Associations between Depression and Self-Reported COVID-19 Symptoms among Adults: Results from Two Population-Based Seroprevalence Studies in Switzerland
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Laboratory Analysis
2.3. Measurements and Procedures
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Future Implications
4.2. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Period | Assessments | ||
|---|---|---|---|
| Geneva | Ticino | ||
| 2013–2019 | Patient Health Questionnaire depression module (PHQ-9). | N/A | |
| 2020 | April | Socio-demographic information, health status, and exposure to SARS-CoV-2-infected individuals. Self-reported COVID-19-compatible symptoms. Serological testing. | N/A |
| May | |||
| June | |||
| July | N/A | Socio-demographic information, health status, and exposure to SARS-CoV-2-infected individuals. Patient Health Questionnaire depression module (PHQ-9). Self-reported COVID-19-compatible symptoms. Serological testing. | |
| 2021 | January | Self-reported COVID-19-compatible symptoms. | Self-reported COVID-19-compatible symptoms. |
| February | |||
| Symptom Categories | Geneva | Ticino | ||
|---|---|---|---|---|
| Baseline (April–June 2020) | Follow-Up (February–January 2021) | Baseline (July 2020) | Follow-Up (February–January 2021) | |
| Systemic symptoms | fatigue, muscular and/or articular pain, loss of appetite | fatigue, muscular and/or articular pain, loss of appetite | fatigue, muscular and/or articular pain, loss of appetite | fatigue, muscular and/or articular pain, loss of appetite |
| Upper airways symptoms | runny and/or itchy and/or stuffy nose, sore throat | runny nose, itchy nose, stuffy nose, sore throat | runny nose, itchy nose, stuffy nose, sore throat | runny nose, itchy nose, stuffy nose, sore throat |
| Gastro-intestinal symptoms | abdominal pain, nausea and/or vomiting, diarrhoea | stomach pain, nausea and/or vomiting, diarrhoea | stomach pain, nausea and/or vomiting, diarrhoea | stomach pain, nausea and/or vomiting, diarrhoea |
| Fever and/or cough | fever, cough | fever (>38 °C), dry cough, heavy cough | fever (>38 °C), dry cough, heavy cough | fever (>38 °C), dry cough, heavy cough |
| Dyspnoea | shortness of breath | shortness of breath | shortness of breath, respiratory distress | shortness of breath, respiratory distress |
| Headache | headache | headache | headache | headache |
| Anosmia/dysgeusia | loss of taste and/or smell | loss of taste and/or smell | loss of taste, loss of smell | loss of taste, loss of smell |
| Symptom Categories | Geneva | Ticino | ||
|---|---|---|---|---|
| Baseline, N = 576 (April–June 2020) | Follow-Up, N = 365 (February–January 2021) | Baseline, N = 581 (July 2020) | Follow-Up, N = 536 (February–January 2021) | |
| Systemic symptoms | 213 (39.9) | 30 (8.2) | 150 (25.8) | 101 (18.8) |
| Upper airways symptoms | 268 (46.5) | 47 (12.9) | 162 (27.9) | 105 (19.6) |
| Gastro-intestinal symptoms | 101 (17.5) | 22 (6.0) | 54 (9.3) | 56 (10.5) |
| Fever and/or cough | 199 (34.6) | 26 (7.1) | 140 (24.1) | 49 (9.1) |
| Dyspnoea | 50 (8.7) | 6 (1.6) | 44 (7.6) | 22 (4.1) |
| Headache | 193 (33.5) | 33 (9.0) | 99 (17.0) | 92 (17.2) |
