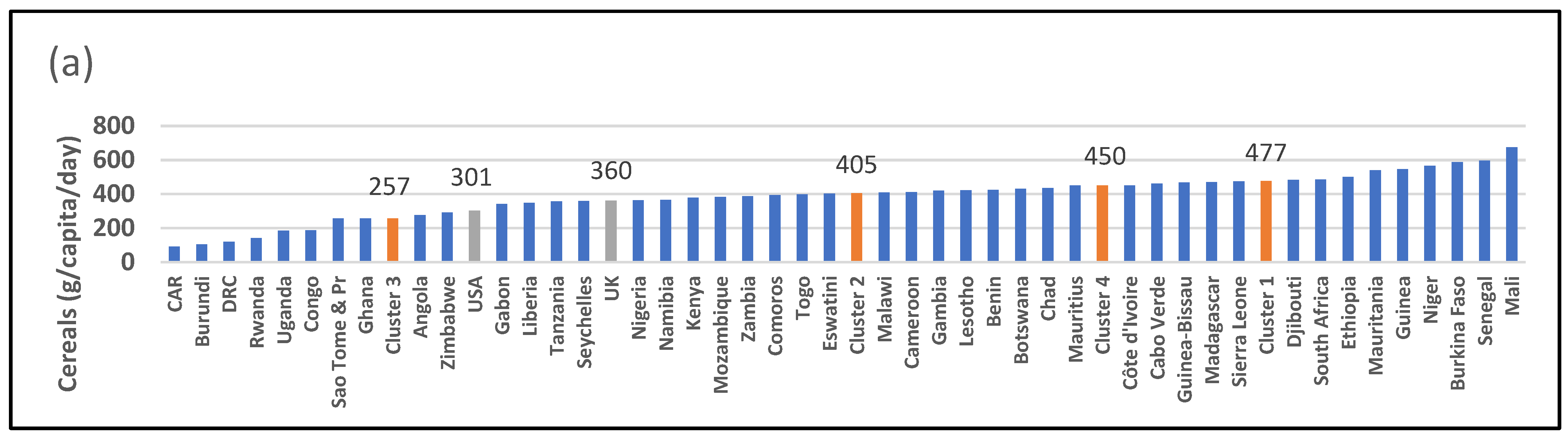
| 2905: Cereals (g/capita/day) | 477 [a] (422–552) | 405 [a] (385–471) | 257 [b] (141–356) | 450 [a] (402–461) | 0.0004 ** | 361 | 301 |
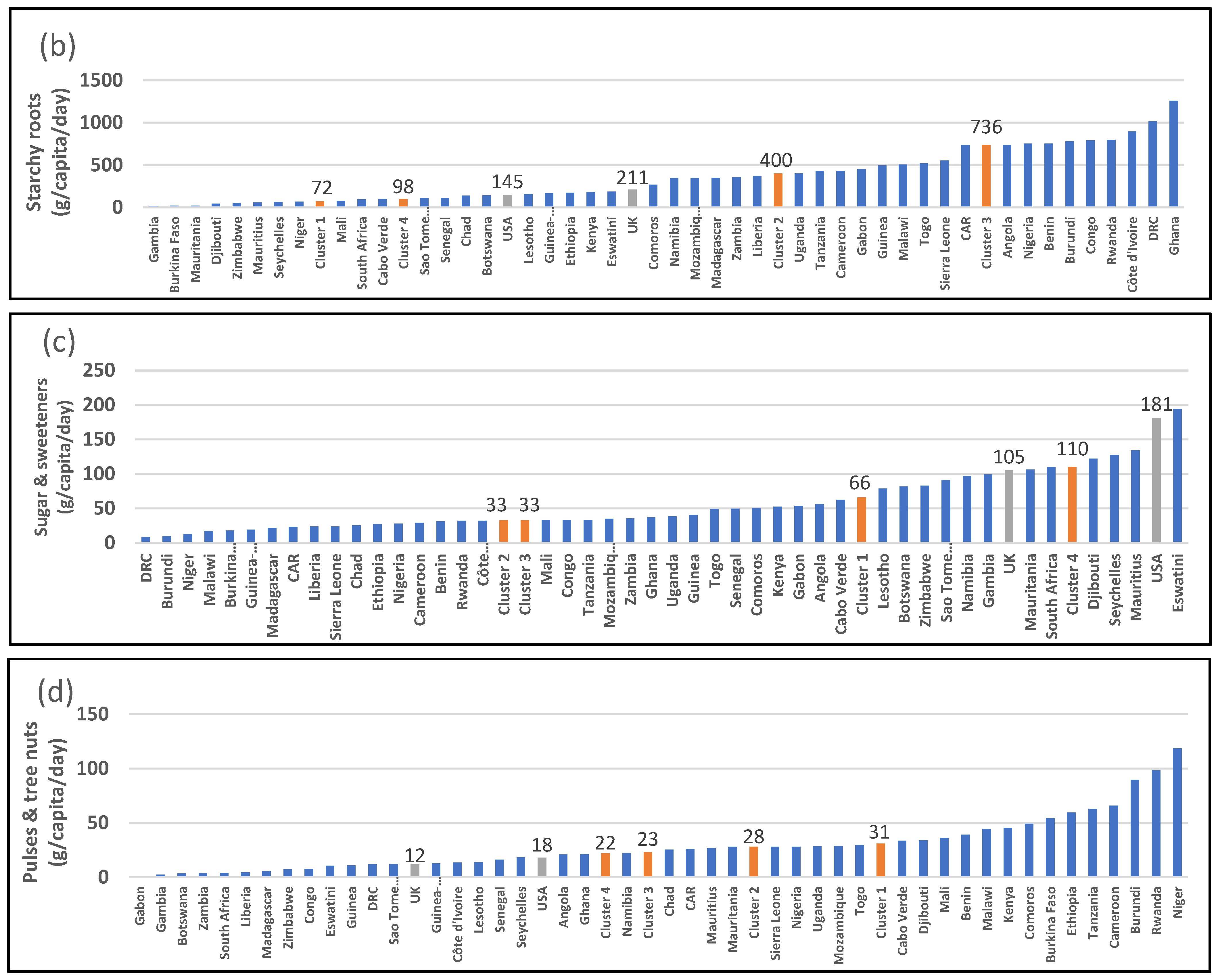
| 2907: Starchy roots (g/capita/day) | 72 [c] (33–163) | 400 [a] [b] (306–535) | 736 [a] (431–796) | 98 [b] [c] (94–186) | <0.001 *** | 211 | 145 |
| 2909: Sugar and sweeteners (g/capita/day) | 66 [b] (25–91) | 33 [b] (26–45) | 33 [b] (23–39) | 110 [a] (97–134) | 0.006 ** | 105 | 181 |
| 2911 + 2912: Pulses and tree nuts (g/capita/day) | 31 (6–50) | 28 (12–34) | 23 (12–44) | 22 (10–27) | 0.870 | 12 | 18 |
| 2914: Vegetable oils (g/capita/day) | 21 [a] [b] (16–34) | 32 [a] [b] (25–35) | 21 [b] (13–25) | 25 [a] (23–42) | 0.011 * | 37 | 55 |
