Fatigue and Arousal Modulations Revealed by Saccade and Pupil Dynamics
Abstract
1. Introduction
2. Methods and Materials
3. Results
3.1. Exp 1: Time-On-Task Effects on Saccade Latency and Metrics in the Visually and Memory-Guided Saccade Task
3.2. Time-On-Task Effects on Tonic Pupil Size and Phasic Pupil Light Reflex Responses
3.3. Tonic Pupil Size Effects on Saccade Latency and Metrics in the Visually and Memory-Guided Saccade Task
3.4. Exp 2: Time-On-Task Effects on Tonic Pupil Size and Phasic Pupil Dilation in the Emotion Sound Task
3.5. Time-On-Task Effects on Tonic Pupil Size and Phasic Pupil Dilation Responses
3.6. Time-On-Task Effects on Saccade Latency and Metrics in the Pro- and Anti-Saccade Task
3.7. Tonic Pupil Size Effects on Saccade Latency and Metrics in the Pro- and Anti-Saccade Task
4. Discussion
4.1. Time-On-Task Effects on Saccades and Pupil Size
4.2. Tonic Pupil Size Effects in Saccade and Phasic Pupillary Responses
4.3. Neural Mechanisms for Coordinated Saccades and Phasic Pupillary Responses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, D.J.; Boehnke, S.E.; Marino, R.A.; Munoz, D.P.; Itti, L. Free Viewing of Dynamic Stimuli by Humans and Monkeys. J. Vis. 2009, 9, 19.1–19.15. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Walker, D.G.; Husain, M.; Kennard, C. Control of Voluntary and Reflexive Saccades. Exp. Brain Res. 2000, 130, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Seideman, J.A.; Stanford, T.R.; Salinas, E. Saccade Metrics Reflect Decision-Making Dynamics during Urgent Choices. Nat. Commun. 2018, 9, 2907. [Google Scholar] [CrossRef] [PubMed]
- Coe, B.C.; Munoz, D.P. Mechanisms of Saccade Suppression Revealed in the Anti-Saccade Task. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160192. [Google Scholar] [CrossRef]
- Munoz, D.P.; Everling, S. Look Away: The Anti-Saccade Task and the Voluntary Control of Eye Movement. Nat. Rev. Neurosci. 2004, 5, 218–228. [Google Scholar] [CrossRef]
- Wang, C.-A.; Nguyen, K.T.; Juan, C.-H. Linking Pupil Size Modulated by Global Luminance and Motor Preparation to Saccade Behavior. Neuroscience 2021, 476, 90–101. [Google Scholar] [CrossRef]
- Balkin, T.J.; Wesensten, N.J. Differentiation of Sleepiness and Mental Fatigue Effects. In Cognitive Fatigue: Multidisciplinary Perspectives on Current Research and Future Applications; American Psychological Association: Washington, DC, USA, 2010. [Google Scholar]
- Coetzer, R.C.; Hancke, G.P. Driver Fatigue Detection: A Survey. In Proceedings of the IEEE AFRICON Conference, Nairobi, Kenya, 23–25 September 2009. [Google Scholar]