| Anosmia/dysgeusia | 39 (6.8) | 6 (1.6) | 25 (4.3) | 6 (1.1) |
| Asymptomatic | 197 (34.2) | 292 (80.0) | 326 (56.1) | 328 (61.2) |
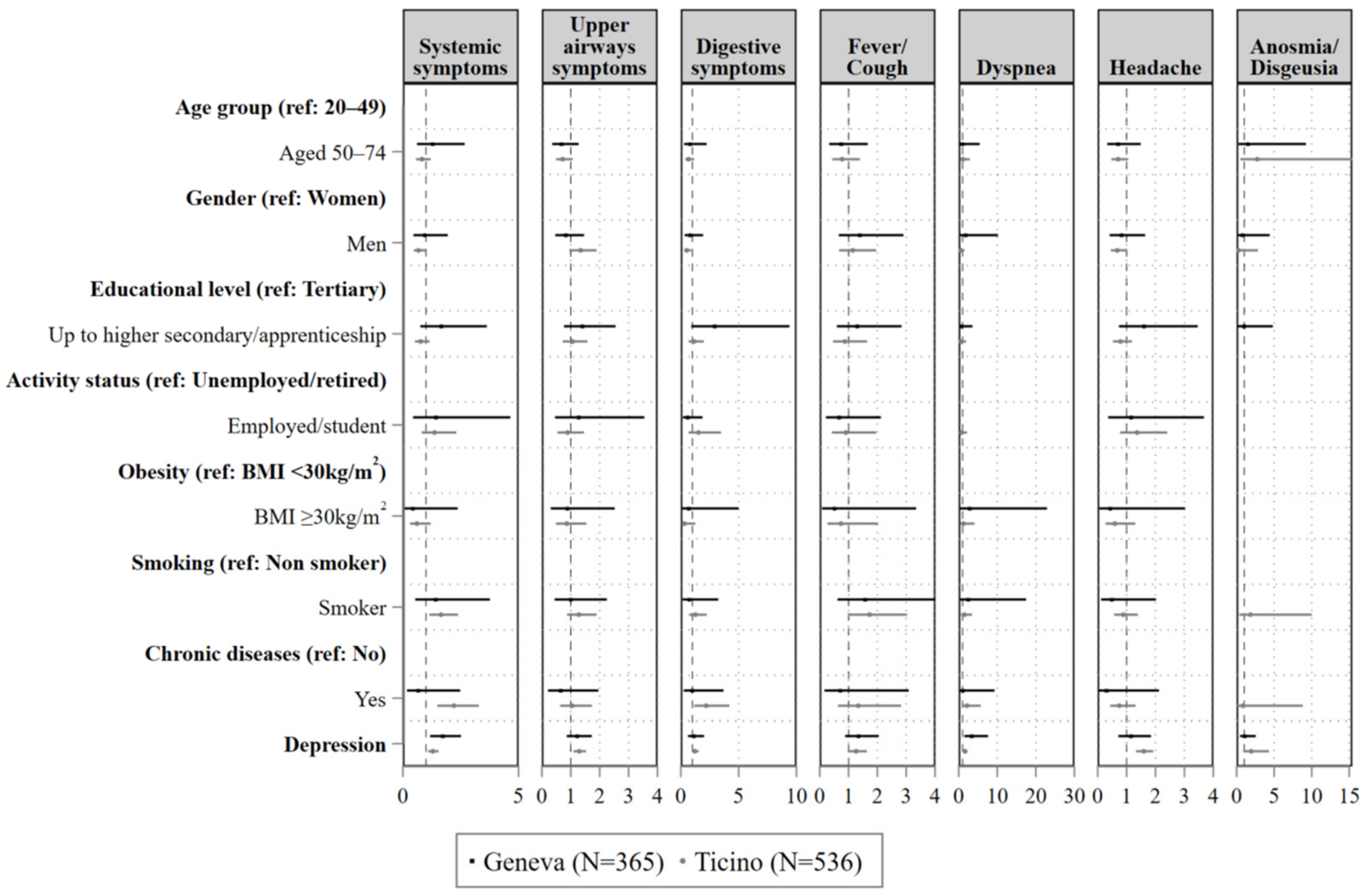
| Systemic Symptoms | Upper Airways Symptoms | Gastro-Intestinal Symptoms | Fever and/or Cough | Dyspnoea | Headache | Anosmia/ Dysgeusia | |
|---|---|---|---|---|---|---|---|
| Age group (ref: Aged 20–49) | |||||||
| Aged 50–64 | 0.98 (0.77, 1.24) | 0.92 (0.76, 1.11) | 0.77 (0.51, 1.17) | 0.98 (0.76, 1.25) | 1.20 (0.65, 2.22) | 0.89 (0.69, 1.14) | 0.99 (0.54, 1.84) |
| Gender (ref: Female) | |||||||
| Male | 0.86 (0.69, 1.07) | 0.84 (0.69, 1.01) | 0.62 (0.42, 0.91) * | 0.82 (0.64, 1.04) | 0.80 (0.45, 1.40) | 0.64 (0.49, 0.82) ** | 1.00 (0.58, 1.75) |
| Educational level (ref: Tertiary) | |||||||
| Up to higher secondary/ apprenticeship | 1.30 (1.03, 1.65) * | 1.05 (0.87, 1.26) | 1.51 (1.01, 2.26) * | 0.95 (0.76, 1.19) | 1.03 (0.58, 1.82) | 1.07 (0.84, 1.35) | 2.51 (1.12, 5.66) |
| Work status (ref: Unemployed/retired) | |||||||
| Employed/student | 0.93 (0.67, 1.27) | 0.97 (0.74, 1.27) | 0.88 (0.54, 1.45) | 0.84 (0.61, 1.16) | 1.02 (0.48, 2.16) | 1.05 (0.73, 1.51) | 0.60 (0.25, 1.47) |
| Obesity (ref: BMI < 30 kg/m2) | |||||||
| BMI ≥ 30 kg/m2 | 0.85 (0.57, 1.26) | 1.16 (0.89, 1.51) | 1.31 (0.78, 2.20) | 0.83 (0.54, 1.27) | 0.96 (0.41, 2.26) | 1.05 (0.72, 1.54) | 0.75 (0.28, 1.99) |
| Smoking (ref: No) | |||||||
| Yes | 1.10 (0.83, 1.45) | 1.05 (0.83, 1.33) | 1.46 (0.97, 2.20) | 0.97 (0.71, 1.34) | 1.66 (0.90, 3.07) | 1.01 (0.74, 1.39) | 0.68 (0.24, 1.94) |
| Chronic diseases (ref: No) | |||||||
| Yes | 1.28 (0.92, 1.76) | 1.03 (0.78, 1.35) | 1.05 (0.62, 1.77) | 1.13 (0.81, 1.58) | 2.52 (1.28, 4.98) ** | 1.08 (0.75, 1.54) | 1.12 (0.34, 3.74) |
| COVID-19 family cases (ref: No) | |||||||
| Yes | 1.11 (0.78, 1.59) | 1.06 (0.77, 1.44) | 0.65 (0.27, 1.55) | 0.98 (0.65, 1.48) | 0.95 (0.35, 2.58) | 1.33 (0.93, 1.90) | 0.90 (0.50, 1.62) |
| Anti-SARS-CoV-2 IgG (ref: Seronegative) | |||||||
| Seropositive | 2.00 (1.56, 2.57) *** | 1.48 (1.17, 1.87) ** | 1.55 (0.87, 2.77) | 2.17 (1.67, 2.80) *** | 2.92 (1.38, 6.19) ** | 1.64 (1.21, 2.21) ** | 18.07 (10.34, 31.60) *** |
| Depression | 1.15 (1.03, 1.30) * | 1.13 (1.03, 1.24) * | 1.43 (1.19, 1.71) *** | 1.15 (1.02, 1.30) * | 1.28 (0.96, 1.71) | 1.13 (0.99, 1.29) | 1.12 (0.84, 1.50) |
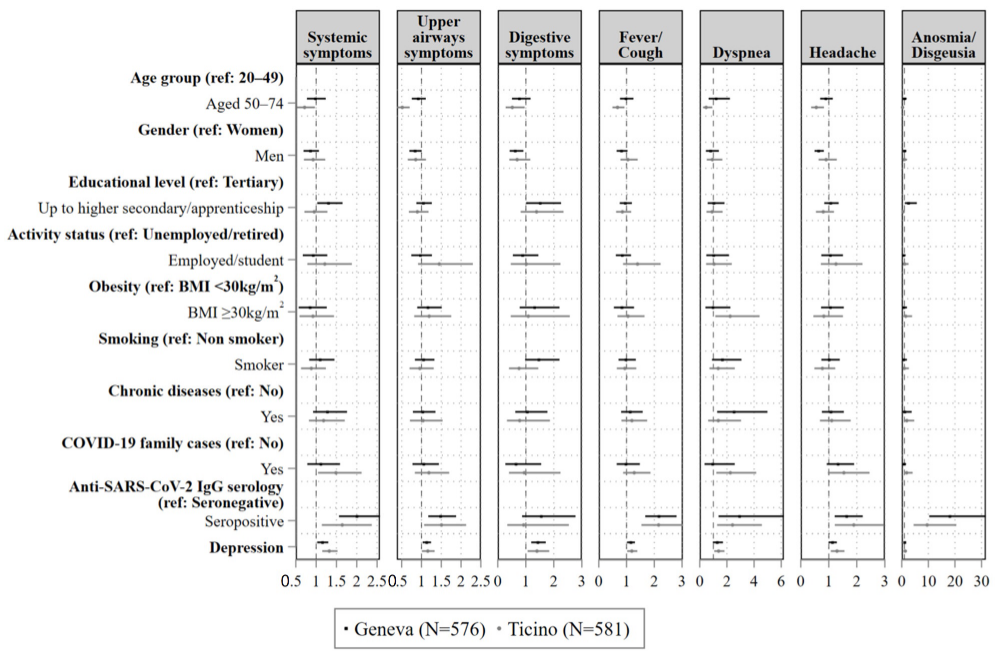
| Systemic Symptoms | Upper Airways Symptoms | Gastro-Intestinal Symptoms | Fever and/or Cough | Dyspnoea | Headache | Anosmia/ Dysgeusia | |
|---|---|---|---|---|---|---|---|
| Age group (ref: Aged 20–49) | |||||||
| Aged 50–64 | 0.72 (0.53, 0.97) * | 0.51 (0.38, 0.7) *** | 0.52 (0.28, 0.96) * | 0.67 (0.49, 0.93) * | 0.47 (0.24, 0.92) * | 0.55 (0.37, 0.83) ** | 0.3 (0.14, 0.65) ** |
| Gender (ref: Women) | |||||||
| Men | 0.93 (0.7, 1.23) | 0.85 (0.65, 1.11) | 0.69 (0.41, 1.16) | 1.05 (0.78, 1.4) | 0.93 (0.51, 1.66) | 0.9 (0.64, 1.29) | 0.99 (0.49, 2) |
| Educational level (ref: Tertiary) | |||||||
| Up to higher secondary/ apprenticeship | 0.95 (0.71, 1.28) | 0.9 (0.68, 1.18) | 1.38 (0.82, 2.34) | 0.85 (0.62, 1.17) | 0.91 (0.49, 1.68) | 0.8 (0.54, 1.19) | 0.45 (0.18, 1.16) |
| Work status (ref: Unemployed/retired) | |||||||