| 2918 + 2919: Fruit and vegetables (g/capita/day) | 154 (71–305) | 217 (152–365) | 341 (197–611) | 308 (180–341) | 0.078 | 433 | 586 |
| 2924: Alcoholic beverages (g/capita/day) | 51 (11–99) | 30 (9–83) | 67 (52–146) | 180 (124–221) | 0.022 * | 243 | 248 |
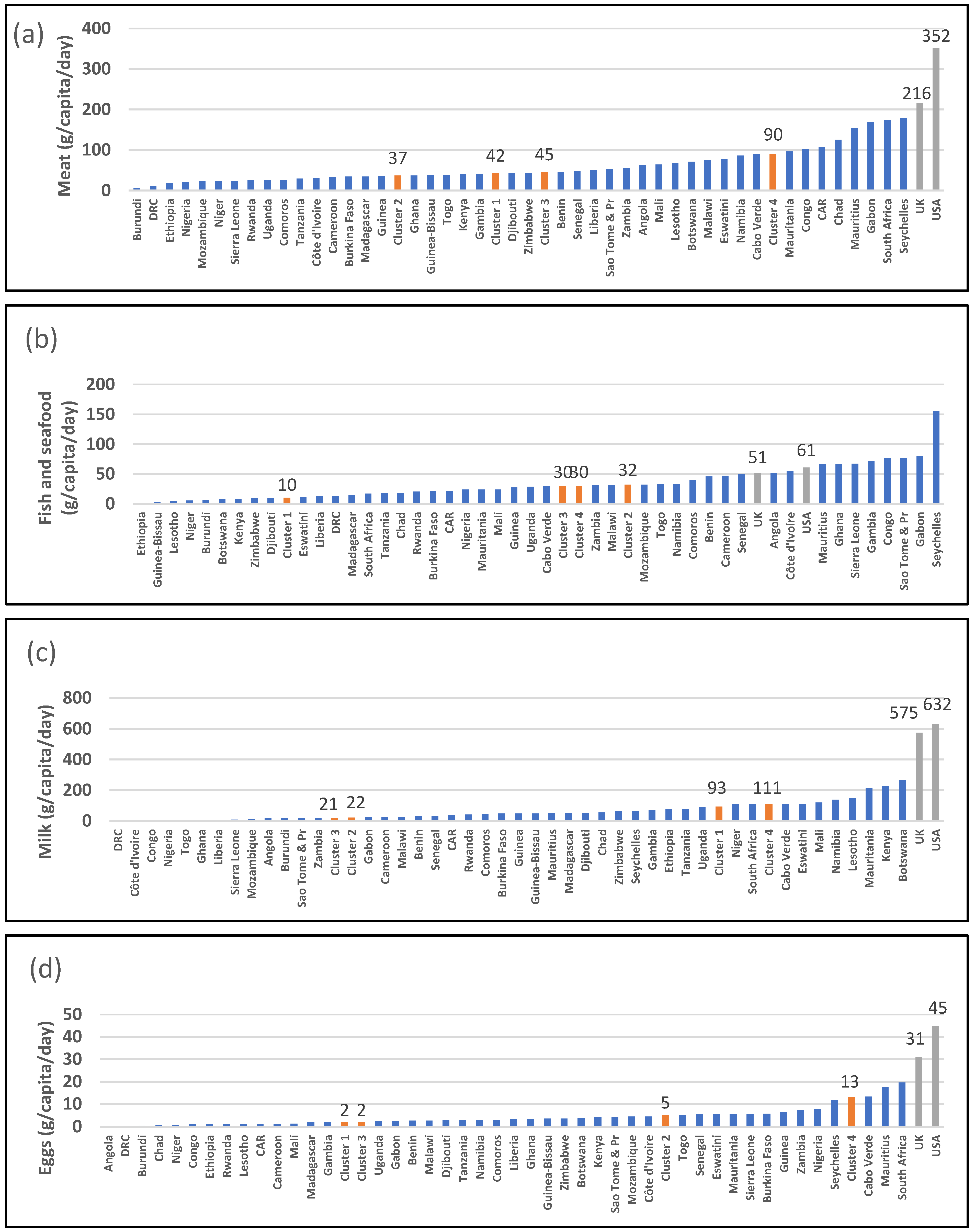
| 2943: Meat (g/capita/day) | 42 [b] (34–66) | 37 [b] (25–47) | 45 [b] (26–102) | 90 [a] (86–153) | 0.015 * | 216 | 352 |
| 2946: Animal fats (g/capita/day) | 2 [b] (1–3) | 1 [b] (1–1) | 1 [b] (0–3) | 3 [a] (2–4) | 0.006 ** | 12 | 10 |
| 2949: Eggs (g/capita/day) | 2 [b] (1–4) | 5 [b] (3–6) | 2 [b] (1–3) | 13 [a] (5–18) | 0.001 ** | 31 | 45 |
| 2948: Dairy (g/capita/day) | 93 [a] (59–181) | 22 [b] (7–40) | 21 [b] (5–42) | 111 [a] (110–111) | <0.001 *** | 575 | 632 |
| 2960: Fish and seafood (g/capita/day) | 10 (6–22) | 32 (26–46) | 30 (19–66) | 30 (17–31) | 0.024 * | 51 | 61 |