- Hu, X.; Lodewijks, G. Detecting Fatigue in Car Drivers and Aircraft Pilots by Using Non-Invasive Measures: The Value of Differentiation of Sleepiness and Mental Fatigue. J. Safety Res. 2020, 72, 173–187. [Google Scholar] [CrossRef]
- Yamada, Y.; Kobayashi, M. Detecting Mental Fatigue from Eye-Tracking Data Gathered While Watching Video: Evaluation in Younger and Older Adults. Artif. Intell. Med. 2018, 91, 39–48. [Google Scholar] [CrossRef]
- Di Stasi, L.L.; Antolí, A.; Cañas, J.J. Main Sequence: An Index for Detecting Mental Workload Variation in Complex Tasks. Appl. Ergon. 2011, 42, 807–813. [Google Scholar] [CrossRef]
- Di Stasi, L.L.; McCamy, M.B.; Macknik, S.L.; Mankin, J.A.; Hooft, N.; Catena, A.; Martinez-Conde, S. Saccadic Eye Movement Metrics Reflect Surgical Residents’ Fatigue. Ann. Surg. 2014, 259, 824–829. [Google Scholar] [CrossRef]
- Zargari Marandi, R.; Madeleine, P.; Omland, Ø.; Vuillerme, N.; Samani, A. Eye Movement Characteristics Reflected Fatigue Development in Both Young and Elderly Individuals. Sci. Rep. 2018, 8, 13148. [Google Scholar] [CrossRef]
- Hirvonen, K.; Puttonen, S.; Gould, K.; Korpela, J.; Koefoed, V.F.; Müller, K. Improving the Saccade Peak Velocity Measurement for Detecting Fatigue. J. Neurosci. Methods 2010, 187, 199–206. [Google Scholar] [CrossRef]
- Di Stasi, L.L.; Renner, R.; Catena, A.; Cañas, J.J.; Velichkovsky, B.M.; Pannasch, S. Towards a Driver Fatigue Test Based on the Saccadic Main Sequence: A Partial Validation by Subjective Report Data. Transp. Res. Part C Emerg. Technol. 2012, 21, 122–133. [Google Scholar] [CrossRef]
- Di Stasi, L.L.; Catena, A.; Cañas, J.J.; Macknik, S.L.; Martinez-Conde, S. Saccadic Velocity as an Arousal Index in Naturalistic Tasks. Neurosci. Biobehav. Rev. 2013, 37, 968–975. [Google Scholar] [CrossRef]
- McDougal, D.H.; Gamlin, P.D. Autonomic Control of the Eye. Compr. Physiol. 2015, 5, 439–473. [Google Scholar] [CrossRef]
- May, P.J.; Reiner, A.; Gamlin, P.D.; May, P.J.; Reiner, A.; Gamlin, P.D. Autonomic Regulation of the Eye. Oxford Res. Encycl. Neurosci. 2019, 1–27. [Google Scholar] [CrossRef]
- Denton, E.J. The Responses of the Pupil of Gekko Gekko to External Light Stimulus. J. Gen. Physiol. 1956, 40, 201–216. [Google Scholar] [CrossRef]
- Campbell, F.W.; Gregory, A.H. Effect of Size of Pupil on Visual Acuity. Nature 1960, 187, 1121–1123. [Google Scholar] [CrossRef]
- Woodhouse, J.M.; Campbell, F.W. The Role of the Pupil Light Reflex in Aiding Adaptation to the Dark. Vision Res. 1975, 15, 649–653. [Google Scholar] [CrossRef]