| Employed/student | 1.21 (0.78, 1.88) | 1.45 (0.91, 2.3) | 1.02 (0.46, 2.24) | 1.4 (0.87, 2.23) | 1.05 (0.46, 2.36) | 1.26 (0.72, 2.2) | 1.06 (0.44, 2.56) |
| Obesity (ref: BMI < 30 kg/m2) | |||||||
| BMI ≥ 30 kg/m2 | 0.92 (0.59, 1.44) | 1.2 (0.82, 1.75) | 1.09 (0.46, 2.57) | 1.05 (0.67, 1.65) | 2.24 (1.14, 4.41) * | 0.82 (0.45, 1.51) | 1.48 (0.56, 3.9) |
| Smoking (ref: No) | |||||||
| Yes | 0.88 (0.63, 1.24) | 0.96 (0.7, 1.31) | 0.76 (0.4, 1.45) | 0.94 (0.65, 1.35) | 1.36 (0.71, 2.58) | 0.77 (0.49, 1.23) | 0.99 (0.37, 2.68) |
| Chronic diseases (ref: No) | |||||||
| Yes | 1.18 (0.82, 1.71) | 1.04 (0.71, 1.53) | 0.78 (0.33, 1.86) | 1.19 (0.81, 1.74) | 1.37 (0.61, 3.05) | 1.1 (0.68, 1.78) | 1.91 (0.77, 4.7) |
| COVID-19 family cases (ref: No) | |||||||
| Yes | 1.49 (1.04, 2.12) * | 1.19 (0.83, 1.7) | 0.95 (0.4, 2.24) | 1.28 (0.88, 1.86) | 2.25 (1.22, 4.16) ** | 1.54 (0.96, 2.46) | 1.85 (0.83, 4.11) |
| Anti-SARS-CoV-2 IgG serology (ref: Seronegative) | |||||||
| Seropositive | 1.64 (1.14, 2.37) ** | 1.51 (1.07, 2.13) * | 0.92 (0.34, 2.54) | 2.16 (1.53, 3.03) *** | 2.42 (1.28, 4.57) ** | 1.89 (1.22, 2.95) ** | 9.55 (4.44, 20.53) *** |
| Depression | 1.33 (1.15, 1.53) *** | 1.16 (1.01, 1.33) * | 1.4 (1.06, 1.84) * | 1.19 (1.02, 1.39) * | 1.39 (1.06, 1.82) * | 1.29 (1.07, 1.56) ** | 1.28 (0.85, 1.91) |
| Systemic Symptoms | Upper Airways Symptoms | Gastro-Intestinal Symptoms | Fever and/or Cough | Dyspnoea | Headache | Anosmia/ Dysgeusia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | χ2 | p-Value | χ2 | p-Value | χ2 | p-Value | χ2 | p-Value | χ2 | p-Value | χ2 | p-Value | χ2 | |
| Depression with anti-SARS-CoV-2 IgG serology | 0.803 | 0.06 | 0.376 | 0.79 | 0.066 | 3.35 | 0.412 | 0.68 | 0.387 | 0.75 | 0.163 | 1.96 | 0.231 | 1.42 |
| Depression with gender | 0.255 | 1.32 | 0.799 | 0.07 | 0.902 | 0.02 | 0.300 | 1.06 | 0.895 | 0.02 | 0.782 | 0.08 | 0.966 | 0.00 |
| Depression with age group | 0.315 | 1.02 | 0.918 | 0.01 | 0.882 | 0.02 | 0.364 | 0.82 | 0.216 | 1.53 | 0.635 | 0.22 | 0.998 | 0.00 |
| Systemic Symptoms | Upper Airways Symptoms | Gastro-Intestinal Symptoms | Fever and/or Cough | Dyspnoea | Headache | Anosmia/ Dysgeusia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | χ2 | p-Value | χ2 | p-Value | χ2 | p-Value | χ2 | p-Value | χ2 | p-Value | χ2 | p-Value | χ2 | |
| Depression with anti-SARS-CoV-2 IgG serology | 0.364 | 0.82 | 0.679 | 0.17 | 0.100 | 2.64 | 0.204 | 1.61 | 0.929 | 0.01 | 0.498 | 0.46 | 0.831 | 0.05 |
| Depression with gender | 0.149 | 2.06 | 0.448 | 0.57 | 0.796 | 0.07 | 0.096 | 2.74 | 0.109 | 2.54 | 0.970 | 0.00 | 0.117 | 2.49 |
| Depression with age group | 0.122 | 2.34 | 0.221 | 1.46 | 0.351 | 0.90 | 0.632 | 0.23 | 0.907 | 0.01 | 0.205 | 1.57 | 0.648 | 0.21 |
| Systemic Symptoms | Upper Airways Symptoms | Gastro-Intestinal Symptoms | Fever and/or Cough | Dyspnoea | Headache | Anosmia/ Dysgeusia | |
|---|---|---|---|---|---|---|---|
| Age group (ref: Aged 20–49) | |||||||
| Aged 50–64 | 1.27 (0.61, 2.67) | 0.67 (0.36, 1.27) | 0.79 (0.28, 2.23) | 0.74 (0.33, 1.67) | 0.96 (0.17, 5.53) | 0.7 (0.33, 1.49) | 1.48 (0.24, 9.23) |
| Gender (ref: Women) | |||||||
