Serum Phospholipids Are Potential Therapeutic Targets of Aqueous Extracts of Roselle (Hibiscus sabdariffa) against Obesity and Insulin Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Herbal Materials and Aqueous Extract Preparation
2.3. Quantification of Total Polyphenols
2.3.1. Quantification of Total Phenolic Compounds
2.3.2. Quantification of Total Flavonoids
2.3.3. Quantification of Monomeric Anthocyanins
2.4. Polyphenol and Organic Acid Profile
2.5. Free Radical Scavenging Assays
2.6. Animals and Treatments
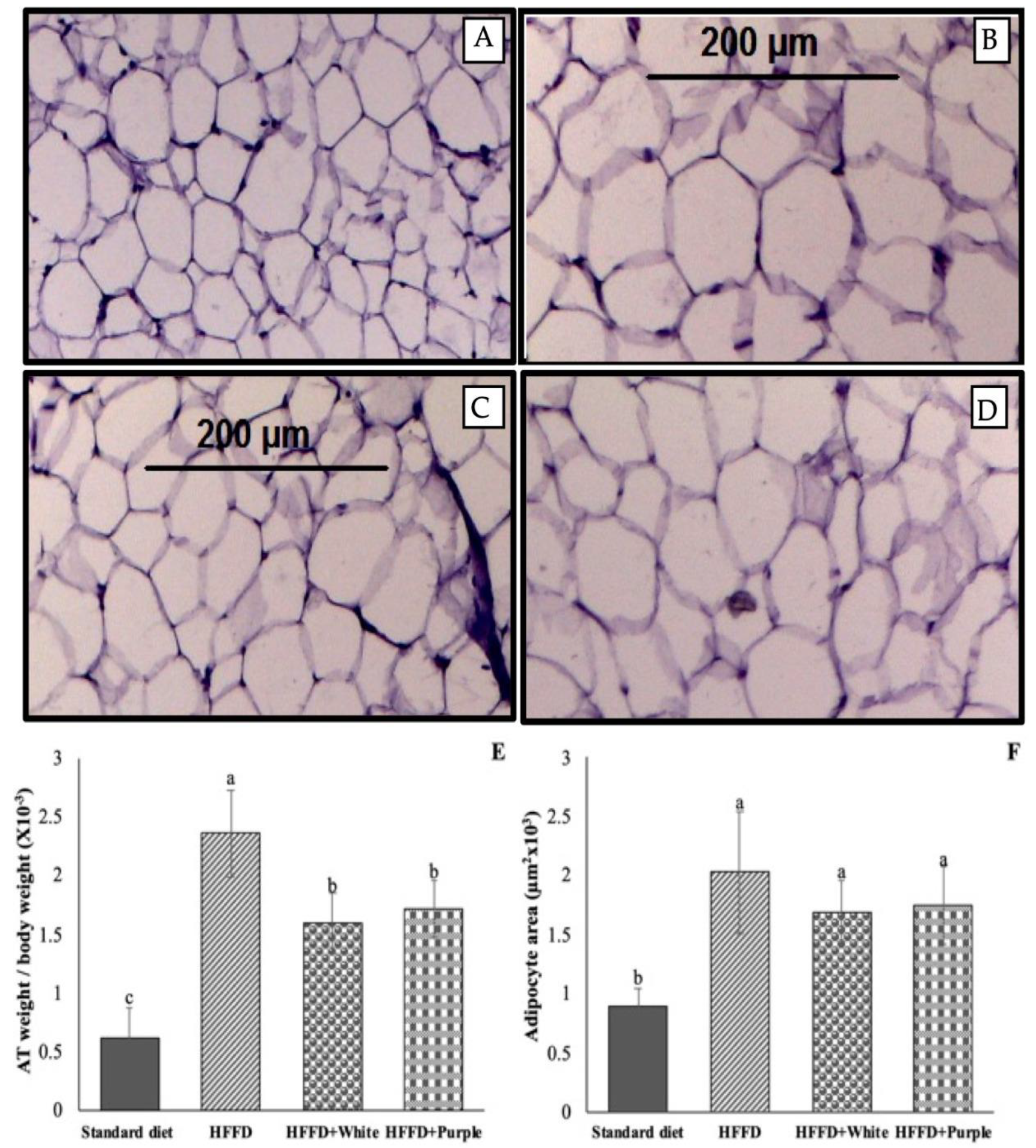
2.7. Histopathology of Mesenteric Adipose Tissue
2.8. Serum Fasting Glucose (FG), Fasting Insulin (FINS), and Free Fatty Acids (FFA)
2.9. Serum Targeted Metabolomics
2.10. Statistical Analysis
3. Results
3.1. Phytochemical Characterization of Alma Blanca and Cuarenteña HSC Aqueous Extracts
3.2. Antioxidant Capacities of Alma Blanca and Cuarenteña HSC Aqueous Extracts