- Laughlin, S.B. Retinal Information Capacity and the Function of the Pupil. Ophthalmic Physiol. Opt. 1992, 12, 161–164. [Google Scholar] [CrossRef]
- Janisse, M.P. Pupil Size and Affect: A Critical Review of the Literature since 1960. Can. Psychol. 1973, 14, 311–329. [Google Scholar] [CrossRef]
- Oliva, M.; Anikin, A. Pupil Dilation Reflects the Time Course of Emotion Recognition in Human Vocalizations. Sci. Rep. 2018, 8, 4871. [Google Scholar] [CrossRef] [PubMed]
- Partala, T.; Surakka, V. Pupil Size Variation as an Indication of Affective Processing. Int. J. Hum. Comput. Stud. 2003, 59, 185–198. [Google Scholar] [CrossRef]
- Bradley, M.M.; Miccoli, L.; Escrig, M.A.; Lang, P.J. The Pupil as a Measure of Emotional Arousal and Autonomic Activation. Psychophysiology 2008, 45, 602–607. [Google Scholar] [CrossRef]
- Morad, Y.; Lemberg, H.; Yofe, N.; Dagan, Y. Pupillography as an Objective Indicator of Fatigue. Curr. Eye Res. 2000, 21, 535–542. [Google Scholar] [CrossRef]
- Kashihara, K.; Okanoya, K.; Kawai, N. Emotional Attention Modulates Microsaccadic Rate and Direction. Psychol. Res. 2014, 78, 166–179. [Google Scholar] [CrossRef]
- Cherng, Y.-G.; Baird, T.; Chen, J.-T.; Wang, C.-A. Background Luminance Effects on Pupil Size Associated with Emotion and Saccade Preparation. Sci. Rep. 2020, 10, 15718. [Google Scholar] [CrossRef]
- Wang, C.-A.; Boehnke, S.E.; White, B.J.; Munoz, D.P. Microstimulation of the Monkey Superior Colliculus Induces Pupil Dilation without Evoking Saccades. J. Neurosci. 2012, 32, 3629–3636. [Google Scholar] [CrossRef]
- Wang, C.-A.; Munoz, D.P. Coordination of Pupil and Saccade Responses by the Superior Colliculus. J. Cogn. Neurosci. 2021, 33, 919–932. [Google Scholar] [CrossRef]
- Joshi, S.; Li, Y.; Kalwani, R.M.; Gold, J.I. Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron 2016, 89, 221–234. [Google Scholar] [CrossRef]
- Ebitz, R.B.; Moore, T. Selective Modulation of the Pupil Light Reflex by Microstimulation of Prefrontal Cortex. J. Neurosci. 2017, 37, 5008–5018. [Google Scholar] [CrossRef]
- Wang, C.-A.; Munoz, D.P. Neural Basis of Location-Specific Pupil Luminance Modulation. Proc. Natl. Acad. Sci. USA 2018, 115, 10446–10451. [Google Scholar] [CrossRef]
- Corneil, B.D.; Munoz, D.P. Overt Responses during Covert Orienting. Neuron 2014, 82, 1230–1243. [Google Scholar] [CrossRef]
- Lehmann, S.J.; Corneil, B.D. Transient Pupil Dilation after Subsaccadic Microstimulation of Primate Frontal Eye Fields. J. Neurosci. 2016, 36, 3765–3776. [Google Scholar] [CrossRef]