| Desert/Semi-Arid (Cluster 1) N = 12 | Tropical Coastal (Cluster 2) N = 12 | Equatorial (Cluster 3) N = 14 | Southern African and Islands (Cluster 4) N = 5 | Kruskal–Wallis p-Value | United Kingdom | United States | |
|---|---|---|---|---|---|---|---|
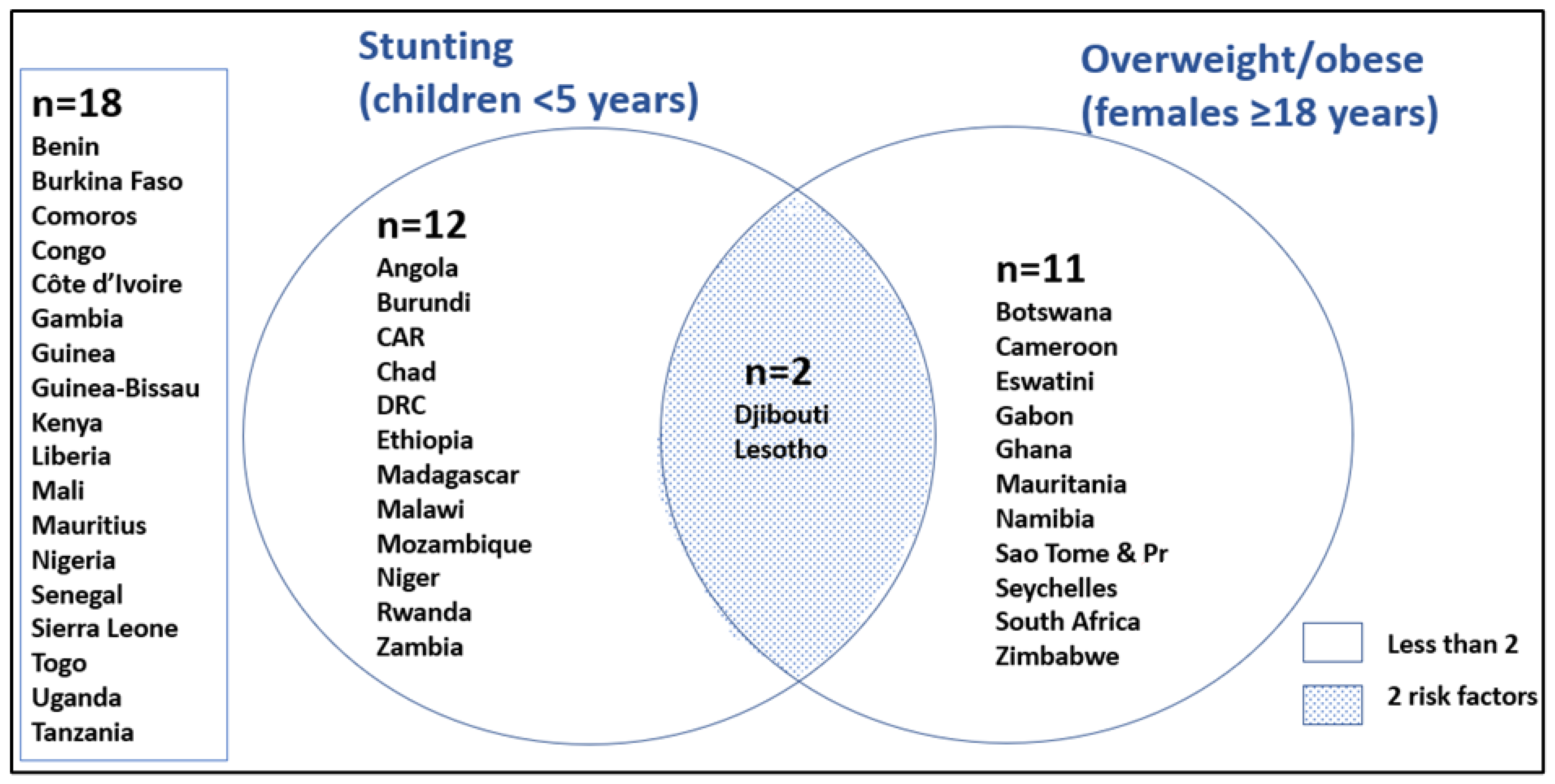
| Child stunting %HAZ < −2SD <5 years, 2021 | 27.7 (23.7–35.7) | 30.1 (28.5–31.9) | 31.8 (21.2–37.8) | 22.1 (17.5–24.1) | 0.1950 | NA | NA |
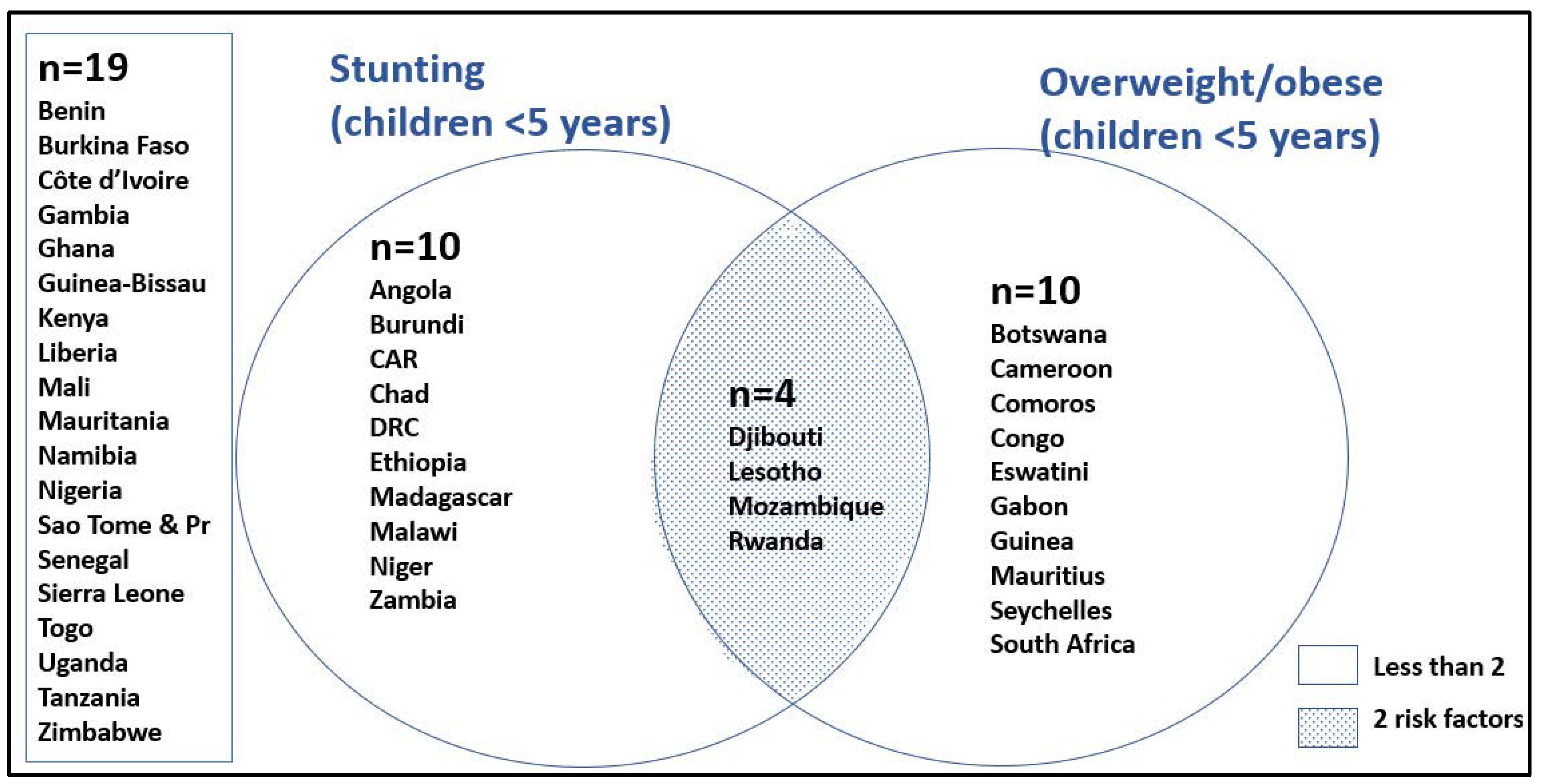
| Child overweight %WHZ > +2SD < 5 years, 2021 | 2.1 [b] (1.5–5.4) | 4.5 [b] (2.1–6.3) | 3.4 [b] (2.3–4.5) | 7.8 [a] (5.3–10.3) | 0.0848 | NA | NA |
| Concurrent stunting and overweight < 5 years, 2021 | 0.8 (0.5–1.8) | 1.8 (0.7–3.5) | 1.2 (0.6–1.6) | 1.7 (1.1–2.6) | 0.3398 | NA | NA |
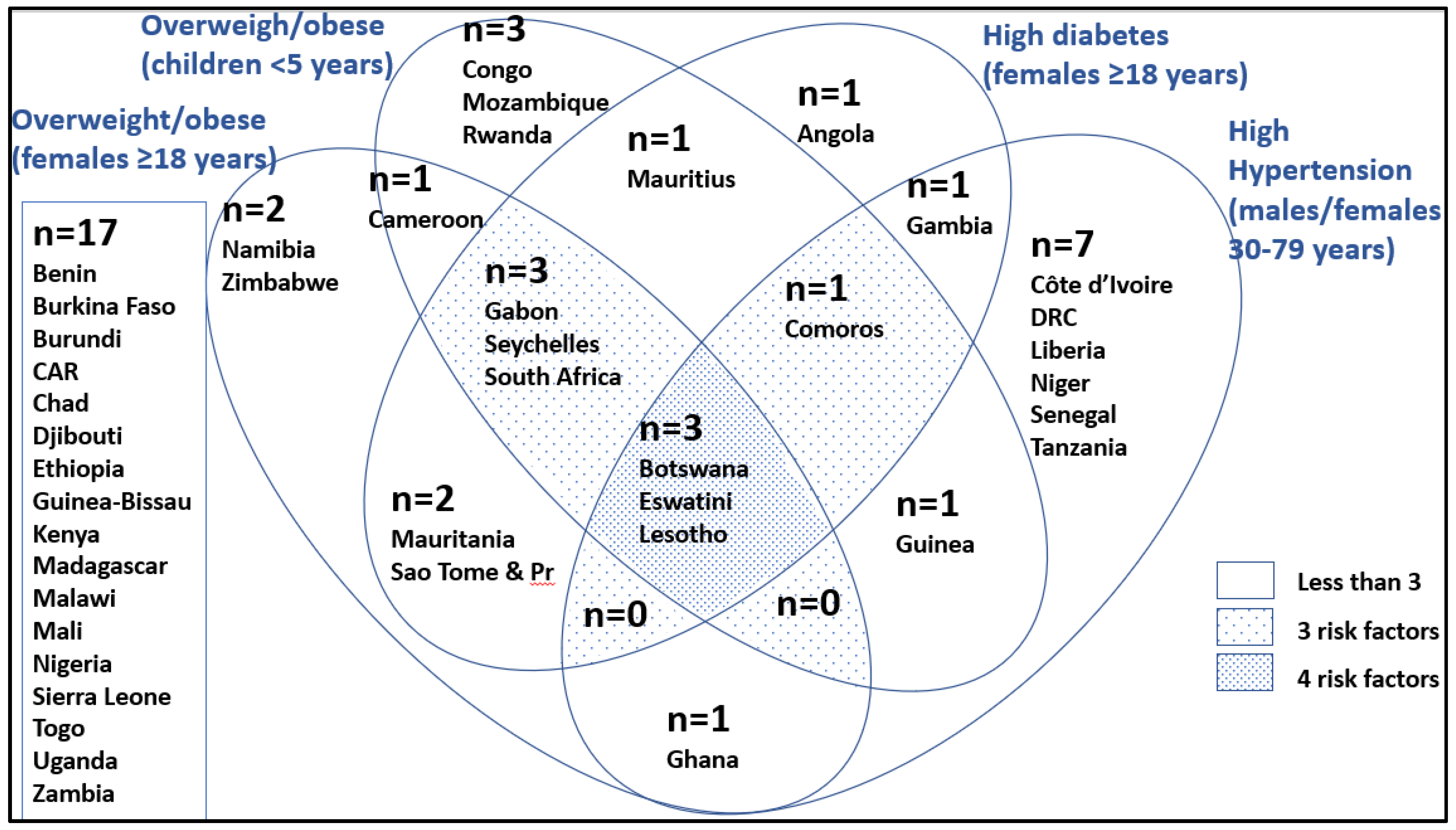
