Pharmacopuncture Effects on Insomnia Disorder: Protocol for a Multi-Site, Randomized, Acupuncture-Controlled, Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
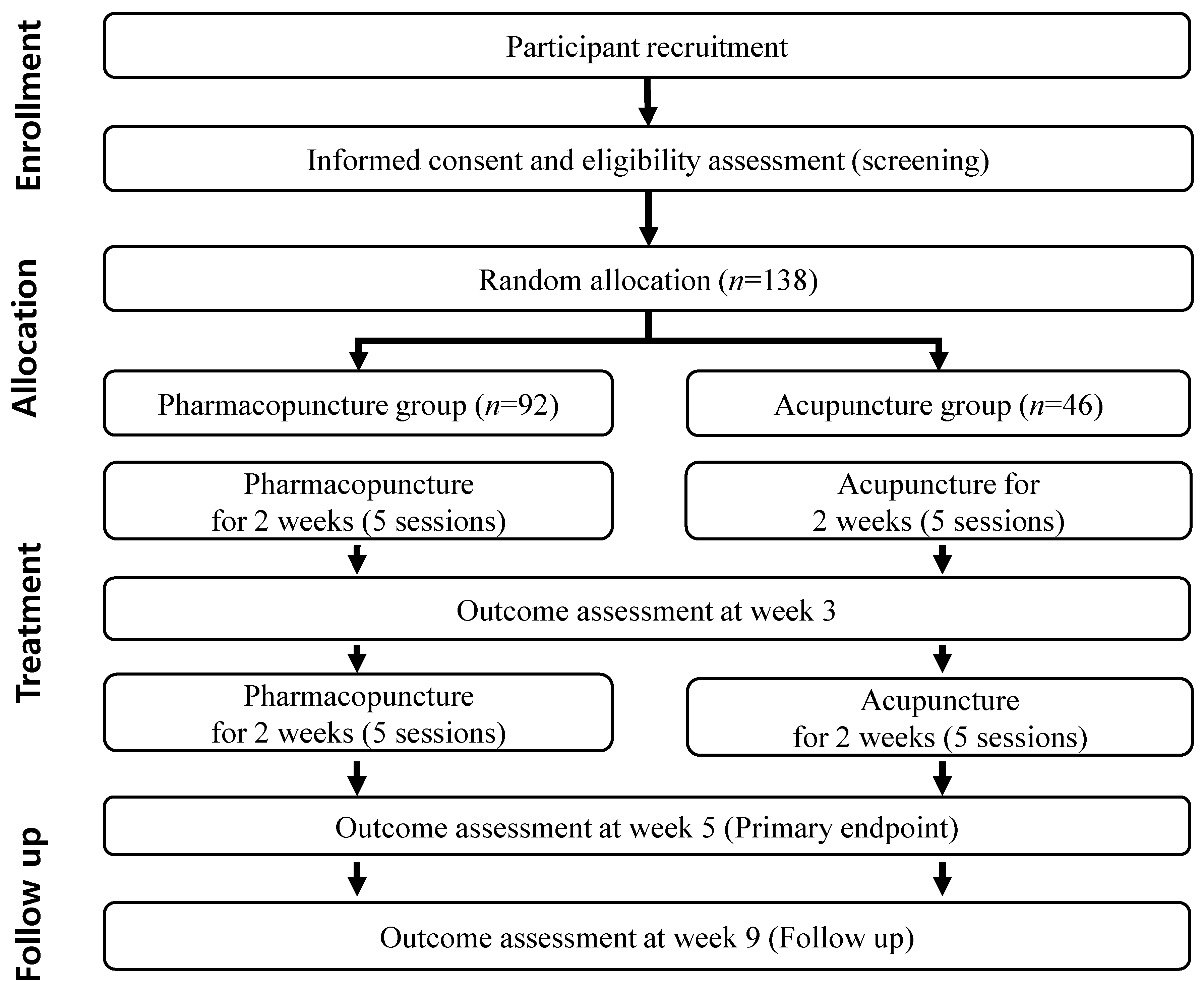
2.2. Participants Recruitment and Study Schedule
2.3. Sample Size
2.4. Eligibility Criteria: Inclusion Criteria
- (1)
- Male and female participants aged 19–80 years.
- (2)
- Total Insomnia Severity Index (ISI) score ≥ 15.
