Exploring the Social Environment with the Eyes: A Review of the Impact of Facial Stimuli on Saccadic Trajectories
Abstract
1. Introduction
Taking Advantage of Saccadic Trajectories
2. Saccadic Curvatures Modulated by Facial Stimuli
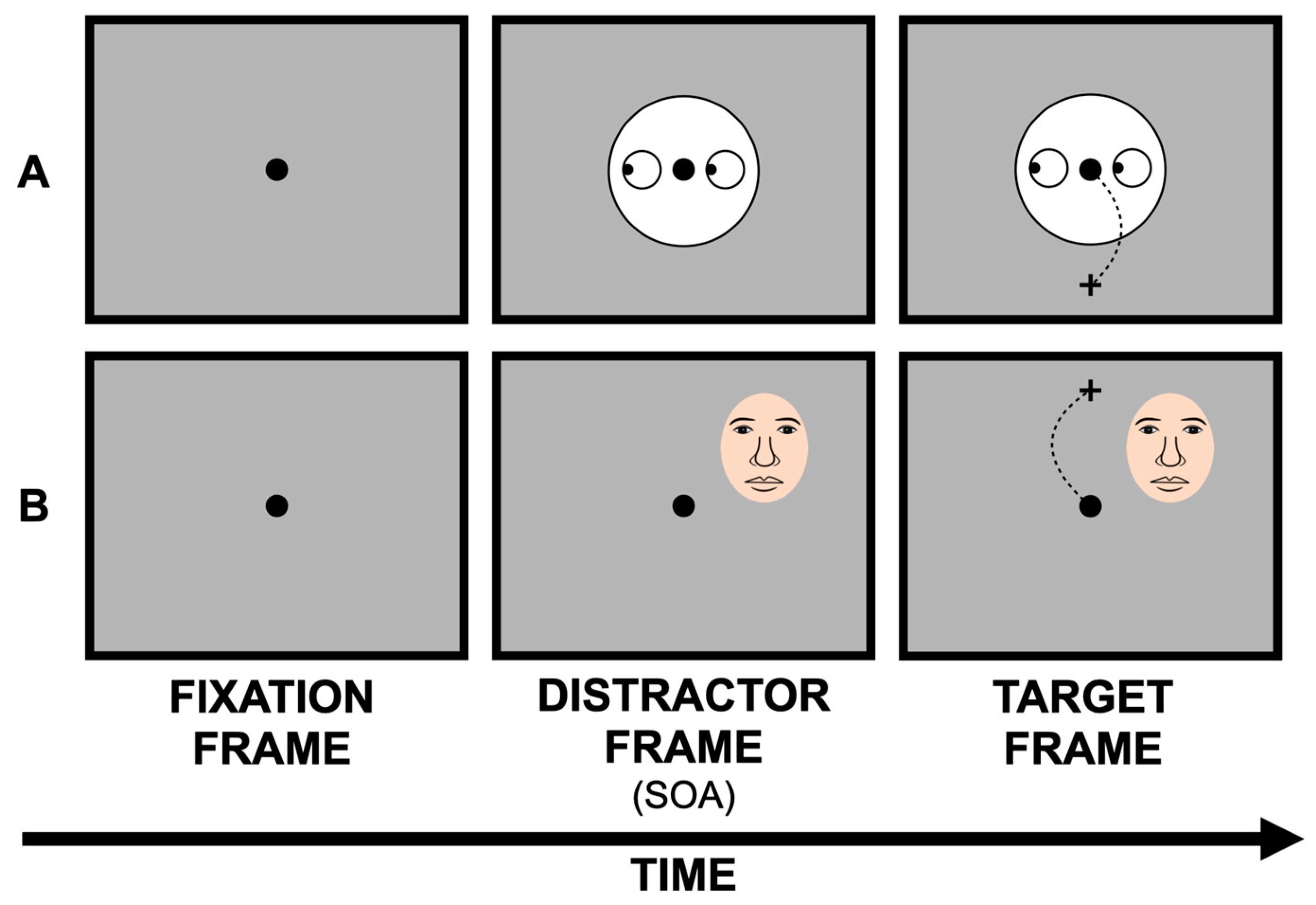
2.1. Centrally Placed Facial Stimuli
2.2. Peripherally Placed Facial Stimuli
3. Discussion and Future Directions
4. Conclusions
Funding
Conflicts of Interest
References
- Frischen, A.; Bayliss, A.P.; Tipper, S.P. Gaze cueing of attention: Visual attention, social cognition, and individual differences. Psychol. Bull. 2007, 133, 694–724. [Google Scholar] [CrossRef] [PubMed]
- Emery, N.J. The eyes have it: The neuroethology, function and evolution of social gaze. Neurosci. Biobehav. Rev. 2000, 24, 581–604. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.; Davis, G.; Ricciardelli, P.; Kidd, P.; Maxwell, E.; Baron-Cohen, S. Gaze perception triggers reflexive visuospatial orienting. Vis. Cogn. 1999, 6, 509–540. [Google Scholar] [CrossRef]
- Friesen, C.K.; Kingstone, A. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychon. Bull. Rev. 1998, 5, 490–495. [Google Scholar] [CrossRef]
- Hietanen, J.K. Does your gaze direction and head orientation shift my visual attention? Neuroreport 1999, 10, 3443–3447. [Google Scholar] [CrossRef] [PubMed]
- Kristjansson, A. The intriguing interactive relationship between visual attention and saccadic eye movements. In The Oxford Handbook of Eye Movements; Oxford University Press: Oxfordshire, UK, 2011; pp. 455–470. [Google Scholar]
- Porter, G.; Hood, B.M.; Troscianko, T.; Macrae, C.N. Females, but not males, show greater pupillary response to direct- than deviated-gaze faces. Perception 2006, 35, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Noguchi, Y.; Kita, S. Attentional shifts by gaze direction in voluntary orienting: Evidence from a microsaccade study. Exp. Brain Res. 2012, 223, 291–300. [Google Scholar] [CrossRef]
- Ricciardelli, P.; Bricolo, E.; Aglioti, S.; Chelazzi, L. My eyes want to look where your eyes are looking: Exploring the tendency to imitate another individual’s gaze. Neuroreport 2002, 13, 2259–2264. [Google Scholar] [CrossRef]
- Kuhn, G.; Benson, V. The influence of eye-gaze and arrow pointing distractor cues on voluntary eye movements. Percept. Psychophys. 2007, 69, 966–971. [Google Scholar] [CrossRef]
- Liuzza, M.T.; Cazzato, V.; Vecchione, M.; Crostella, F.; Caprara, G.V.; Aglioti, S.M. Follow my eyes: The gaze of politicians reflexively captures the gaze of ingroup voters. PLoS ONE 2011, 6, e25117. [Google Scholar] [CrossRef]
- Ciardo, F.; Marino, B.F.M.; Actis-Grosso, R.; Rossetti, A.; Ricciardelli, P. Face age modulates gaze following in young adults. Sci. Rep. 2014, 4, 4746. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, G.; Kingstone, A. Look away! Eyes and arrows engage oculomotor responses automatically. Atten. Percept. Psychophys. 2009, 71, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Mares, I.; Smith, M.L.; Johnson, M.H.; Senju, A. Direct gaze facilitates rapid orienting to faces: Evidence from express saccades and saccadic potentials. Biol. Psychol. 2016, 121, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Dalmaso, M.; Galfano, G.; Castelli, L. The impact of same- and other-race gaze distractors on the control of saccadic eye movements. Perception 2015, 44, 1020–1028. [Google Scholar] [CrossRef]