| Men | 0.94 (0.46, 1.94) | 0.82 (0.46, 1.46) | 0.8 (0.33, 1.93) | 1.39 (0.66, 2.9) | 1.77 (0.31, 10.19) | 0.82 (0.41, 1.64) | 0.77 (0.14, 4.41) |
| Educational level (ref: Tertiary) | |||||||
| Up to higher secondary/ apprenticeship | 0.61 (0.28, 1.33) | 0.72 (0.39, 1.31) | 0.34 (0.11, 1.1) | 0.77 (0.35, 1.69) | 1.66 (0.28, 9.84) | 0.63 (0.29, 1.36) | 1.03 (0.21, 5.10) |
| Work status a (ref: Unemployed/retired) | |||||||
| Employed/student | 1.42 (0.44, 4.65) | 1.27 (0.45, 3.54) | 0.59 (0.18, 1.88) | 0.67 (0.21, 2.12) | - | 1.15 (0.36, 3.68) | - |
| Obesity a (ref: BMI < 30 kg/m2) | |||||||
| BMI ≥ 30 kg/m2 | 0.42 (0.07, 2.37) | 0.88 (0.31, 2.52) | 0.68 (0.09, 5) | 0.51 (0.08, 3.34) | 2.82 (0.35, 22.88) | 0.43 (0.06, 3.02) | - |
| Smoking a (ref: No) | |||||||
| Yes | 1.41 (0.53, 3.76) | 0.99 (0.44, 2.25) | 0.72 (0.16, 3.24) | 1.57 (0.61, 4.01) | 2.42 (0.33, 17.47) | 0.49 (0.12, 2.02) | - |
| Chronic diseases a (ref: No) | |||||||
| Yes | 0.66 (0.17, 2.48) | 0.64 (0.21, 1.96) | 0.97 (0.26, 3.69) | 0.71 (0.16, 3.09) | 1.04 (0.12, 9.29) | 0.3 (0.04, 2.12) | - |
| Depression | 1.72 (1.18, 2.51) ** | 1.22 (0.86, 1.73) | 1.11 (0.61, 2.01) | 1.34 (0.88, 2.05) | 3.34 (1.46, 7.62) ** | 1.15 (0.71, 1.85) | 1.07 (0.44, 2.55) |
| Systemic Symptoms | Upper Airways Symptoms | Gastro-Intestinal Symptoms | Fever and/or Cough | Dyspnoea | Headache | Anosmia/ Dysgeusia | |
|---|---|---|---|---|---|---|---|
| Age group (ref: Aged 20–49) | |||||||
| Aged 50–64 | 0.82 (0.57, 1.2) | 0.72 (0.49, 1.07) | 0.67 (0.39, 1.16) | 0.78 (0.43, 1.39) | 1.12 (0.43, 2.88) | 0.71 (0.47, 1.06) | 2.75 (0.49, 15.41) |
| Gender (ref: Women) | |||||||
| Men | 0.68 (0.47, 0.97) * | 1.34 (0.95, 1.9) | 0.52 (0.3, 0.89) * | 1.15 (0.67, 1.96) | 0.63 (0.27, 1.5) | 0.68 (0.45, 1.02) | 0.27 (0.03, 2.84) |
| Educational level a (ref: Tertiary) | |||||||
| Up to higher secondary/ apprenticeship | 1.3 (0.86, 1.95) | 0.94 (0.63, 1.39) | 0.87 (0.5, 1.5) | 1.15 (0.61, 2.16) | 1.56 (0.53, 4.63) | 1.27 (0.84, 1.9) | - |
| Work status a (ref: Unemployed/retired) | |||||||
| Employed/student | 1.37 (0.81, 2.31) | 0.89 (0.54, 1.46) | 1.53 (0.67, 3.48) | 0.91 (0.42, 1.96) | 0.76 (0.27, 2.15) | 1.36 (0.77, 2.4) | - |
| Obesity a (ref: BMI < 30 kg/m2) | |||||||
| BMI ≥ 30 kg/m2 | 0.61 (0.31, 1.2) | 0.86 (0.48, 1.54) | 0.31 (0.08, 1.25) | 0.73 (0.27, 2.02) | 1.21 (0.36, 4.08) | 0.6 (0.27, 1.3) | - |
| Smoking (ref: No) | |||||||
| Yes | 1.65 (1.14, 2.38) ** | 1.28 (0.87, 1.89) | 1.27 (0.72, 2.25) | 1.73 (0.98, 3.03) | 1.46 (0.61, 3.47) | 0.89 (0.57, 1.39) | 1.84 (0.34, 10.01) |
| Chronic diseases (ref: No) | |||||||
| Yes | 2.21 (1.49, 3.28) *** | 1.05 (0.63, 1.73) | 2.2 (1.15, 4.2) * | 1.34 (0.63, 2.82) | 2.18 (0.84, 5.7) | 0.75 (0.43, 1.31) | 0.84 (0.08, 8.81) |
| Depression | 1.29 (1.09, 1.54) ** | 1.29 (1.10, 1.53) ** | 1.22 (0.95, 1.55) | 1.26 (0.98, 1.64) | 1.63 (1.16, 2.31) ** | 1.59 (1.32, 1.92) *** | 1.95 (0.88, 4.31) |
References
- Cénat, J.M.; Blais-Rochette, C.; Kokou-Kpolou, C.K.; Noorishad, P.-G.; Mukunzi, J.N.; McIntee, S.-E.; Dalexis, R.D.; Goulet, M.-A.; Labelle, P.R. Prevalence of symptoms of depression, anxiety, insomnia, posttraumatic stress disorder, and psychological distress among populations affected by the COVID-19 pandemic: A systematic review and meta-analysis. Psychiatry Res. 2021, 295, 113599. [Google Scholar] [CrossRef] [PubMed]