- Wang, C.-A.; Munoz, D.P. A Circuit for Pupil Orienting Responses: Implications for Cognitive Modulation of Pupil Size. Curr. Opin. Neurobiol. 2015, 33, 134–140. [Google Scholar] [CrossRef]
- Hsu, T.-Y.; Hsu, Y.-F.; Wang, H.-Y.; Wang, C.-A. Role of the Frontal Eye Field in Human Pupil and Saccade Orienting Responses. Eur. J. Neurosci. 2021, ejn.15253. [Google Scholar] [CrossRef]
- Bradshaw, J. Pupil Size as a Measure of Arousal during Information Processing. Nature 1967, 216, 515–516. [Google Scholar] [CrossRef]
- Wang, C.-A.; Baird, T.; Huang, J.; Coutinho, J.D.; Brien, D.C.; Munoz, D.P. Arousal Effects on Pupil Size, Heart Rate, and Skin Conductance in an Emotional Face Task. Front. Neurol. 2018, 9, 1029. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Cohen, J.D. An Integrative Theory of Locus Coeruleus-Norepinephrine Function: Adaptive Gain and Optimal Performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef]
- Reimer, J.; McGinley, M.J.; Liu, Y.; Rodenkirch, C.; Wang, Q.; McCormick, D.A.; Tolias, A.S. Pupil Fluctuations Track Rapid Changes in Adrenergic and Cholinergic Activity in Cortex. Nat. Commun. 2016, 7, 13289. [Google Scholar] [CrossRef]
- Alnæs, D.; Sneve, M.H.; Espeseth, T.; Endestad, T.; van de Pavert, S.H.P.; Laeng, B. Pupil Size Signals Mental Effort Deployed during Multiple Object Tracking and Predicts Brain Activity in the Dorsal Attention Network and the Locus Coeruleus. J. Vis. 2014, 14, 1. [Google Scholar] [CrossRef]
- Murphy, P.R.; O’Connell, R.G.; O’Sullivan, M.; Robertson, I.H.; Balsters, J.H. Pupil Diameter Covaries with BOLD Activity in Human Locus Coeruleus. Hum. Brain Mapp. 2014, 35, 4140–4154. [Google Scholar] [CrossRef]
- Varazzani, C.; San-Galli, A.; Gilardeau, S.; Bouret, S. Noradrenaline and Dopamine Neurons in the Reward/Effort Trade-Off: A Direct Electrophysiological Comparison in Behaving Monkeys. J. Neurosci. 2015, 35, 7866–7877. [Google Scholar] [CrossRef]
- Samuels, E.R.; Szabadi, E. Functional Neuroanatomy of the Noradrenergic Locus Coeruleus: Its Roles in the Regulation of Arousal and Autonomic Function Part II: Physiological and Pharmacological Manipulations and Pathological Alterations of Locus Coeruleus Activity in Humans. Curr. Neuropharmacol. 2008, 6, 254–285. [Google Scholar] [CrossRef]
- Samuels, E.; Szabadi, E. Functional Neuroanatomy of the Noradrenergic Locus Coeruleus: Its Roles in the Regulation of Arousal and Autonomic Function Part I: Principles of Functional Organisation. Curr. Neuropharmacol. 2008, 6, 235–253. [Google Scholar] [CrossRef]