- Porciello, G.; Holmes, B.S.; Liuzza, M.T.; Crostella, F.; Aglioti, S.M.; Bufalari, I. Interpersonal Multisensory Stimulation reduces the overwhelming distracting power of self-gaze: Psychophysical evidence for ‘engazement’. Sci. Rep. 2014, 4, 6669. [Google Scholar] [CrossRef]
- Dalmaso, M.; Castelli, L.; Galfano, G. Early saccade planning cannot override oculomotor interference elicited by gaze and arrow distractors. Psychon. Bull. Rev. 2020, 27, 990–997. [Google Scholar] [CrossRef]
- Zhang, X.; Dalmaso, M.; Castelli, L.; Fu, S.; Galfano, G. Cross-cultural asymmetries in oculomotor interference elicited by gaze distractors belonging to Asian and White faces. Sci. Rep. 2021, 11, 20410. [Google Scholar] [CrossRef]
- Van der Stigchel, S. Recent advances in the study of saccade trajectory deviations. Vision Res. 2010, 50, 1619–1627. [Google Scholar] [CrossRef]
- Van der Stigchel, S.; Meeter, M.; Theeuwes, J. Eye movement trajectories and what they tell us. Neurosci. Biobehav. Rev. 2006, 30, 666–679. [Google Scholar] [CrossRef]
- Yarbus, A.L. Eye Movements and Vision; Springer: New York, NY, USA, 1967; ISBN 978-1-4899-5381-0. [Google Scholar]
- Tatler, B.W.; Wade, N.J.; Kwan, H.; Findlay, J.M.; Velichkovsky, B.M. Yarbus, eye movements, and vision. I-Perception 2010, 1, 7–27. [Google Scholar] [CrossRef]
- Sheliga, B.M.; Riggio, L.; Rizzolatti, G. Orienting of attention and eye movements. Exp. Brain Res. 1994, 98, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.; Walker, R. Curved saccade trajectories: Voluntary and reflexive saccades curve away from irrelevant distractors. Exp. Brain Res. 2001, 139, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, C.J.H.; Gilchrist, I.D. Target similarity affects saccade curvature away from irrelevant onsets. Exp. Brain Res. 2003, 152, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Mulckhuyse, M.; Van der Stigchel, S.; Theeuwes, J. Early and late modulation of saccade deviations by target distractor similarity. J. Neurophysiol. 2009, 102, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- McPeek, R.M.; Han, J.H.; Keller, E.L. Competition between saccade goals in the superior colliculus produces saccade curvature. J. Neurophysiol. 2003, 89, 2577–2590. [Google Scholar] [CrossRef] [PubMed]
- Port, N.L.; Wurtz, R.H. Sequential activity of simultaneously recorded neurons in the superior colliculus during curved saccades. J. Neurophysiol. 2003, 90, 1887–1903. [Google Scholar] [CrossRef]
- McSorley, E.; Haggard, P.; Walker, R. Time course of oculomotor inhibition revealed by saccade trajectory modulation. J. Neurophysiol. 2006, 96, 1420–1424. [Google Scholar] [CrossRef]
- Walker, R.; McSorley, E. The influence of distractors on saccade target selection: Saccade trajectory effects. J. Eye Mov. Res. 2008, 2, 1–13. [Google Scholar] [CrossRef]
- Walker, R.; Deubel, H.; Schneider, W.X.; Findlay, J.M. Effect of remote distractors on saccade programming: Evidence for an extended fixation zone. J. Neurophysiol. 1997, 78, 1108–1119. [Google Scholar] [CrossRef]
- Dalmaso, M.; Castelli, L.; Galfano, G. Social modulators of gaze-mediated orienting of attention: A review. Psychon. Bull. Rev. 2020, 27, 833–855. [Google Scholar] [CrossRef]