- (3)
- Diagnosis of insomnia disorder according to DSM-5 criteria.
- (4)
- Voluntary participation and signing of an informed consent form after adequate explanation of the purpose and procedure of this clinical trial and any risks and potential for adverse effects from this trial procedure.
2.5. Eligibility Criteria: Exclusion Criteria
- (1)
- Changes in the type or dosage of regularly used sleeping pills within the last 4 weeks
- (2)
- Korean medicine treatment (acupuncture, moxibustion, cupping, and herbal medicine) to improve insomnia within the last 4 weeks.
- (3)
- Initiation of health supplements or other non-pharmacological therapies (e.g., cognitive behavioral therapy and meditation) to improve insomnia within the last 4 weeks or plans to start the therapies during the clinical trial period.
- (4)
- Current participation in other clinical trials with interventions within the last 4 weeks
- (5)
- Shift workers.
- (6)
- Obvious pain that interferes with sleep or diseases causing insomnia.
- (7)
- Treatment for unstable (controllable) schizophrenia, bipolar disorder, and other mental disorders during the last 6 months or an anxiety or depression subscale score of ≥11 on the Hospital Anxiety and Depression Scale.
- (8)
- Current treatment for serious chronic diseases or terminal diseases (malignant tumor, tuberculosis, chronic liver disease, interstitial pneumonia, chronic renal disease, chronic heart disease, and other rare metabolic diseases).
- (9)
- Abnormal hormone levels on thyroid function tests (abnormal free T4 and thyroid stimulating hormone < 0.1 uIU/mL or >5.1 uIU/mL).
- (10)
- Clinically significant abnormalities in blood chemistry (aspartate aminotransferase and alanine aminotransferase > 2 times the upper limit; serum creatinine ≥ 1.5 times the normal upper limit).
- (11)
- Electrolyte abnormalities (5% more or less than the normal range).
- (12)
- Hypertension not controlled with antihypertensive drugs (systolic blood pressure >160 mmHg and diastolic blood pressure > 90 mmHg).
- (13)
- Diabetes mellitus not controlled with oral hypoglycemic agents or insulin-dependent diabetes (hemoglobin A1c level ≥ 7%).
- (14)
- Use of hemostatic agents (e.g., Greenmono, Advate, Monoclate-P, Facnyne, and BenFix) for cardiovascular diseases or hemostatic disorders.
- (15)
- Pregnancy, lactation, and disagreement with contraception methods (dual contraception, intrauterine contraceptive devices, and spermicides) during the clinical trial.
- (16)
- Difficulty with adherence to the study protocol.
- (17)
- Deemed unsuitable for participation by the investigator.
2.6. Randomization, Allocation Concealment, and Blinding
2.7. Intervention
2.7.1. Experiment Group: Pharmacopuncture
2.7.2. Control Group: Acupuncture
2.8. Concomitant Treatment
2.9. Outcome Measures
2.9.1. Primary Outcome
2.9.2. Secondary Outcomes
2.9.3. Quality of Life Assessment
2.9.4. Cost Data
2.9.5. Additional Evaluation
2.9.6. Safety Assessment
2.10. Data Collection and Management
2.11. Data Monitoring and Auditing
2.12. Criteria for Discontinuation and Withdrawal
2.13. Statistical Analysis
2.14. Confidentiality
2.15. Dissemination Policy
2.16. Trial Status