- Breton-Provencher, V.; Sur, M. Active Control of Arousal by a Locus Coeruleus GABAergic Circuit. Nat. Neurosci. 2019, 22, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.E.; Yizhar, O.; Chikahisa, S.; Nguyen, H.; Adamantidis, A.; Nishino, S.; Deisseroth, K.; de Lecea, L. Tuning Arousal with Optogenetic Modulation of Locus Coeruleus Neurons. Nat. Neurosci. 2010, 13, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Bull. World Health Organ. 2001, 79, 373–374. [Google Scholar]
- Hsu, Y.-F.; Baird, T.; Wang, C.-A. Investigating Cognitive Load Modulation of Distractor Processing Using Pupillary Luminance Responses in the Anti-saccade Paradigm. Eur. J. Neurosci. 2020, 52, 3061–3073. [Google Scholar] [CrossRef]
- Brown, M.R.; DeSouza, J.F.; Goltz, H.C.; Ford, K.; Menon, R.S.; Goodale, M.A.; Everling, S. Comparison of Memory- and Visually Guided Saccades Using Event-Related FMRI. J. Neurophysiol. 2004, 91, 873–889. [Google Scholar] [CrossRef]
- Hikosaka, O.; Wurtz, R.H. Visual and Oculomotor Functions of Monkey Substantia Nigra Pars Reticulata. III. Memory-Contingent Visual and Saccade Responses. J. Neurophysiol. 1983, 49, 1268–1284. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, O.; Wurtz, R.H. Modification of Saccadic Eye Movements by GABA-Related Substances. I. Effect of Muscimol and Bicuculline in Monkey Superior Colliculus. J. Neurophysiol. 1985, 53, 266–291. [Google Scholar] [CrossRef]
- Takikawa, Y.; Kawagoe, R.; Miyashita, N.; Hikosaka, O. Presaccadic Omnidirectional Burst Activity in the Basal Interstitial Nucleus in the Monkey Cerebellum. Exp. Brain Res. 1998, 121, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.C.; Van Gisbergen, J.A.M.; Cools, A.R. A Parametric Analysis of Human Saccades in Different Experimental Paradigms. Vision Res. 1987, 27, 1745–1762. [Google Scholar] [CrossRef]
- Wang, C.-A.; Huang, J.; Yep, R.; Munoz, D.P. Comparing Pupil Light Response Modulation between Saccade Planning and Working Memory. J. Cogn. 2018, 1, 33. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.M.; Lang, P.J. The International Affective Digitized Sounds (2nd Edition; IADS-2): Affective Ratings of Sounds and Instruction Manual; Technical Report B-3; University of Florida: Gainesville, FL, USA, 2007. [Google Scholar]
- Chen, J.-T.; Yep, R.; Hsu, Y.-F.; Cherng, Y.-G.; Wang, C.-A. Investigating Arousal, Saccade Preparation, and Global Luminance Effects on Microsaccade Behavior. Front. Hum. Neurosci. 2021, 15, 95. [Google Scholar] [CrossRef]
- Munoz, D.P.; Broughton, J.R.; Goldring, J.E.; Armstrong, I.T. Age-Related Performance of Human Subjects on Saccadic Eye Movement Tasks. Exp. Brain Res. Hirnforschung. Exp. Cereb. 1998, 121, 391–400. [Google Scholar] [CrossRef]