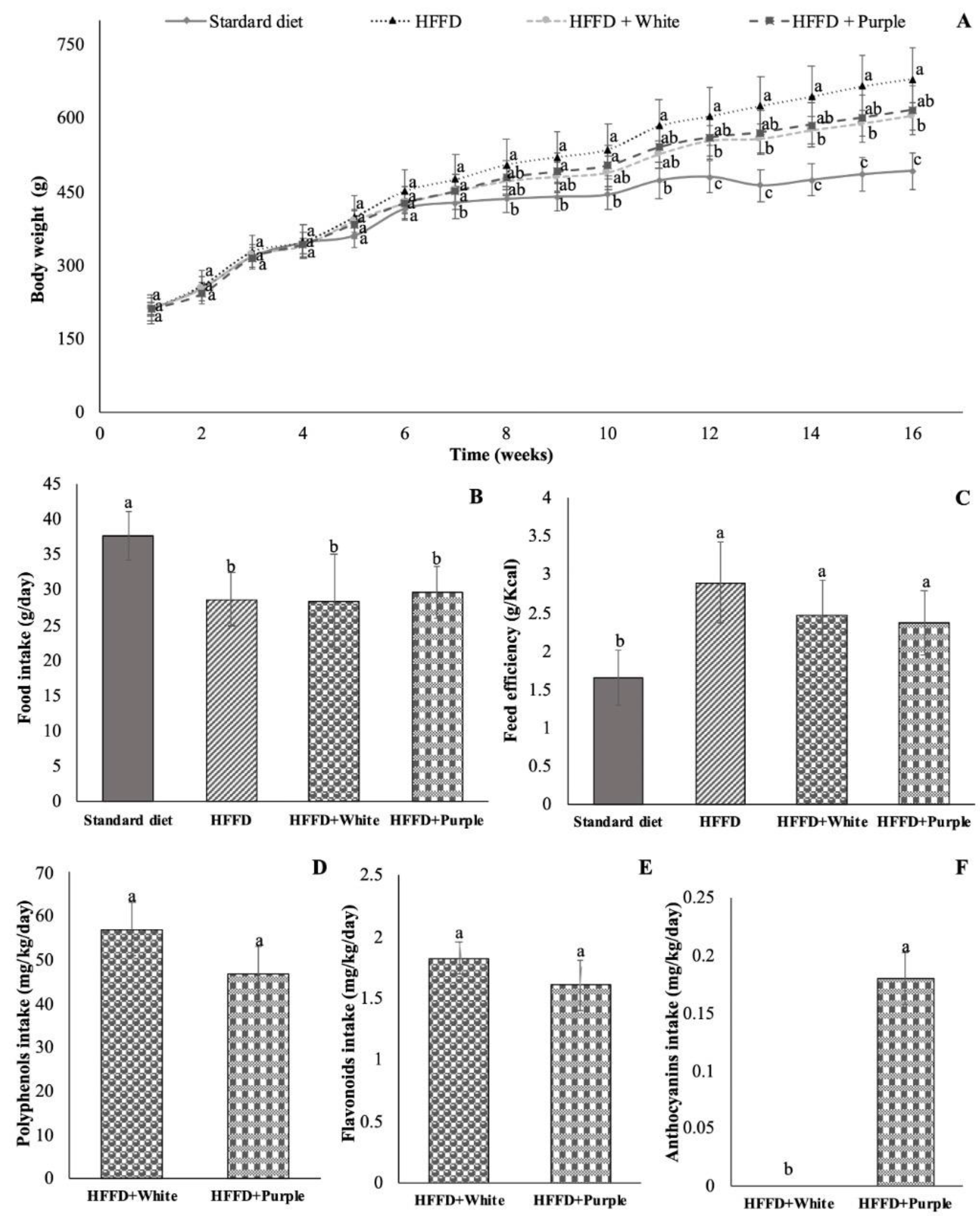
3.3. Effect of Alma Blanca and Cuarenteña HSC Aqueous Extracts on Obesity and Its Complications in HFFD-Fed Rats
3.4. Insulin Resistance
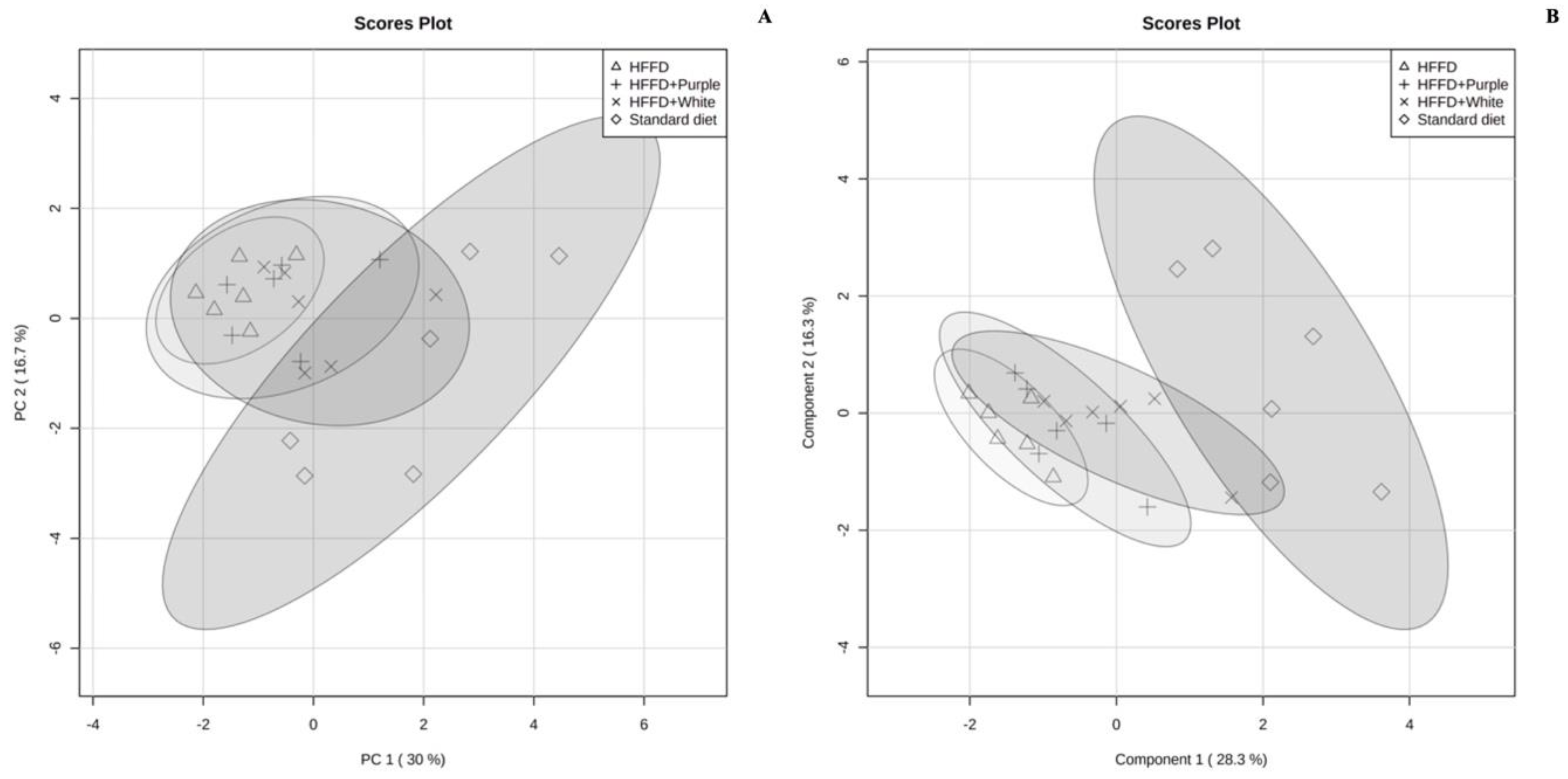
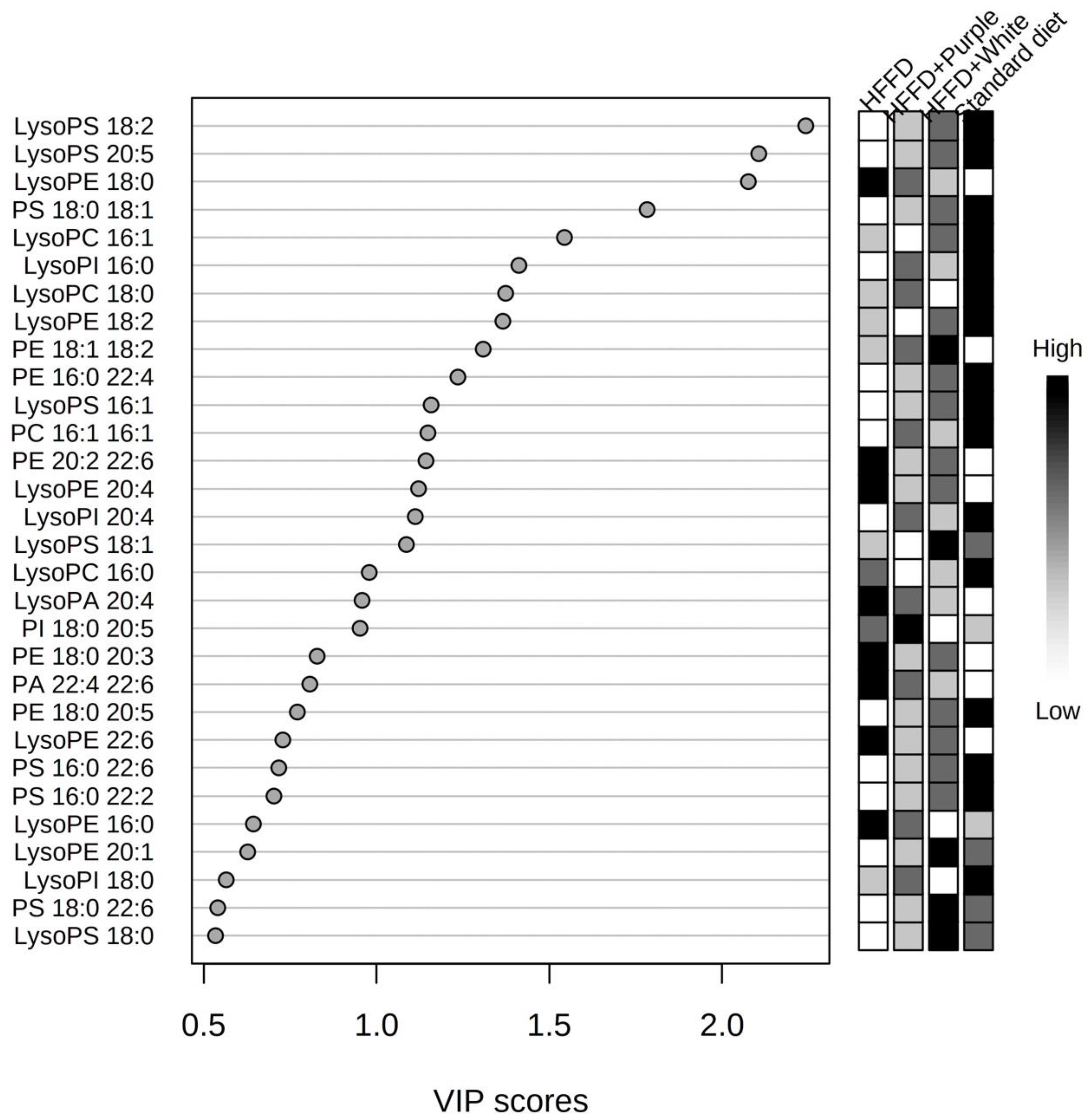
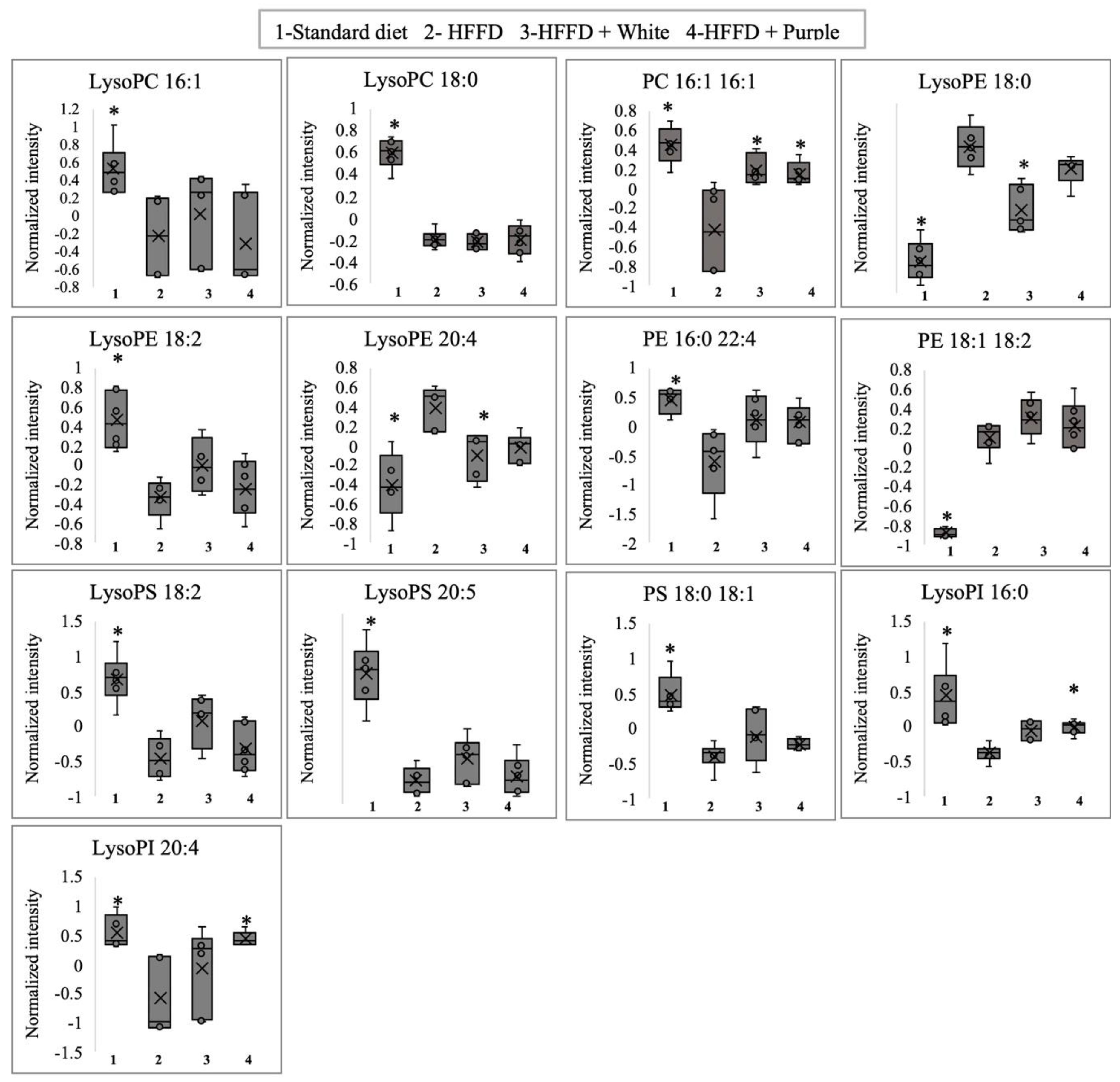
3.5. Serum Targeted Metabolomics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Gil, A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics 2019, 15, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; Segura-Carretero, A.; Contreras, M.D. Phenolic compounds as natural and multifunctional anti-obesity agents: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1212–1229. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Zarrabal, O.; Hayward-Jones, P.M.; Orta-Flores, Z.; Nolasco-Hipólito, C.; Barradas-Dermitz, D.M.; Aguilar-Uscanga, M.G.; Pedroza-Hernández, M.F. Effect of Hibiscus sabdariffa L. dried calyx ethanol extract on fat absorption-excretion, and body weight implication in rats. J. Biomed. Biotechnol. 