| Females 18 years and older, overweight BMI ≥ 25, age-standardised, 2017 | 37.0 [b] (29.6–48.7) | 36.0 [b] (35.7–37.3) | 34.9 [b] (31.5–39.5) | 51.9 [a] (41.4–52.6) | 0.3130 | 60.0 | 63.2 |
| Child anaemia # <5 years, 2019 Hb < 110 g/L | 52.1 [a] (43.3–72.0) | 68.6 [a] (63.6–72.4) | 58.8 [a] (56.1–62.4) | 44.1 [b] (42.7–44.4) | 0.0025 ** | 15.5 | 6.1 |
| Women 15–49 years Anaemia #, 2019 Hb < 110 g/L (pregnant) and Hb < 120 g/L (non-pregnant) | 37.8 [a] (28.7–49.5) | 48.0 [a] (41.6–50.6) | 44.2 [a] (35.4–46.8) | 25.2 [b] (24.3–30.5) | 0.0098 ** | 11.1 | 11.8 |
| Hypertension prevalence ## in male and female, age-standardised 30–79 years, 2019, mmHg | 36.7 (33.1–42.4) | 36.7 (33.8–40.2) | 35.6 (32.1–38.2) | 38.1 (32.7–48.8) | 0.8144 | 37.7 | 29.8 |