- Tudge, L.; McSorley, E.; Brandt, S.A.; Schubert, T. Setting things straight: A comparison of measures of saccade trajectory deviation. Behav. Res. Methods 2017, 49, 2127–2145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ludwig, C.J.H.; Gilchrist, I.D. Measuring saccade curvature: A curve-fitting approach. Behav. Res. Methods. Instrum. Comput. 2002, 34, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Cercenelli, L.; Tiberi, G.; Bortolani, B.; Giannaccare, G.; Fresina, M.; Campos, E.; Marcelli, E. Gaze Trajectory Index (GTI): A novel metric to quantify saccade trajectory deviation using eye tracking. Comput. Biol. Med. 2019, 107, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Senju, A.; Johnson, M.H. The eye contact effect: Mechanisms and development. Trends Cogn. Sci. 2009, 13, 127–134. [Google Scholar] [CrossRef]
- Vuilleumier, P. How brains beware: Neural mechanisms of emotional attention. Trends Cogn. Sci. 2005, 9, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Böckler, A.; van der Wel, R.P.R.D.; Welsh, T.N. Catching eyes: Effects of social and nonsocial cues on attention capture. Psychol. Sci. 2014, 25, 720–727. [Google Scholar] [CrossRef] [PubMed]
- von Grünau, M.; Anston, C. The detection of gaze direction: A stare-in-the-crowd effect. Perception 1995, 24, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Senju, A.; Hasegawa, T. Direct gaze captures visuospatial attention. Vis. Cogn. 2005, 12, 127–144. [Google Scholar] [CrossRef]
- Dalmaso, M.; Castelli, L.; Galfano, G. Attention holding elicited by direct-gaze faces is reflected in saccadic peak velocity. Exp. Brain Res. 2017, 235, 3319–3332. [Google Scholar] [CrossRef]
- Syrjämäki, A.H.; Hietanen, J.K. Social inclusion, but not exclusion, delays attentional disengagement from direct gaze. Psychol. Res. 2018, 84, 1126–1138. [Google Scholar] [CrossRef]
- Kingstone, A.; Klein, R.M. What are human express saccades? Percept. Psychophys. 1993, 54, 260–273. [Google Scholar] [CrossRef]
- Schiller, P.H.; Sandell, J.H.; Maunsell, J.H. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J. Neurophysiol. 1987, 57, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Mathôt, S. Pupillometry: Psychology, Physiology, and Function. J. Cogn. 2018, 1, 16. [Google Scholar] [CrossRef]
- McKay, K.T.; Grainger, S.A.; Coundouris, S.P.; Skorich, D.P.; Phillips, L.H.; Henry, J.D. Visual attentional orienting by eye gaze: A meta-analytic review of the gaze-cueing effect. Psychol. Bull. 2021, 147, 1269–1289. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, F.; Ristic, J. How attention gates social interactions. Ann. N. Y. Acad. Sci. 2018, 1426, 179–198. [Google Scholar] [CrossRef] [PubMed]
- Nummenmaa, L.; Hietanen, J.K. Gaze distractors influence saccadic curvature: Evidence for the role of the oculomotor system in gaze-cued orienting. Vis. Res. 2006, 46, 3674–3680. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hermens, F.; Walker, R. Gaze and arrow distractors influence saccade trajectories similarly. Q. J. Exp. Psychol. 2010, 63, 2120–2140. [Google Scholar] [CrossRef] [PubMed]
- Galfano, G.; Dalmaso, M.; Marzoli, D.; Pavan, G.; Coricelli, C.; Castelli, L. Eye gaze cannot be ignored (but neither can arrows). Q. J. Exp. Psychol. 2012, 65, 1895–1910. [Google Scholar] [CrossRef] [PubMed]
- West, G.L.; Al-Aidroos, N.; Susskind, J.; Pratt, J. Emotion and action: The effect of fear on saccadic performance. Exp. Brain Res. 2011, 209, 153–158. [Google Scholar] [CrossRef]