2009, 2009, 394592. [Google Scholar] [CrossRef]
- Chang, H.C.; Peng, C.H.; Yeh, D.M.; Kao, E.S.; Wang, C.J. Hibiscus sabdariffa extract inhibits obesity and fat accumulation, and improves liver steatosis in humans. Food Funct. 2014, 5, 734–739. [Google Scholar] [CrossRef]
- Bule, M.; Albelbeisi, A.H.; Nikfar, S.; Amini, M.; Abdollahi, M. The antidiabetic and antilipidemic effects of Hibiscus sabdariffa: A systematic review and meta-analysis of randomized clinical trials. Food Res. Int. 2020, 130, 108980. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Peng, C.H.; Chan, K.C.; Yang, Y.S.; Huang, C.N.; Wang, C.J. The hypolipidemic effect of Hibiscus sabdariffa polyphenols via inhibiting lipogenesis and promoting hepatic lipid clearance. J. Agric. Food Chem. 2010, 58, 850–859. [Google Scholar] [CrossRef]
- Herranz-López, M.; Fernández-Arroyo, S.; Pérez-Sanchez, A.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Menéndez, J.A.; Alonso-Villaverde, C.; Segura-Carretero, A.; Joven, J.; Micol, V. Synergism of plant-derived polyphenols in adipogenesis: Perspectives and implications. Phytomedicine 2012, 19, 253–261. [Google Scholar] [CrossRef]
- Mayasari, N.R.; Susetyowati; Wahyuningsih, M.S.H. Probosuseno, Antidiabetic effect of rosella-stevia tea on prediabetic women in Yogyakarta, Indonesia. J. Am. Coll. Nutr. 2018, 37, 373–379. [Google Scholar] [CrossRef]
- Diez-Echave, P.; Vezza, T.; Rodríguez-Nogales, A.; Ruiz-Malagón, A.J.; Hidalgo-García, L.; Garrido-Mesa, J.; Molino-Tijeras, J.A.; Romero, M.; Robles-Vera, I.; Pimentel-Moral, S.; et al. The prebiotic properties of Hibiscus sabdariffa extract contribute to the beneficial effects in diet-induced obesity in mice. Food Res. Int. 2020, 127, 108722. [Google Scholar] [CrossRef]
- Beltrán-Debón, R.; Rodríguez-Gallego, E.; Fernández-Arroyo, S.; Senan-Campos, O.; Massucci, F.A.; Hernández-Aguilera, A.; Sales-Pardo, M.; Guimerà, R.; Camps, J.; Menendez, J.A.; et al. The acute impact of polyphenols from Hibiscus sabdariffa in metabolic homeostasis: An approach combining metabolomics and gene-expression analyses. Food Funct. 2015, 6, 2957–2966. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Flores, R.; Serrano-Altamirano, V.; Navarro-Galindo, S.; Ovando-Cruz, M.E.; Vázquez-García, E.; Barrios-Ayala, A.; Michel-Aceves, A.C.; Guzmán-Maldonado, S.H.; Otero-Sánchez, M.A. Variedades mexicanas de jamaica (Hibiscus sabdariffa L.) ‘Alma Blanca’y‘Rosalíz’ de color claro, y ‘Cotzaltzin’ y ‘Tecoanapa’ de color rojo. Rev. Fitotec. Mex. 2014, 37, 181–185. Available online: https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0187-73802014000200009 (accessed on 9 October 2022).