- Phiri, P.; Ramakrishnan, R.; Rathod, S.; Elliot, K.; Thayanandan, T.; Sandle, N.; Haque, N.; Chau, S.W.; Wong, O.W.; Chan, S.S.; et al. An evaluation of the mental health impact of SARS-CoV-2 on patients, general public and healthcare professionals: A systematic review and meta-analysis. EClinicalMedicine 2021, 34, 100806. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.C.d.S.; Jaspal, R. Understanding the mental health burden of COVID-19 in the United Kingdom. Psychol. Trauma Theory Res. Pract. Policy 2020, 12, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Newby, J.M.; O’Moore, K.; Tang, S.; Christensen, H.; Faasse, K. Acute mental health responses during the COVID-19 pandemic in Australia. PLoS ONE 2020, 15, e0236562. [Google Scholar] [CrossRef]
- Bendau, A.; Petzold, M.B.; Pyrkosch, L.; Mascarell Maricic, L.; Betzler, F.; Rogoll, J.; Große, J.; Ströhle, A.; Plag, J. Associations between COVID-19 related media consumption and symptoms of anxiety, depression and COVID-19 related fear in the general population in Germany. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 283–291. [Google Scholar] [CrossRef]
- Schweda, A.; Weismüller, B.; Bäuerle, A.; Dörrie, N.; Musche, V.; Fink, M.; Kohler, H.; Teufel, M.; Skoda, E.-M. Phenotyping mental health: Age, community size, and depression differently modulate COVID-19-related fear and generalized anxiety. Compr. Psychiatry 2020, 104, 152218. [Google Scholar] [CrossRef]
- Yan, L.; Gan, Y.; Ding, X.; Wu, J.; Duan, H. The relationship between perceived stress and emotional distress during the COVID-19 outbreak: Effects of boredom proneness and coping style. J. Anxiety Disord. 2020, 77, 102328. [Google Scholar] [CrossRef]
- Haug, T.T.; Mykletun, A.; Dahl, A.A. The Association Between Anxiety, Depression, and Somatic Symptoms in a Large Population: The HUNT-II Study. Psychosom. Med. 2004, 66, 845–851. [Google Scholar] [CrossRef]
- Vaccarino, A.L.; Sills, T.L.; Evans, K.R.; Kalali, A.H. Prevalence and association of somatic symptoms in patients with Major Depressive Disorder. J. Affect. Disord. 2008, 110, 270–276. [Google Scholar] [CrossRef]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines; World Health Organization: Geneva, Switzerland, 1992; Available online: https://apps.who.int/iris/handle/10665/37958 (accessed on 9 November 2022).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Stringhini, S.; Wisniak, A.; Piumatti, G.; Azman, A.S.; Lauer, S.A.; Baysson, H.; de Ridder, D.; Petrovic, D.; Schrempft, S.; Marcus, K.; et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): A population-based study. Lancet 2020, 396, 313–319. [Google Scholar] [CrossRef]
- West, E.A.; Anker, D.; Amati, R.; Richard, A.; Wisniak, A.; Butty, A.; Albanese, E.; Bochud, M.; Chiolero, A.; Crivelli, L.; et al. Corona Immunitas: Study protocol of a nationwide program of SARS-CoV-2 seroprevalence and seroepidemiologic studies in Switzerland. Int. J. Public Health 2020, 65, 1529–1548. [Google Scholar] [CrossRef] [PubMed]