- Karatekin, C.; Bingham, C.; White, T. Oculomotor and Pupillometric Indices of Pro- and Antisaccade Performance in Youth-Onset Psychosis and Attention Deficit/Hyperactivity Disorder. Schizophr. Bull. 2010, 36, 1167–1186. [Google Scholar] [CrossRef]
- Mathôt, S.; Fabius, J.; Van Heusden, E.; Van der Stigchel, S. Safe and Sensible Preprocessing and Baseline Correction of Pupil-Size Data. Behav. Res. Methods 2018, 50, 94–106. [Google Scholar] [CrossRef]
- Bala, A.D.; Takahashi, T.T. Pupillary Dilation Response as an Indicator of Auditory Discrimination in the Barn Owl. J. Comp. Physiol. Sens. Neural Behav. Physiol. 2000, 186, 425–434. [Google Scholar] [CrossRef]
- Moresi, S.; Adam, J.J.; Rijcken, J.; Van Gerven, P.W.; Kuipers, H.; Jolles, J. Pupil Dilation in Response Preparation. Int. J. Psychophysiol. 2008, 67, 124–130. [Google Scholar] [CrossRef]
- JASP Team JASP (Version 0.10.2). [Computer Software]. 2019. Available online: https://jasp-stats.org/previous-versions/ (accessed on 26 July 2022).
- de Gee, J.W.; Knapen, T.; Donner, T.H. Decision-Related Pupil Dilation Reflects Upcoming Choice and Individual Bias. Proc. Natl. Acad. Sci. USA 2014, 111, E618–E625. [Google Scholar] [CrossRef]
- Nassar, M.R.; Rumsey, K.M.; Wilson, R.C.; Parikh, K.; Heasly, B.; Gold, J.I. Rational Regulation of Learning Dynamics by Pupil-Linked Arousal Systems. Nat. Neurosci. 2012, 15, 1040–1046. [Google Scholar] [CrossRef]
- Lacey, J.I. The Evaluation of Autonomic Responses: Toward a General Solution. Ann. N. Y. Acad. Sci. 1956, 67, 125–163. [Google Scholar] [CrossRef]
- Wang, C.A.; Munoz, D.P. Differentiating Global Luminance, Arousal and Cognitive Signals on Pupil Size and Microsaccades. Eur. J. Neurosci. 2021, 54, 7560–7574. [Google Scholar] [CrossRef]
- van Steenbergen, H.; Band, G.P.H.; Hommel, B. Threat But Not Arousal Narrows Attention: Evidence from Pupil Dilation and Saccade Control. Front. Psychol. 2011, 2, 281. [Google Scholar] [CrossRef]
- Crommelinck, M.; Roucoux, A. Characteristics of Cat’s Eye Saccades in Different States of Alertness. Brain Res. 1976, 103, 574–578. [Google Scholar] [CrossRef]
- DiGirolamo, G.J.; Patel, N.; Blaukopf, C.L. Arousal Facilitates Involuntary Eye Movements. Exp. Brain Res. 2016, 234, 1967–1976. [Google Scholar] [CrossRef]
- McSorley, E.; Morriss, J. What You See Is What You Want to See: Motivationally Relevant Stimuli Can Interrupt Current Resource Allocation. Cogn. Emot. 2017, 31, 168–174. [Google Scholar] [CrossRef]