| Females 18 years and older, type 2 diabetes ###, 2014, age- standardised | 7.0 [b] (5.4–8.7) | 6.9 [b] (6.4–7.2) | 6.3 [b] (6.0–7.6) | 11.3 [a] (8.0–12.6) | 0.0291 * | 4.9 | 6.4 |
| Females’ total cholesterol, age-standardised, 18 years and older, 2018, mmol/L | 4.1 (4.1–4.3) | 4.2 (4.2–4.2) | 4.1 (4.1–4.2) | 4.2 (4.1–4.4) | 0.5390 | 4.8 | 4.7 |
| Females’ LDL-cholesterol, age-standardised, 18 years and older, 2018, mmol/L | 2.9 (2.8–3.0) | 2.9 (2.9–3.0) | 2.9 (2.8–3.0) | 2.9 (2.9–3.1) | 0.8756 | 3.2 | 3.2 |
| Females’ HDL-cholesterol, age-standardised, 18 years and older, 2018, mmol/L | 1.2 [a] [b] (1.1–1.2) | 1.1 [b] (1.1–1.2) | 1.2 [a] [b] (1.1–1.2) | 1.3 [a] (1.2–1.3) | 0.0335 * | 1.7 | 1.6 |
| Desert/Semi-Arid (Cluster 1) N = 12 | Tropical Coastal (Cluster 2) N = 12 | Equatorial (Cluster 3) N = 14 | Southern African and Islands (Cluster 4) N = 5 | Kruskal–Wallis p-Value | United Kingdom | United States | |