- Guessous, I.; Bochud, M.; Theler, J.M.; Gaspoz, J.M.; Pechère-Bertschi, A. 1999–2009 Trends in Prevalence, Unawareness, Treatment and Control of Hypertension in Geneva, Switzerland. PLoS ONE 2012, 7, e39877. [Google Scholar] [CrossRef] [PubMed]
- Digital Platform Specchio-COVID19. Available online: https://www.specchio-covid19.ch/ (accessed on 4 July 2022).
- The Corona Immunitas Study. Available online: https://www.corona-immunitas.ch/en/ (accessed on 4 July 2022).
- Fenwick, C.; Croxatto, A.; Coste, A.T.; Pojer, F.; André, C.; Pellaton, C.; Farina, A.; Campos, J.; Hacker, D.; Lau, K.; et al. Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J. Virol. 2021, 95, e01828-20. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Löwe, B.; Kroenke, K.; Herzog, W.; Gräfe, K. Measuring depression outcome with a brief self-report instrument: Sensitivity to change of the Patient Health Questionnaire (PHQ-9). J. Affect. Disord. 2004, 81, 61–66. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Samejima, F. Graded Response Model. In Handbook of Modern Item Response Theory; van der Linden, W.J., Hambleton, R.K., Eds.; Springer: New York, NY, USA, 1997; pp. 85–100. Available online: https://doi.org/10.1007/978-1-4757-2691-6_5 (accessed on 9 November 2022).
- Richard, A.; Wisniak, A.; Perez-Saez, J.; Garrison-Desany, H.; Petrovic, D.; Piumatti, G.; Baysson, H.; Picazio, A.; Pennacchio, F.; De Ridder, D.; et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies, risk factors for infection and associated symptoms in Geneva, Switzerland: A population-based study. Scand. J. Public Health 2021, 50, 124–135. [Google Scholar] [CrossRef]
- Barros, A.J.; Hirakata, V.N. Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio. BMC Med. Res. Methodol. 2003, 3, 21. [Google Scholar] [CrossRef]
- Coutinho, L.M.S.; Scazufca, M.; Menezes, P.R. Methods for estimating prevalence ratios in cross-sectional studies. Rev. Saúde Pública 2008, 42, 992–998. [Google Scholar] [CrossRef]
- Aiken, L.S.; West, S.G.; Reno, R.R. Multiple Regression: Testing and Interpreting Interactions; SAGE: Thousand Oaks, CA, USA, 1991; 228p. [Google Scholar]
- Giorli, A.; Ferretti, F.; Biagini, C.; Salerni, L.; Bindi, I.; Dasgupta, S.; Pozza, A.; Gualtieri, G.; Gusinu, R.; Coluccia, A.; et al. A Literature Systematic Review with Meta-Analysis of Symptoms Prevalence in COVID-19: The Relevance of Olfactory Symptoms in Infection Not Requiring Hospitalization. Curr. Treat. Options Neurol. 2020, 22, 36. [Google Scholar] [CrossRef]
- Karni, N.; Klein, H.; Asseo, K.; Benjamini, Y.; Israel, S.; Nammary, M.; Olshtain-Pops, K.; Nir-Paz, R.; Hershko, A.; Muszkat, M.; et al. Self-Rated Smell Ability Enables Highly Specific Predictors of COVID-19 Status: A Case–Control Study in Israel. Open Forum Infect. Dis. 2020, 8, ofaa589. [Google Scholar] [CrossRef] [PubMed]
- Struyf, T.; Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Leeflang, M.M.; Spijker, R.; Hooft, T.; Emperador, D.; Domen, J.; et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database Syst. Rev. 2022. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Coronavirus Disease (COVID-19). 2021. Available online: https://www.who.int/health-topics/coronavirus#tab=tab_1 (accessed on 9 June 2021).