- Mulckhuyse, M. The influence of emotional stimuli on the oculomotor system: A review of the literature. Cogn. Affect. Behav. Neurosci. 2018, 18, 411–425. [Google Scholar] [CrossRef]
- Laidlaw, K.E.W.; Badiudeen, T.A.; Zhu, M.J.H.; Kingstone, A. A fresh look at saccadic trajectories and task irrelevant stimuli: Social relevance matters. Vis. Res. 2015, 111, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Gao, X.; Wang, Z. Faces distort eye movement trajectories, but the distortion is not stronger for your own face. Exp. Brain Res. 2015, 233, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Hungr, C.J.; Hunt, A.R. Rapid communication: Physical self-similarity enhances the gaze-cueing effect. Q. J. Exp. Psychol. 2012, 65, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Dalmaso, M.; Castelli, L.; Scatturin, P.; Galfano, G. Trajectories of social vision: Eye contact increases saccadic curvature. Vis. Cogn. 2017, 25, 358–365. [Google Scholar] [CrossRef]
- Schmidt, L.J.; Belopolsky, A.V.; Theeuwes, J. The presence of threat affects saccade trajectories. Vis. Cogn. 2012, 20, 284–299. [Google Scholar] [CrossRef]
- Petrova, K.; Wentura, D. Upper-lower visual field asymmetries in oculomotor inhibition of emotional distractors. Vision Res. 2012, 62, 209–219. [Google Scholar] [CrossRef]
- McSorley, E.; Van Reekum, C.M. The time course of implicit affective picture processing: An eye movement study. Emotion 2013, 13, 769–773. [Google Scholar] [CrossRef]
- Mulckhuyse, M.; Crombez, G.; Van der Stigchel, S. Conditioned fear modulates visual selection. Emotion 2013, 13, 529–536. [Google Scholar] [CrossRef]
- Nummenmaa, L.; Hyönä, J.; Calvo, M.G. Emotional scene content drives the saccade generation system reflexively. J. Exp. Psychol. Hum. Percept. Perform. 2009, 35, 305–323. [Google Scholar] [CrossRef]
- Previc, F.H. Functional specialization in the lower and upper visual fields in humans: Its ecological origins and neurophysiological implications. Behav. Brain Sci. 1990, 13, 519–542. [Google Scholar] [CrossRef]
- Azarian, B.; Esser, E.G.; Peterson, M.S. Watch out! Directional threat-related postures cue attention and the eyes. Cogn. Emot. 2016, 30, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Dalmaso, M.; Castelli, L.; Galfano, G. Self-related shapes can hold the eyes. Q. J. Exp. Psychol. 2019, 72, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Yankouskaya, A.; Palmer, D.; Stolte, M.; Sui, J.; Humphreys, G.W. Self-bias modulates saccadic control. Q. J. Exp. Psychol. 2017, 70, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.G.R.; Tatler, B.B.W. Do as eye say: Gaze cueing and language in a real-world social interaction. J. Vis. 2013, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, U.J.; Vogeley, K.; Schilbach, L. From gaze cueing to dual eye-tracking: Novel approaches to investigate the neural correlates of gaze in social interaction. Neurosci. Biobehav. Rev. 2013, 37, 2516–2528. [Google Scholar] [CrossRef]
- Ristic, J.; Mottron, L.; Friesen, C.K.; Iarocci, G.; Burack, J.A.; Kingstone, A. Eyes are special but not for everyone: The case of autism. Cogn. Brain Res. 2005, 24, 715–718. [Google Scholar] [CrossRef]