|---|---|---|---|---|---|---|---|
| GDP Current USD, 2020 | 996.0 [b] (784.0–1828.0) | 1139.0 [b] (710.5–1416.0) | 1086.0 [b] (710.0–2276.0) | 5010.0 [a] (3916.0–6625.0) | 0.0102 * | USD 2.8 million | USD 21.0 million |
| % Over 65 years (2020) | 3.1 [b] (2.5–4.0) | 2.9 [b] (2.8–3.1) | 2.7 [b] (2.5–3.0) | 4.8 [a] (4.0–5.5) | 0.0040 ** | 18.7 | 16.6 |
| % Urban dwellers | 35.4 (28.5–59.0) | 44.4 (40.0–50.2) | 42.2 (23.5–66.8) | 52.0 (40.8–66.7) | 0.8288 | 83.9 | 82.7 |
| % Informal urban dwellers | 57.1 (46.5–64.3) | 54.5 (50.1–69.4) | 48.3 (42.1–65.1) | 32.1 (25.6–42.3) | 0.1369 | - | - |
| Gini coefficient | 41.2 [b] (36.0–46.1) | 38.0 [b] (35.2–46.0) | 41.4 [a] [b] (38.5–43.7) | 54.6 [a] (42.4–59.1) | 0.2046 | 35.1 | 41.5 |