- Larkin, D.; Martin, C.R. The interface between chronic fatigue syndrome and depression: A psychobiological and neurophysiological conundrum. Neurophysiol. Clin./Clin. Neurophysiol. 2017, 47, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Matte, D.L.; Pizzichini, M.M.M.; Hoepers, A.T.C.; Diaz, A.P.; Karloh, M.; Dias, M.; Pizzichini, E. Prevalence of depression in COPD: A systematic review and meta-analysis of controlled studies. Respir. Med. 2016, 117, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Ciharova, M.; Quero, S.; Miguel, C.; Driessen, E.; Harrer, M.; Purgato, M.; Ebert, D.; Karyotaki, E. The Contribution of “Individual Participant Data” Meta-Analyses of Psychotherapies for Depression to the Development of Personalized Treatments: A Systematic Review. J. Pers. Med. 2022, 12, 93. [Google Scholar] [CrossRef]
- Cuijpers, P.; Reynolds, C.F., III. Increasing the Impact of Prevention of Depression—New Opportunities. JAMA Psychiatry 2022, 79, 11–12. [Google Scholar] [CrossRef]
- Antonelli, M.; Capdevila, J.; Chaudhari, A.; Granerod, J.; Canas, L.; Graham, M.; Klaser, K.; Modat, M.; Molteni, E.; Murray, B.; et al. Optimal symptom combinations to aid COVID-19 case identification: Analysis from a community-based, prospective, observational cohort. J. Infect. 2021, 82, 384–390. [Google Scholar] [CrossRef]
- Menni, C.; Sudre, C.H.; Steves, C.J.; Ourselin, S.; Spector, T.D. Quantifying additional COVID-19 symptoms will save lives. Lancet 2020, 395, e107–e108. [Google Scholar] [CrossRef]
- Menni, C.; Valdes, A.M.; Freidin, M.B.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Ganesh, S.; Varsavsky, T.; Cardoso, M.J.; El-Sayed Moustafa, J.S.; et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020, 26, 1037–1040. [Google Scholar] [CrossRef]
- Sudre, C.H.; Lee, K.A.; Ni Lochlainn, M.; Varsavsky, T.; Murray, B.; Graham, M.S.; Menni, C.; Modat, M.; Bowyer, R.C.E.; Nguyen, L.H.; et al. Symptom clusters in COVID-19: A potential clinical prediction tool from the COVID Symptom Study app. Sci. Adv. 2021, 7, eabd4177. [Google Scholar] [CrossRef]
- Daniali, H.; Flaten, M.A. What Psychological Factors Make Individuals Believe They Are Infected by Coronavirus 2019? Frontiers in Psychology. 2021. Available online: https://www.frontiersin.org/articles/10.3389/fpsyg.2021.667722 (accessed on 9 November 2022).
- Daniali, H.; Flaten, M.A. Experiencing COVID-19 symptoms without the disease: The role of nocebo in reporting of symptoms. Scand. J. Public Health 2022, 50, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Mitsikostas, D.D.; Blease, C.; Carlino, E.; Colloca, L.; Geers, A.L.; Howick, J.; Evers, A.W.M.; Flaten, M.A.; Kelley, J.M.; Kirsch, I.; et al. European Headache Federation recommendations for placebo and nocebo terminology. J. Headache Pain 2020, 21, 117. [Google Scholar] [CrossRef] [PubMed]
- Colizzi, M.; Bortoletto, R.; Silvestri, M.; Mondini, F.; Puttini, E.; Cainelli, C.; Gaudino, R.; Ruggeri, M.; Zoccante, L. Medically unexplained symptoms in the times of COVID-19 pandemic: A case-report. Brain Behav. Immun.-Health 2020, 5, 100073. [Google Scholar] [CrossRef] [PubMed]
- Shevlin, M.; Nolan, E.; Owczarek, M.; McBride, O.; Murphy, J.; Gibson Miller, J.; Hartman, T.K.; Levita, L.; Mason, L.; Martinez, A.P.; et al. COVID-19-related anxiety predicts somatic symptoms in the UK population. Br. J. Health Psychol. 2020, 25, 875–882. [Google Scholar] [CrossRef]
- Willis, C.; Chalder, T. Concern for COVID-19 cough, fever and impact on mental health. What about risk of Somatic Symptom Disorder? J. Ment. Health 2021, 30, 551–555. [Google Scholar] [CrossRef]
- Yifan, T.; Ying, L.; Chunhong, G.; Jing, S.; Rong, W.; Zhenyu, L.; Zejuan, G.; Peihung, L. Symptom Cluster of ICU Nurses Treating COVID-19 Pneumonia Patients in Wuhan, China. J. Pain Symptom Manag. 2020, 60, e48–e53. [Google Scholar] [CrossRef]
- Bhattacharyya, N.; Wasan, A. Do Anxiety and Depression Confound Symptom Reporting and Diagnostic Accuracy in Chronic Rhinosinusitis? Ann. Otol. Rhinol. Laryngol. 2008, 117, 18–23. [Google Scholar] [CrossRef]