- Wang, C.-A.; Blohm, G.; Huang, J.; Boehnke, S.E.; Munoz, D.P. Multisensory Integration in Orienting Behavior: Pupil Size, Microsaccades, and Saccades. Biol. Psychol. 2017, 129, 36–44. [Google Scholar] [CrossRef]
- Simola, J.; Le Fevre, K.; Torniainen, J.; Baccino, T. Affective Processing in Natural Scene Viewing: Valence and Arousal Interactions in Eye-Fixation-Related Potentials. Neuroimage 2015, 106, 21–33. [Google Scholar] [CrossRef]
- Gandhi, N.J.; Katnani, H.A. Motor Functions of the Superior Colliculus. Annu. Rev. Neurosci. 2011, 34, 205–231. [Google Scholar] [CrossRef]
- Krauzlis, R.J.; Lovejoy, L.P.; Zenon, A. Superior Colliculus and Visual Spatial Attention. Annu. Rev. Neurosci. 2013, 36, 165–182. [Google Scholar] [CrossRef]
- Edwards, S.B.; Ginsburgh, C.L.; Henkel, C.K.; Stein, B.E. Sources of Subcortical Projections to the Superior Colliculus in the Cat. J. Comp. Neurol. 1979, 184, 309–329. [Google Scholar] [CrossRef]
- Li, L.L.; Feng, X.; Zhou, Z.; Zhang, H.; Shi, Q.; Lei, Z.; Shen, P.; Yang, Q.; Zhao, B.; Chen, S.; et al. Stress Accelerates Defensive Responses to Looming in Mice and Involves a Locus Coeruleus-Superior Colliculus Projection. Curr. Biol. 2018, 28, 859–871. [Google Scholar] [CrossRef]
- Segraves, M.A.; Goldberg, M.E. Functional Properties of Corticotectal Neurons in the Monkey’s Frontal Eye Field. J. Neurophysiol. 1987, 58, 1387–1419. [Google Scholar] [CrossRef]
- White, B.J.; Munoz, D.P. The Superior Colliculus BT—Oxford Handbook of Eye Movements. In Oxford Handbook of Eye Movements; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Moschovakis, A.K.; Karabelas, A.B.; Highstein, S.M. Structure-Function Relationships in the Primate Superior Colliculus. II. Morphological Identity of Presaccadic Neurons. J. Neurophysiol. 1988, 60, 263–302. [Google Scholar] [CrossRef]
- Rodgers, C.K.; Munoz, D.P.; Scott, S.H.; Pare, M.; Paré, M. Discharge Properties of Monkey Tectoreticular Neurons. J. Neurophysiol. 2006, 95, 3502–3511. [Google Scholar] [CrossRef][Green Version]
- May, P.J.; Warren, S.; Bohlen, M.O.; Barnerssoi, M.; Horn, A.K.E. A Central Mesencephalic Reticular Formation Projection to the Edinger-Westphal Nuclei. Brain Struct. Funct. 2016, 221, 4073–4089. [Google Scholar] [CrossRef]
- May, P.J. The Mammalian Superior Colliculus: Laminar Structure and Connections. Prog. Brain Res. 2006, 151, 321–378. [Google Scholar] [CrossRef]
- Barnerssoi, M.; May, P.J.; Horn, A.K.E. GABAergic Innervation of the Ciliary Ganglion in Macaque Monkeys—A Light and Electron Microscopic Study. J. Comp. Neurol. 2017, 525, 1517–1531. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-A.; White, B.; Munoz, D.P. Pupil-Linked Arousal Signals in the Midbrain Superior Colliculus. J. Cogn. Neurosci. 2022, 34, 1340–1354. [Google Scholar] [CrossRef] [PubMed]