- Camacho, R.R. Project “Selección de Variedades de Jamaica (hibiscus sabdariffa L.) en Base de su Perfil de Compuestos Bioactivos Para el Desarrollo de Productos de Alto Valor Agregado”. Componente de Innovación agroalimentaria 2016 SAGARPA. DF1600000640. 2016. Available online: https://www.agricultura.gob.mx/sites/default/files/sagarpa/document/2018/09/04/1316/180904-primer-listado-innovacion-agroalimentaria.pdf (accessed on 9 October 2022).
- Borrás-Linares, I.; Fernández-Arroyo, S.; Arráez-Roman, D.; Palmeros-Suárez, P.A.; del Val-Díaz, R.; Andrade-Gonzáles, I.; Fernández-Gutiérrez, A.; Gómez-Leyva, J.F.; Segura-Carretero, A. Characterization of phenolic compounds, anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican Roselle (Hibiscus sabdariffa). Ind. Crops Prd. 2015, 69, 385–394. [Google Scholar] [CrossRef]
- Morales-Luna, E.; Pérez-Ramírez, I.F.; Salgado, L.M.; Castaño-Tostado, E.; Gómez-Aldapa, C.A.; Reynoso-Camacho, R. The main beneficial effect of roselle (Hibiscus sabdariffa) on obesity is not only related to its anthocyanin content. J. Sci. Food Agric. 2019, 99, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Cid-Ortega, S.; Guerrero-Beltrán, J.A. Roselle calyces (Hibiscus sabdariffa), an alternative to the food and beverages industries: A review. J. Food Sci. Technol. 2015, 52, 6859–6869. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Oxidants and antioxidants, Pt A. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Vincieri, F.F.; Romani, A. Antiradical activity and polyphenol composition of local Brassicaceae edible varieties. Food Chem. 2006, 99, 464–469. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Amaya-Cruz, D.; Peréz-Ramírez, I.F.; Pérez-Jiménez, J.; Nava, G.M.; Reynoso-Camacho, R. Comparison of the bioactive potential of roselle (Hibiscus sabdariffa L.) calyx and its by-product: Phenolic characterization by UPLC-QTOF MSE and their anti-obesity effect in vivo. Food Res. Int. 2019, 126, 108589. [Google Scholar] [CrossRef]
- Williams, W.B.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- de los Ríos, E.A.; Ruiz-Herrera, X.; Tinoco-Pantoja, V.; López-Barrera, F.; de la Escalera, G.M.; Clapp, C.; Macotela, Y. Impaired prolactin actions mediate altered offspring metabolism induced by maternal high-fat feeding during lactation. FASEB J. 2018, 32, 3457–3470. [Google Scholar] [CrossRef] [PubMed]
- Duncombe, W.G. The colorimetric micro-determination of non-esterified fatty acids in plasma. Clin. Chim. Acta 1964, 9, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Zamora, A.; Delgado-Andrade, C.; Rufián-Henares, J.A. Antioxidant capacity, total phenols and color profile during the storage of selected plants used for infusion. Food Chem. 2016, 199, 339–346. [Google Scholar] [CrossRef]
- Beye, C.; Hiligsmann, S.; Tounkara, L.S.; Thonart, P. Anthocyanin content of two Hibiscus sabdariffa cultivars grown in Senegal. Agron. Afr. 2017, 29, 63–68. Available online: https://www.ajol.info/index.php/aga/article/view/163170 (accessed on 9 October 2022).