| Annual population growth (%) (2020) | 2.6 [a] (1.8–2.9) | 2.6 [a] (2.4–2.8) | 2.5 [a] (2.4–3.0) | 1.1 [b] (1.0–1.3) | 0.0081 ** | 0.6 | 1.0 |
| Birth rate (2020) | 4.0 [a] (3.3–5.1) | 4.5 [a] (4.2–4.7) | 4.5 [a] (4.1–4.8) | 2.4 [b] (2.2–2.9) | 0.0043 ** | 1.6 | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nel, J.H.; Steyn, N.P. The Nutrition Transition and the Double Burden of Malnutrition in Sub-Saharan African Countries: How Do These Countries Compare with the Recommended LANCET COMMISSION Global Diet? Int. J. Environ. Res. Public Health 2022, 19, 16791. https://doi.org/10.3390/ijerph192416791
Nel JH, Steyn NP. The Nutrition Transition and the Double Burden of Malnutrition in Sub-Saharan African Countries: How Do These Countries Compare with the Recommended LANCET COMMISSION Global Diet? International Journal of Environmental Research and Public Health. 2022; 19(24):16791. https://doi.org/10.3390/ijerph192416791
Chicago/Turabian StyleNel, Johanna H., and Nelia P. Steyn. 2022. "The Nutrition Transition and the Double Burden of Malnutrition in Sub-Saharan African Countries: How Do These Countries Compare with the Recommended LANCET COMMISSION Global Diet?" International Journal of Environmental Research and Public Health 19, no. 24: 16791. https://doi.org/10.3390/ijerph192416791
APA StyleNel, J. H., & Steyn, N. P. (2022). The Nutrition Transition and the Double Burden of Malnutrition in Sub-Saharan African Countries: How Do These Countries Compare with the Recommended LANCET COMMISSION Global Diet? International Journal of Environmental Research and Public Health, 19(24), 16791. https://doi.org/10.3390/ijerph192416791