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Taylor, D.J.; Mallory, L.J.; Lichstein, K.L.; Durrence, H.H.; Riedel, B.W.; Bush, A.J. Comorbidity of chronic insomnia with medical problems. Sleep 2007, 30, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Angst, J.; Gamma, A.; Ajdacic, V.; Eich, D.; Rössler, W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep 2008, 31, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Schutte-Rodin, S.; Broch, L.; Buysse, D.; Dorsey, C.; Sateia, M. Clinical Guideline for the Evaluation and Management of Chronic Insomnia in Adults. J. Clin. Sleep Med. 2008, 4, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Aernout, E.; Benradia, I.; Hazo, J.-B.; Sy, A.; Askevis-Leherpeux, F.; Sebbane, D.; Roelandt, J.-L. International study of the prevalence and factors associated with insomnia in the general population. Sleep Med. 2021, 82, 186–192. [Google Scholar] [CrossRef]
- Cho, Y.W.; Shin, W.C.; Yun, C.H.; Hong, S.B.; Kim, J.; Earley, C.J. Epidemiology of insomnia in Korean adults: Prevalence and associated factors. J. Clin. Neurol. 2009, 5, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Bolge, S.C.; Doan, J.F.; Kannan, H.; Baran, R.W. Association of insomnia with quality of life, work productivity, and activity impairment. Qual. Life Res. 2009, 18, 415–422. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.A.; Coulouvrat, C.; Fitzgerald, T.; Hajak, G.; Roth, T.; Shahly, V.; Shillington, A.; Stephenson, J.J.; Walsh, J.K. Insomnia, Comorbidity, and Risk of Injury Among Insured Americans: Results from the America Insomnia Survey. Sleep 2012, 35, 825–834. [Google Scholar] [CrossRef]
- Khurshid, K.A. Comorbid insomnia and psychiatric disorders: An update. Innov. Clin. Neurosci. 2018, 15, 28–32. [Google Scholar]
- Chien, K.-L.; Chen, P.-C.; Hsu, H.-C.; Su, T.-C.; Sung, F.-C.; Chen, M.-F.; Lee, Y.-T. Habitual Sleep Duration and Insomnia and the Risk of Cardiovascular Events and All-cause Death: Report from a Community-Based Cohort. Sleep 2010, 33, 177–184. [Google Scholar] [CrossRef]
- Fernandez-Mendoza, J.; Vgontzas, A.N. Insomnia and its Impact on Physical and Mental Health. Curr. Psychiatry Rep. 2013, 15, 418. [Google Scholar] [CrossRef]
- Olfson, M.; Wall, M.; Liu, S.-M.; Morin, C.M.; Blanco, C. Insomnia and Impaired Quality of Life in the United States. J. Clin. Psychiatry 2018, 79, 9151. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, S.H.; Kim, B.K.; Lim, J.H. A review on clinical research trend in treatment of chai-Hu-Shu-Gan-San (Sihosogan-san) for insomnia. J. Orient. Neuropsychiatr. 2020, 31, 25–38. [Google Scholar]
- Phelan, J.C.; Link, B.; Tehranifar, P. Social Conditions as Fundamental Causes of Health Inequalities: Theory, Evidence, and Policy Implications. J. Health Soc. Behav. 2010, 51, S28–S40. [Google Scholar] [CrossRef] [PubMed]
- Sosso, F.A.E.; Kreidlmayer, M.; Pearson, D.; Bendaoud, I. Towards A Socioeconomic Model of Sleep Health among the Canadian Population: A Systematic Review of the Relationship between Age, Income, Employment, Education, Social Class, Socioeconomic Status and Sleep Disparities. Eur. J. Investig. Health Psychol. Educ. 2022, 12, 1143–1167. [Google Scholar] [CrossRef]
- Bendaoud, I.; Sosso, F.A.E. Socioeconomic Position and Excessive Daytime Sleepiness: A Systematic Review of Social Epidemiological Studies. Clocks Sleep 2022, 4, 240–259. [Google Scholar] [CrossRef]
- Sosso, F.A.E.; Holmes, S.D.; Weinstein, A.A. Influence of socioeconomic status on objective sleep measurement: A systematic review and meta-analysis of actigraphy studies. Sleep Health 2021, 7, 417–428. [Google Scholar] [CrossRef]
- Sateia, M.J.; Buysse, D.J.; Krystal, A.D.; Neubauer, D.N.; Heald, J.L. Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 307–349. [Google Scholar] [CrossRef]
- Qaseem, A.; Kansagara, D.; Forciea, M.A.; Cooke, M.; Denberg, T.D. Clinical Guidelines Committee of the American College of Physicians. Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2016, 165, 125–133. [Google Scholar] [CrossRef]