- Pragalyaashree, M.M.; Tiroutchelvame, D.; Sashikumar, S. Degradation kinetics of anthocyanin extracted from roselle calyces (Hibiscus sabdariffa). J. Appl. Pharm. Sci. 2018, 8, 57–63. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Obanijesu, E.O.; Alara, J.A.; Mudalip, S.K.A. Extract-rich in flavonoids from Hibiscus sabdariffa calyces: Optimizing microwave-assisted extraction method and characterization through LC-Q-TOF-MS analysis. J. Food Process Eng. 2020, 43, e13339. [Google Scholar] [CrossRef]
- da Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.–A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef]
- Moyano, G.; Sáyago-Ayerdi, S.G.; Largo, C.; Caz, V.; Santamaria, M.; Tabernero, M. Potential use of dietary fibre from Hibiscus sabdariffa and Agave tequilana in obesity management. J. Funct. Foods 2016, 21, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, L.; Liao, C.; Chen, L.; Wang, J.; Zeng, L. Effects of brewing conditions on the phytochemical composition, sensory qualities and antioxidant activity of green tea infusion: A study using response surface methodology. Food Chem. 2018, 269, 24–34. [Google Scholar] [CrossRef]
- Villalpando-Arteaga, E.V.; Mendieta-Condado, E.; Esquivel-Solís, H.; Canales-Aguirre, A.A.; Gálvez-Gastélum, F.J.; Mateos-Díaz, J.C.; Rodríguez-González, J.A.; Márquez-Aguirre, A.L. Hibiscus sabdariffa L. aqueous extract attenuates hepatic steatosis through down-regulation of PPAR-γ and SREBP-1c in diet-induced obese mice. Food Funct. 2013, 4, 618–626. [Google Scholar] [CrossRef] [PubMed]
- El-Shiekh, R.A.; Ashour, R.M.; El-Haleim, E.A.A.; Ahmed, K.A.; Abdel-Sattar, E. Hibiscus sabdariffa L.: A potent natural neuroprotective agent for the prevention of streptozotocin-induced Alzheimer’s disease in mice. Biomed. Pharmacother. 2020, 128, 110303. [Google Scholar] [CrossRef] [PubMed]
- Alkhalidy, H.; Moore, W.; Wang, Y.; Luo, J.; McMillan, R.P.; Zhen, W.; Zhou, K.; Liu, D. The flavonoid kaempferol ameliorates streptozotocin-induced diabetes by suppressing hepatic glucose production. Molecules 2018, 23, 2338. [Google Scholar] [CrossRef] [PubMed]
- Russo, B.; Picconi, F.; Malandrucco, I.; Frontoni, S. Flavonoids and insulin-resistance: From molecular evidences to clinical trials. Int. J. Mol. Sci. 2019, 20, 2061. [Google Scholar] [CrossRef] [PubMed]
- Zagayko, A.L.; Kravchenko, G.B.; Fylymonenko, V.P.; Krasilnikova, O.A. Effect of apple polyphenol concentrate on lipid metabolism in rats under experimental insulin resistance. Wiad. Lek. 2017, 70, 200–204. [Google Scholar]
- Barber, M.N.; Risis, S.; Yang, C.; Meikle, P.J.; Staples, M.; Febbraio, M.A.; Bruce, C.R. Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS ONE 2012, 7, e41456. [Google Scholar] [CrossRef]
- Heimerl, S.; Fischer, M.; Baessler, A.; Liebisch, G.; Sigruener, A.; Wallner, S.; Schmitz, G. Alterations of plasma lysophosphatidylcholine species in obesity and weight loss. PLoS ONE 2014, 9, e111348. [Google Scholar] [CrossRef]
- Rauschert, S.; Uhl, O.; Koletzko, B.; Kirchberg, F.; Mori, T.A.; Huang, R.C.; Lawrence, J.B.; Hellmuth, C.; Oddy, W.H. Lipidomics reveals associations of phospholipids with obesity and insulin resistance in young adults. J. Clin. Endocrinol. Metab. 2016, 101, 871–879. [Google Scholar] [CrossRef]