- Chan, A.S.W.; Ho, J.M.C.; Li, J.S.F.; Tam, H.L.; Tang, P.M.K. Impacts of COVID-19 Pandemic on Psychological Well-Being of Older Chronic Kidney Disease Patients. Front. Med. 2021, 8, 666973. [Google Scholar] [CrossRef]
- Do, B.N.; Tran, T.V.; Phan, D.T.; Nguyen, H.C.; Nguyen, T.T.P.; Nguyen, H.C.; Ha, T.H.; Dao, H.K.; Trinh, M.V.; Do, T.V.; et al. Health Literacy, eHealth Literacy, Adherence to Infection Prevention and Control Procedures, Lifestyle Changes, and Suspected COVID-19 Symptoms Among Health Care Workers During Lockdown: Online Survey. J. Med. Internet Res. 2020, 22, e22894. [Google Scholar] [CrossRef]
| Geneva | Ticino | |||
|---|---|---|---|---|
| Baseline (April–June 2020) | Follow-Up (February–January 2021) | Baseline (July 2020) | Follow-Up (February–January 2021) | |
| N | 576 | 365 | 581 | 536 |
| Age, years, mean (SD) | 46 (11) | 46 (11) | 46 (11) | 46 (11) |
| Age group (years) | ||||
| 20–49 | 333 (57.8) | 212 (58.1) | 327 (56.3) | 293 (54.7) |
| 50–64 | 243 (42.2) | 153 (41.9) | 254 (43.7) | 243 (45.3) |
| Gender | ||||
| Female | 333 (57.8) | 222 (60.8) | 328 (56.5) | 310 (57.8) |
| Male | 243 (42.2) | 143 (39.2) | 253 (43.5) | 226 (42.2) |
| Educational level | ||||
| Up to higher secondary/apprenticeship | 195 (33.9) | 122 (33.4) | 374 (64.4) | 345 (64.4) |
| Tertiary | 381 (66.2) | 243 (66.6) | 207 (35.6) | 191 (35.6) |
| Work status | ||||
| Unemployed/retired | 70 (12.2) | 38 (10.4) | 93 (16.0) | 87 (16.2) |
| Employed/student | 506 (87.8) | 327 (89.6) | 488 (84.0) | 449 (83.8) |
| Obese (BMI ≥ 30 kg/m2) | 47 (8.2) | 24 (6.6) | 62 (10.7) | 57 (10.6) |
| Smoking | ||||
| No | 487 (84.5) | 320 (87.7) | 453 (78.0) | 421 (78.5) |
| Yes | 89 (15.5) | 45 (12.3) | 128 (22.0) | 115 (21.5) |
| Chronic diseases | ||||
| No | 506 (87.9) | 324 (88.8) | 486 (83.7) | 454 (84.7) |
| Yes | 70 (12.1) | 41 (11.2) | 95 (16.3) | 82 (15.3) |
| COVID-19 family cases a | ||||
| No | 535 (92.9) | - | 525 (90.4) | - |
| Yes | 41 (7.1) | - | 56 (9.6) | - |
| Anti-SARS-CoV-2 IgG seropositive a | 47 (8.2) | - | 47 (8.1) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piumatti, G.; Amati, R.; Richard, A.; Baysson, H.; Purgato, M.; Guessous, I.; Stringhini, S.; Albanese, E., on behalf of the SEROCoV-POP; Specchio-COVID19 Study Group; the Corona Immunitas Ticino Working Group. Associations between Depression and Self-Reported COVID-19 Symptoms among Adults: Results from Two Population-Based Seroprevalence Studies in Switzerland. Int. J. Environ. Res. Public Health 2022, 19, 16696. https://doi.org/10.3390/ijerph192416696
Piumatti G, Amati R, Richard A, Baysson H, Purgato M, Guessous I, Stringhini S, Albanese E on behalf of the SEROCoV-POP, Specchio-COVID19 Study Group, the Corona Immunitas Ticino Working Group. Associations between Depression and Self-Reported COVID-19 Symptoms among Adults: Results from Two Population-Based Seroprevalence Studies in Switzerland. International Journal of Environmental Research and Public Health. 2022; 19(24):16696. https://doi.org/10.3390/ijerph192416696
Chicago/Turabian StylePiumatti, Giovanni, Rebecca Amati, Aude Richard, Hélène Baysson, Marianna Purgato, Idris Guessous, Silvia Stringhini, Emiliano Albanese on behalf of the SEROCoV-POP, Specchio-COVID19 Study Group, and the Corona Immunitas Ticino Working Group. 2022. "Associations between Depression and Self-Reported COVID-19 Symptoms among Adults: Results from Two Population-Based Seroprevalence Studies in Switzerland" International Journal of Environmental Research and Public Health 19, no. 24: 16696. https://doi.org/10.3390/ijerph192416696
APA StylePiumatti, G., Amati, R., Richard, A., Baysson, H., Purgato, M., Guessous, I., Stringhini, S., Albanese, E., on behalf of the SEROCoV-POP, Specchio-COVID19 Study Group, & the Corona Immunitas Ticino Working Group. (2022). Associations between Depression and Self-Reported COVID-19 Symptoms among Adults: Results from Two Population-Based Seroprevalence Studies in Switzerland. International Journal of Environmental Research and Public Health, 19(24), 16696. https://doi.org/10.3390/ijerph192416696