- Riemann, D.; Baum, E.; Cohrs, S.; Crönlein, T.; Hajak, G.; Hertenstein, E.; Klose, P.; Langhorst, J.; Mayer, G.; Nissen, C.; et al. S3-Leitlinie Nicht erholsamer Schlaf/Schlafstörungen: Kapitel “Insomnie bei Erwachsenen”. Somnologie 2017, 21, 2–44. [Google Scholar] [CrossRef]
- Cumming, R.G.; Le Couteur, D.G. Benzodiazepines and risk of hip fractures in older people: A review of the evidence. CNS Drugs 2003, 17, 825–837. [Google Scholar] [CrossRef]
- Puustinen, J.; Lähteenmäki, R.; Polo-Kantola, P.; Salo, P.; Vahlberg, T.; Lyles, A.; Neuvonen, P.J.; Partinen, M.; Räihä, I.; Kivelä, S.-L. Effect of withdrawal from long-term use of temazepam, zopiclone or zolpidem as hypnotic agents on cognition in older adults. Eur. J. Clin. Pharmacol. 2014, 70, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Fluyau, D.; Revadigar, N.; Manobianco, B.E. Challenges of the pharmacological management of benzodiazepine withdrawal, dependence, and discontinuation. Ther. Adv. Psychopharmacol. 2018, 8, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, N.; VanderMeer, B.; Friesen, C.; Bialy, L.; Tubman, M.; Ospina, M.; Klassen, T.; Witmans, M. The Efficacy and Safety of Drug Treatments for Chronic Insomnia in Adults: A Meta-analysis of RCTs. J. Gen. Intern. Med. 2007, 22, 1335–1350. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.D.; Gehrman, P.; Perlis, M.; Umscheid, C.A. Comparative effectiveness of cognitive behavioral therapy for insomnia: A systematic review. BMC Fam. Pract. 2012, 13, 40. [Google Scholar] [CrossRef]
- Trauer, J.M.; Qian, M.Y.; Doyle, J.S.; Rajaratnam, S.M.W.; Cunnington, D. Cognitive behavioral therapy for chronic insomnia: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 163, 191–204. [Google Scholar] [CrossRef]
- Rybarczyk, B.; Mack, L.; Harris, J.H.; Stepanski, E. Testing two types of self-help CBT-I for insomnia in older adults with arthritis or coronary artery disease. Rehabil. Psychol. 2011, 56, 257–266. [Google Scholar] [CrossRef]
- Ong, J.C.; Crawford, M.; Dawson, S.C.; Fogg, L.F.; Turner, A.D.; Wyatt, J.K.; I Crisostomo, M.; Chhangani, B.S.; A Kushida, C.; Edinger, J.D.; et al. A randomized controlled trial of CBT-I and PAP for obstructive sleep apnea and comorbid insomnia: Main outcomes from the MATRICS study. Sleep 2020, 43, zsaa041. [Google Scholar] [CrossRef]
- Koffel, E.; Bramoweth, A.D.; Ulmer, C.S. Increasing access to and utilization of cognitive behavioral therapy for insomnia (CBT-I): A narrative review. J. Gen. Intern. Med. 2018, 33, 955–962. [Google Scholar] [CrossRef]
- Spiegelhalder, K.; Acker, J.; Baumeister, H.; Büttner-Teleaga, A.; Danker-Hopfe, H.; Ebert, D.D.; Fietze, I.; Frase, L.; Klein, S.; Lehr, D.; et al. Digitale Behandlungsangebote für Insomnie—Eine Übersichtsarbeit. Somnologie 2020, 24, 106–114. [Google Scholar] [CrossRef]
- Lim, J.H.; Jeong, J.H.; Kim, S.H.; Kim, K.O.; Lee, S.Y.; Lee, S.H.; Kim, B.K. The pilot survey of the perception on the practice pattern, diagnosis, and treatment on Korean medicine insomnia: Focusing on the difference between Korean medical neuropsychiatry specialists and Korean medical general practitioners. Evid. Based Complement. Alternat. Med. 2018, 2018, 9152705. [Google Scholar] [CrossRef]
- Lim, B.M. Korean medicine coverage in the National Health Insurance in Korea: Present situation and critical issues. Integr. Med. Res. 2013, 2, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; He, Y.H.; Huang, X.X.; Liu, Y.F.; Yu, H.B. The effects of acupuncture versus sham/placebo acupuncture for insomnia: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Clin. Pract. 2020, 41, 101253. [Google Scholar] [CrossRef] [PubMed]