- Kuhn, G.; Benson, V.; Fletcher-Watson, S.; Kovshoff, H.; McCormick, C.A.; Kirkby, J.; Leekam, S.R. Eye movements affirm: Automatic overt gaze and arrow cueing for typical adults and adults with autism spectrum disorder. Exp. Brain Res. 2010, 201, 155–165. [Google Scholar] [CrossRef]
- Johnson, B.P.; Lum, J.A.G.; Rinehart, N.J.; Fielding, J. Ocular motor disturbances in autism spectrum disorders: Systematic review and comprehensive meta-analysis. Neurosci. Biobehav. Rev. 2016, 69, 260–279. [Google Scholar] [CrossRef]
- McFadyen, J.; Dolan, R.J.; Garrido, M.I. The influence of subcortical shortcuts on disordered sensory and cognitive processing. Nat. Rev. Neurosci. 2020, 21, 264–276. [Google Scholar] [CrossRef]
- Jure, R. Autism Pathogenesis: The Superior Colliculus. Front. Neurosci. 2019, 12, 1029. [Google Scholar] [CrossRef]
- Kleinhans, N.M.; Richards, T.; Johnson, L.C.; Weaver, K.E.; Greenson, J.; Dawson, G.; Aylward, E. FMRI evidence of neural abnormalities in the subcortical face processing system in ASD. Neuroimage 2011, 54, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Hietanen, J.K.; Leppänen, J.M. Does Facial Expression Affect Attention Orienting by Gaze Direction Cues? J. Exp. Psychol. Hum. Percept. Perform. 2003, 29, 1228–1243. [Google Scholar] [CrossRef] [PubMed]
- Bonifacci, P.; Ricciardelli, P.; Lugli, L.; Pellicano, A. Emotional attention: Effects of emotion and gaze direction on overt orienting of visual attention. Cogn. Process. 2008, 9, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, G.; Tipples, J. Increased gaze following for fearful faces. It depends on what you’re looking for! Psychon. Bull. Rev. 2011, 18, 89–95. [Google Scholar] [CrossRef]
- Matsunaka, R.; Hiraki, K. Rapid saccadic response with fearful gaze cue. PLoS ONE 2019, 14, e0212450. [Google Scholar] [CrossRef]
- Lupianez, J.; Klein, R.M.; Bartolomeo, P. Inhibition of return: Twenty years after. Cogn. Neuropsychol. 2006, 23, 1003–1014. [Google Scholar] [CrossRef][Green Version]
- Okamoto-Barth, S.; Kawai, N. The role of attention in the facilitation effect and another “inhibition of return”. Cognition 2006, 101, B42–B50. [Google Scholar] [CrossRef]
- Frischen, A.; Tipper, S.P. Orienting attention via observed gaze shift evokes longer term inhibitory effects: Implications for social interactions, attention, and memory. J. Exp. Psychol. Gen. 2004, 133, 516–533. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalmaso, M. Exploring the Social Environment with the Eyes: A Review of the Impact of Facial Stimuli on Saccadic Trajectories. Int. J. Environ. Res. Public Health 2022, 19, 16615. https://doi.org/10.3390/ijerph192416615
Dalmaso M. Exploring the Social Environment with the Eyes: A Review of the Impact of Facial Stimuli on Saccadic Trajectories. International Journal of Environmental Research and Public Health. 2022; 19(24):16615. https://doi.org/10.3390/ijerph192416615
Chicago/Turabian StyleDalmaso, Mario. 2022. "Exploring the Social Environment with the Eyes: A Review of the Impact of Facial Stimuli on Saccadic Trajectories" International Journal of Environmental Research and Public Health 19, no. 24: 16615. https://doi.org/10.3390/ijerph192416615
APA StyleDalmaso, M. (2022). Exploring the Social Environment with the Eyes: A Review of the Impact of Facial Stimuli on Saccadic Trajectories. International Journal of Environmental Research and Public Health, 19(24), 16615. https://doi.org/10.3390/ijerph192416615