- Strauch, C.; Wang, C.-A.; Einhäuser, W.; Van der Stigchel, S.; Naber, M. Pupillometry as an Integrated Readout of Distinct Attentional Networks. Trends Neurosci. 2022, 45, 635–647. [Google Scholar] [CrossRef] [PubMed]

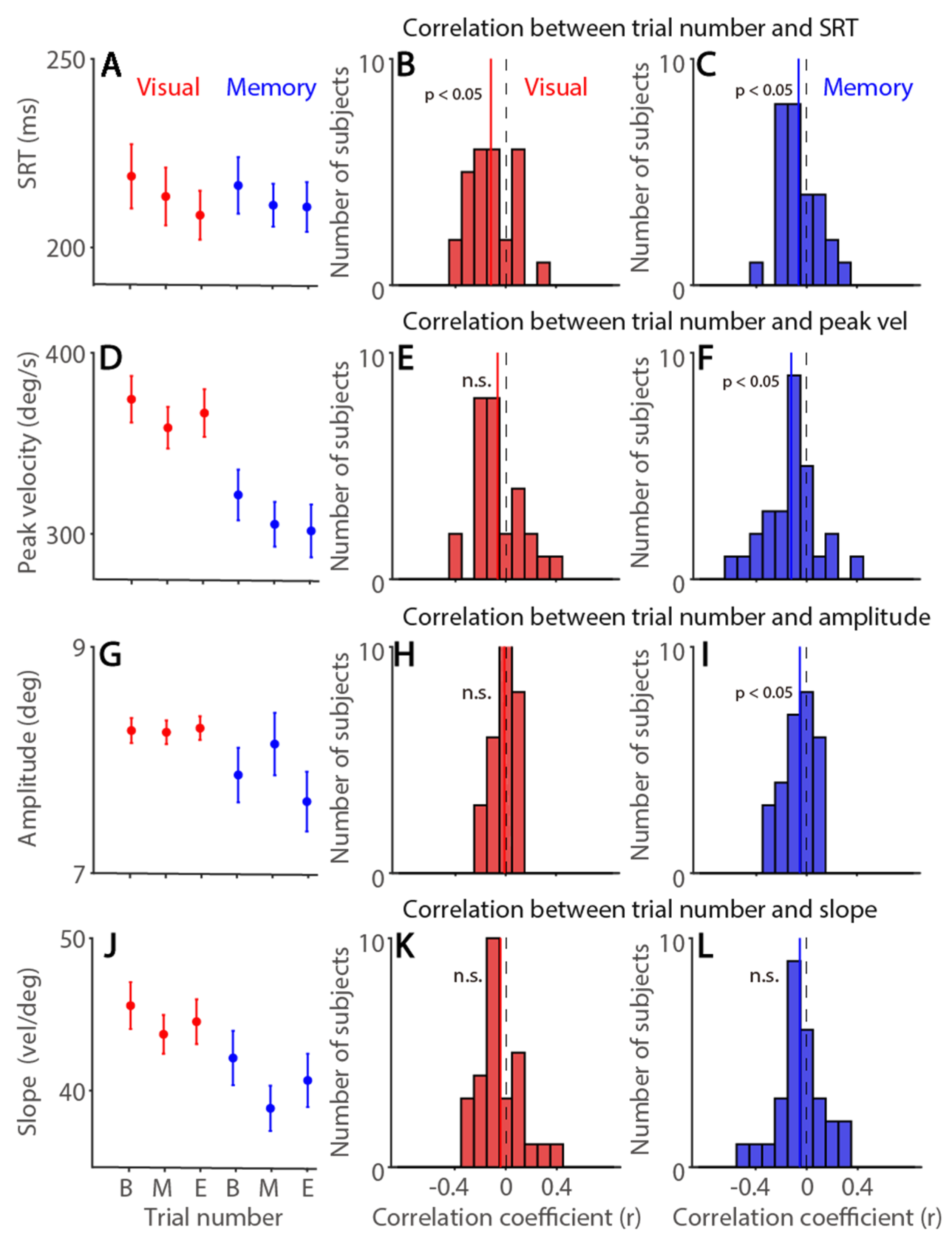

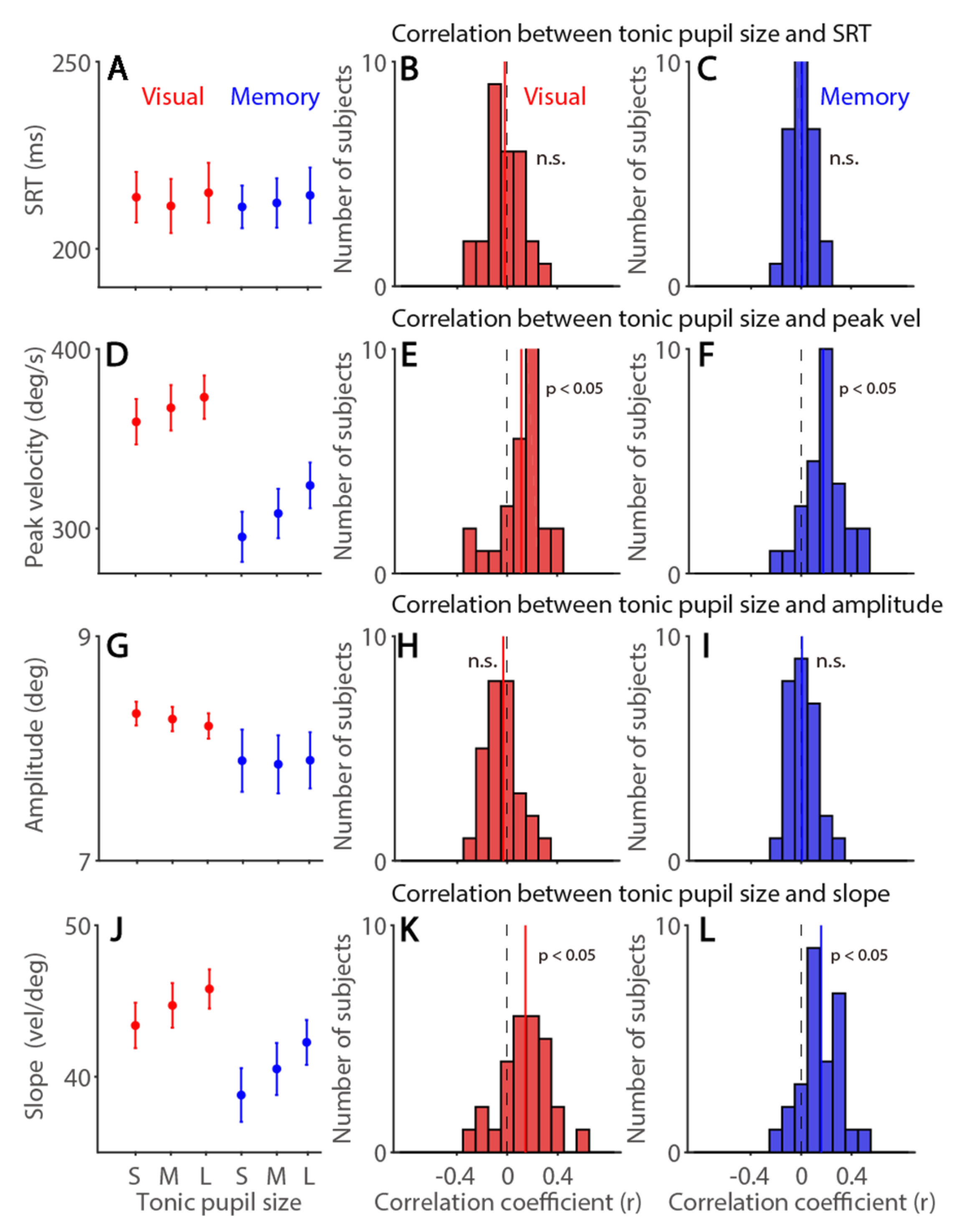

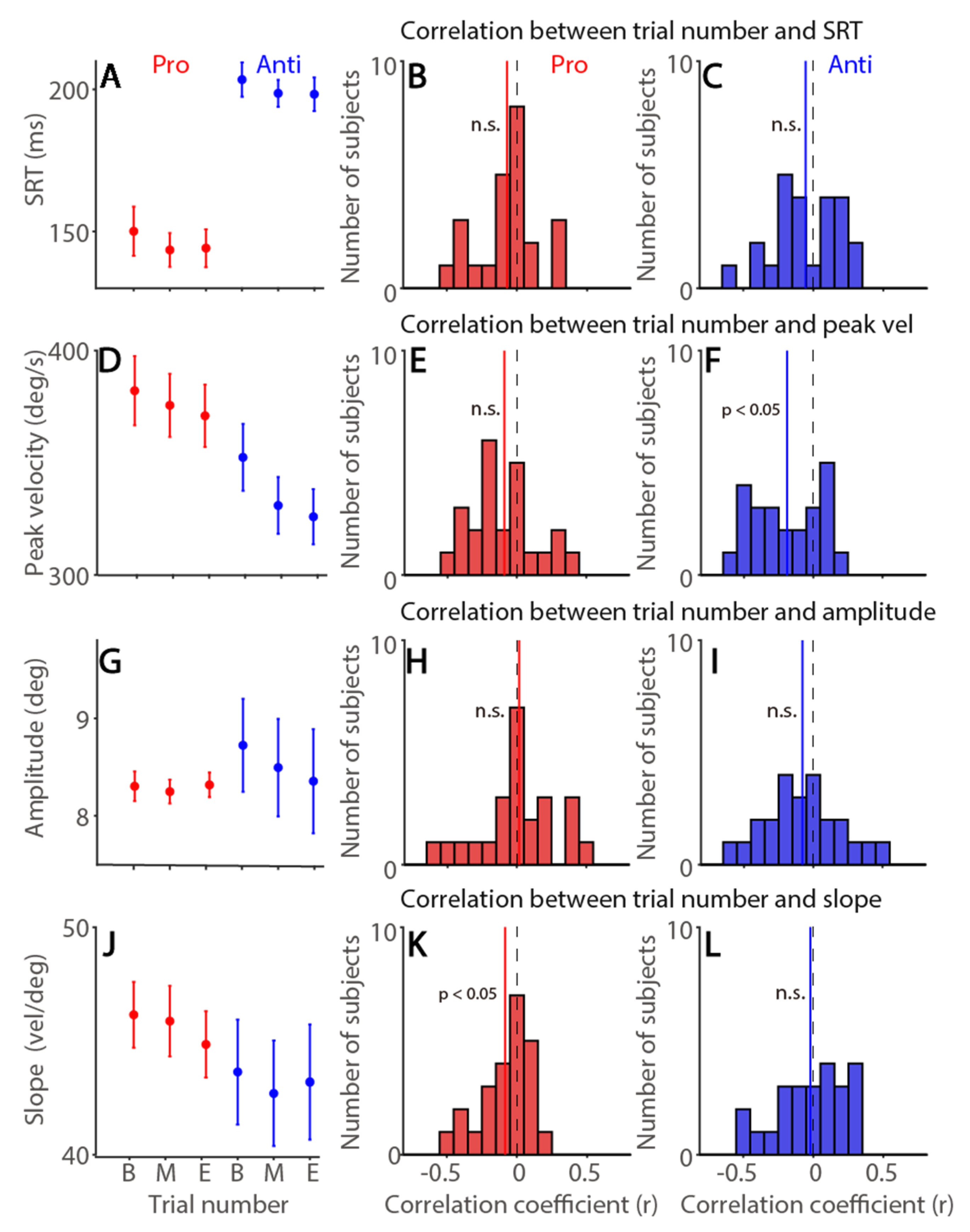
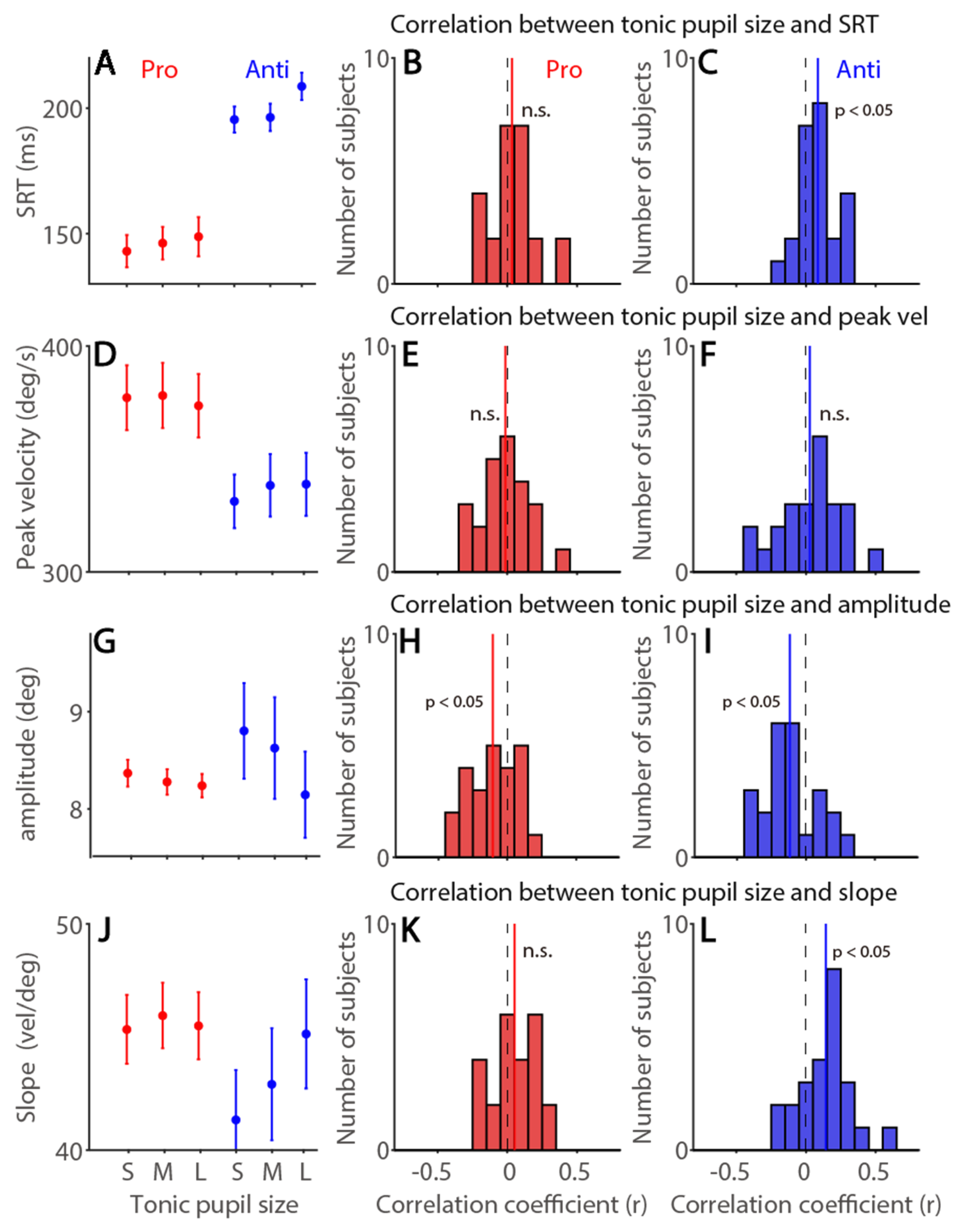
| Exp. 1 | SRT | Peak Vel | Amplitude | Slope | Tonic pu | Phasic pu | |
|---|---|---|---|---|---|---|---|
| Visual | Trial number | −0.12 * (71%) | −0.06 (68%) | −0.01 (46%) | −0.04 (68%) | −0.36 * (89%) | −0.15 * (75%) |
| Memory | −0.06 * (71%) | −0.12 * (79%) | −0.05 * (61%) | −0.05 (57%) | −0.37 * (86%) | −0.16 * (68%) | |
| Visual | Tonic pupil size | −0.02 (57%) | 0.11 * (82%) | −0.03 (68%) | 0.14 * (82%) | −0.65 * (93%) | |
| Memory | 0.01 (54%) | 0.18 * (89%) | 0.01 (47%) | 0.16 * (79%) | −0.65 * (93%) |
| Exp. 2 | SRT | Peak Vel | Amplitude | Slope | Tonic pu | Phasic pu | |
|---|---|---|---|---|---|---|---|
| Pro | Trial number | −0.07 (63%) | −0.09 (67%) | 0.02 (54%) | −0.08 * (63%) | −0.18 * (71%) | −0.08 * (79%) |
| Anti | −0.06 (54%) | −0.19 * (75%) | −0.08 (71%) | −0.02 (54%) | |||
| Pro | Tonic pupil size | 0.03 (50%) | −0.01 (63%) | −0.11 * (71%) | 0.05 (62%) | −0.45 * (100%) | |
| Anti | 0.09 * (79%) | −0.03 (58%) | −0.12 * (75%) | 0.14 * (75%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-T.; Kuo, Y.-C.; Hsu, T.-Y.; Wang, C.-A. Fatigue and Arousal Modulations Revealed by Saccade and Pupil Dynamics. Int. J. Environ. Res. Public Health 2022, 19, 9234. https://doi.org/10.3390/ijerph19159234
Chen J-T, Kuo Y-C, Hsu T-Y, Wang C-A. Fatigue and Arousal Modulations Revealed by Saccade and Pupil Dynamics. International Journal of Environmental Research and Public Health. 2022; 19(15):9234. https://doi.org/10.3390/ijerph19159234
Chicago/Turabian StyleChen, Jui-Tai, Ying-Chun Kuo, Tzu-Yu Hsu, and Chin-An Wang. 2022. "Fatigue and Arousal Modulations Revealed by Saccade and Pupil Dynamics" International Journal of Environmental Research and Public Health 19, no. 15: 9234. https://doi.org/10.3390/ijerph19159234
APA StyleChen, J.-T., Kuo, Y.-C., Hsu, T.-Y., & Wang, C.-A. (2022). Fatigue and Arousal Modulations Revealed by Saccade and Pupil Dynamics. International Journal of Environmental Research and Public Health, 19(15), 9234. https://doi.org/10.3390/ijerph19159234