- The Society of Korean Medicine Neuropsychiatry. Clinical Practice Guideline of Korean Medicine Insomnia Disorder; Koonja: Seoul, Republic of Korea, 2021; pp. 5–6. [Google Scholar]
- Park, J.M.; Lee, H.S.; Shin, B.C.; Lee, M.S.; Kim, B.R.; Kim, J.I. Pharmacopuncture in Korea: A systematic review and meta-analysis of randomized controlled trials. Evid. Based Complement. Alternat. Med. 2016, 2016, 4683121. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Cho, J.H.; Lim, J.H.; Kim, B.K. Study on current usage status of pharmacopuncture for insomnia among Korean medicine doctors. J. Orient. Neuropsychiatr. 2021, 32, 141–154. [Google Scholar]
- Jo, M.W.; Lim, J.H.; Kim, B.K. A systematic review and meta-analysis of pharmacopuncture treatment for insomnia disorder. J. Orient. Neuropsychiatr. 2021, 32, 185–206. [Google Scholar]
- Chandereng, T.; Wei, X.D.; Chappell, R. Imbalanced randomization in clinical trials. Stat. Med. 2020, 39, 2185–2196. [Google Scholar] [CrossRef]
- Torgerson, D.J.; Torgerson, C.J. Designing Randomised Trials in Health, Education and the Social Sciences: An Introduction; Palgrave MacMillan: London, UK, 2008; pp. 108–113. [Google Scholar]
- Xian, M.L. Acupoint Injection Combined with Acupuncture for Insomnia with Cardiacsplenic Asthenia Research Protocol for Clinical Trial. Master’s Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 2014. [Google Scholar]
- Clinical Research Information Service. Available online: https://cris.nih.go.kr/cris/search/detailSearch.do/19026 (accessed on 21 October 2022).
- Lee, B.; Kim, B.K.; Kim, H.J.; Jung, I.C.; Kim, A.R.; Park, H.J.; Kwon, O.J.; Lee, J.H.; Kim, J.H. Efficacy and safety of electroacupuncture for insomnia disorder: A multicenter, randomized, assessor-blinded, controlled trial. Nat. Sci. Sleep 2020, 12, 1145–1159. [Google Scholar] [CrossRef]
- Lee, B.; Kim, B.K.; Kim, M.; Kim, A.R.; Park, H.J.; Kwon, O.J.; Lee, J.H.; Kim, J.H. Electroacupuncture for treating cancer-related insomnia: A multicenter, assessor-blinded, randomized controlled, pilot clinical trial. BMC Complement. Med. Ther. 2022, 22, 77. [Google Scholar] [CrossRef]
- Sohn, S.I.; Kim, D.H.; Lee, M.Y.; Cho, Y.W. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012, 16, 803–812. [Google Scholar] [CrossRef]
- Cho, Y.W.; Song, M.L.; Morin, C.M. Validation of a Korean version of the insomnia severity index. J. Clin. Neurol. 2014, 10, 210–215. [Google Scholar] [CrossRef]
- Morin, C.M.; Vallières, A.; Guay, B.; Ivers, H.; Savard, J.; Mérette, C.; Bastien, C.; Baillargeon, L. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: A randomized controlled trial. JAMA 2009, 301, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Kim, H.; Yun, Y.-G.; Lee, S.; Jeon, J.-H.; Kim, B.-K.; Lee, E.J.; Jung, I.C. Preliminary Study to Develop the Instrument on Pattern Identifications for Insomnia. J. Orient. Neuropsychiatr. 2016, 27, 223–234. [Google Scholar] [CrossRef][Green Version]
- Cheong, M.J.; Lee, G.-E.; Lee, Y.; Bae, K.-H.; Kang, Y.; Kim, J.-H.; Lyu, Y.-S.; Kang, H.W. Validation of the Core Seven-Emotions Inventory—short form. Integr. Med. Res. 2019, 8, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Carney, C.E.; Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Krystal, A.D.; Lichstein, K.L.; Morin, C.M. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep 2012, 35, 287–302. [Google Scholar] [CrossRef]
- Kim, S.-H.; Ahn, J.; Ock, M.; Shin, S.; Park, J.; Luo, N.; Jo, M.-W. The EQ-5D-5L valuation study in Korea. Qual. Life Res. 2016, 25, 1845–1852. [Google Scholar] [CrossRef]
- Kim, S.; Won, C.W.; Kim, B.S.; Yoo, J.; Byun, S.; Jang, H.C.; Cho, B.L.; Son, S.J.; Lee, J.H.; Park, Y.S.; et al. EuroQol Visual Analogue Scale (EQ-VAS) as a Predicting Tool for Frailty in Older Korean Adults: The Korean Frailty and Aging Cohort Study (KFACS). J. Nutr. Health Aging 2018, 22, 1275–1280. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Lee, H.K.; Jo, M.W.; Choi, S.H.; Kim, Y.J.; Oh, K.W. Development and psychometric evaluation of measurement instrument for Korean health-related quality of life. Public Health Wkly. Rep. 2016, 9, 447–454. [Google Scholar]
- Reilly, M.C.; Zbrozek, A.S.; Dukes, E.M. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993, 4, 353–365. [Google Scholar] [CrossRef]
- Korean Pharmacopuncture Institute. Pharmacopuncturology: Principles and Clinical Application; Elsevier Korea: Seoul, Republic of Korea, 2012. [Google Scholar]
| Time Point | Screening | Enrollment Allocation (V1) Treatment: Total 10 Sessions (V1–10) | Post-Treatment Evaluation | Follow-Up | |||
|---|---|---|---|---|---|---|---|
| Week −1 | Week 0 | Weeks 1–4 | Week 5 | Week 9 | |||
| V1 | V2–5 | V6 | V7–10 | V11 | V12 | ||
| Eligibility screening | O | ||||||
| Informed consent | O | ||||||
| Vital signs | O | O | O | O | O | O | O |
| Socioeconomic status, medical history | O | ||||||
| ISI, HADS | O | ||||||
| Laboratory tests and electrocardiogram | O | O | |||||
| Randomized allocation | O | ||||||
| Pharmacopuncture (experimental) | ← 5 times per 2 weeks → | ||||||
| Acupuncture (control) | ← 5 times per 2 weeks → | ||||||
| ISI | O | O | O | O | O | ||
| PSQI | O | O | O | O | |||
| PIT for insomnia | O | O | |||||
| CSEI-S | O | O | |||||
| VAS of subjective symptoms accompanying insomnia | O | O | O | O | |||
| Quality of life (EQ-5D-5L, EQ-VAS, SF-36, HINT-8), Cost data | O | O | O | ||||
| Body composition | O | O | |||||
| Request for additional treatment | O | ||||||
| Sleep diary and actigraphy | O | O | O | O | O | O | O |
| Adverse event check | O | O | O | O | O | O | |
| PSG, melatonin, cortisol * | O | O | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.-H.; Lee, J.-H.; Kwon, C.-Y.; Lee, S.-H.; Kang, C.-W.; Cho, E.; Kim, H.-W.; Cho, J.-H.; Kim, B.-K. Pharmacopuncture Effects on Insomnia Disorder: Protocol for a Multi-Site, Randomized, Acupuncture-Controlled, Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 16688. https://doi.org/10.3390/ijerph192416688
Lim J-H, Lee J-H, Kwon C-Y, Lee S-H, Kang C-W, Cho E, Kim H-W, Cho J-H, Kim B-K. Pharmacopuncture Effects on Insomnia Disorder: Protocol for a Multi-Site, Randomized, Acupuncture-Controlled, Clinical Trial. International Journal of Environmental Research and Public Health. 2022; 19(24):16688. https://doi.org/10.3390/ijerph192416688
Chicago/Turabian StyleLim, Jung-Hwa, Jae-Hyok Lee, Chan-Young Kwon, Sang-Hyup Lee, Chang-Wan Kang, Eun Cho, Hyun-Woo Kim, Jun-Hee Cho, and Bo-Kyung Kim. 2022. "Pharmacopuncture Effects on Insomnia Disorder: Protocol for a Multi-Site, Randomized, Acupuncture-Controlled, Clinical Trial" International Journal of Environmental Research and Public Health 19, no. 24: 16688. https://doi.org/10.3390/ijerph192416688
APA StyleLim, J.-H., Lee, J.-H., Kwon, C.-Y., Lee, S.-H., Kang, C.-W., Cho, E., Kim, H.-W., Cho, J.-H., & Kim, B.-K. (2022). Pharmacopuncture Effects on Insomnia Disorder: Protocol for a Multi-Site, Randomized, Acupuncture-Controlled, Clinical Trial. International Journal of Environmental Research and Public Health, 19(24), 16688. https://doi.org/10.3390/ijerph192416688