- del Bas, J.M.; Caimari, A.; Rodriguez-Naranjo, M.I.; Childs, C.E.; Chavez, C.P.; West, A.L.; Miles, E.A.; Arola, L.; Calder, P.C. Impairment of lysophospholipid metabolism in obesity: Altered plasma profile and desensitization to the modulatory properties of n–3 polyunsaturated fatty acids in a randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 266–279. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Seppänen-Laakso, T.; Kankainen, M.; Maukonen, J.; Törrönen, R.; Kolehmainen, M.; Leppänen, T.; Moilanen, E.; Nohynek, L.; Aura, A.-M.; et al. Effects of ellagitannin-rich berries on blood lipids, gut microbiota, and urolithin production in human subjects with symptoms of metabolic syndrome. Mol. Nutr. Food Res. 2013, 57, 2258–2263. [Google Scholar] [CrossRef]
| Rt (min) | Molecular Formula | Observed Mass (Da) | Concentration | ||
|---|---|---|---|---|---|
| White | Purple | ||||
| Monomeric anthocyanins 1 | ------- | ------- | ------- | ND | 1.3 ± 0.08 |
| Total flavonoids 2 | ------- | ------- | ------- | 13.5 ± 0.72 a | 11.93 ± 0.77 a |
| Total phenolic compounds 3 | ------- | ------- | ------- | 417.0 ± 24.4 a | 343.4 ± 24.4 b |
| Anthocyanins 4 | |||||
| Delphinidin sambubioside + | 2.25 | C26H29O16 | 597.1455 | ND | 23.58 ± 0.84 |
| Delphinidin hexoside + | 2.70 | C21H21O12 | 465.1070 | ND | 0.01 ± 0.00 |
| Cyanidin hexoside + | 2.95 | C21H21O11 | 449.1117 | ND | 0.01 ± 0.01 |
| Flavones 4 | |||||
| Chrysoeriol hexoside | 4.31 | C22H22O11 | 462.1157 | 0.02 ± 0.00 | 0.05 ± 0.00 |
| Chrysoeriol apiosyl-hexoside | 4.85 | C27H30O15 | 594.1577 | 0.82 ± 0.02 | 0.18 ± 0.01 |
| Flavonols 4 | |||||
| Kaempferol pentosyl-hexoside + | 2.53 | C26H28O15 | 580.1417 | 3.19 ± 0.12 | 0.03 ± 0.00 |
| Myricetin hexoside + | 3.41 | C21H20O13 | 480.0903 | 0.63 ± 0.03 | 6.71 ± 0.81 |
| Quercetin rutinoside (rutin) *,+ | 3.95 | C27H30O16 | 610.1528 | 1.66 ± 0.10 | 9.02 ± 0.21 |
| Quercetin hexoside + | 4.18 | C21H20O12 | 464.0960 | 1.69 ± 0.14 | 9.68 ± 1.39 |
| (Iso)-rhamnetin rutinoside | 4.31 | C22H22O11 | 462.1157 | 0.08 ± 0.00 | 0.04 ± 0.00 |
| Kaempferol dihexoside | 4.74 | C27H30O16 | 610.1514 | 0.10 ± 0.00 | 0.57 ± 0.20 |
| Kaempferol hexoside-rhamnoside + | 4.85 | C27H30O15 | 594.1577 | 0.31 ± 0.01 | 1.44 ± 0.03 |
| Quercetin hexoside-rhamnoside | 5.04 | C27H30O16 | 610.1511 | 0.06 ± 0.01 | 0.20 ± 0.01 |
| Myricetin rhamnoside | 5.09 | C21H20O12 | 464.0954 | 0.09 ± 0.01 | 0.54 ± 0.10 |
| Kaempferol hexoside + | 5.14 | C21H20O11 | 448.1011 | 0.16 ± 0.01 | 0.55 ± 0.06 |
| Myricetin *,+ | 5.64 | C15H10O8 | 318.0379 | 0.02 ± 0.00 | 2.70 ± 3.79 |
| Quercetin *,+ | 8.74 | C15H10O7 | 302.0421 | 0.04 ± 0.01 | 3.77 ± 6.49 |
| Hydroxybenzoic acids 4 | |||||
| Dihydroxybenzoic acid hexoside | 1.82 | C13H16O9 | 316.0787 | 14.52 ± 1.32 | 6.63 ± 0.14 |
| Vanillic acid *,+ | 1.89 | C8H8O4 | 168.0416 | 0.36 ± 0.03 | 0.14 ± 0.01 |
| 3,4-Dihydroxybenzoic acid (protocatechuic acid) *,+ | 1.90 | C7H6O4 | 154.0261 | 0.10 ± 0.00 | 0.09 ± 0.00 |
| Hydroxybenzoic acid (isomer I) + | 2.39 | C7H6O3 | 138.0313 | 0.17 ± 0.01 | 0.15 ± 0.01 |
| Hydroxybenzoic acid (isomer II) + | 5.12 | C7H6O3 | 138.0316 | 0.45 ± 0.00 | 0.15 ± 0.01 |
| Hydroxycinnamic acids 4 | |||||
| 5-caffeoylquinic acid (chlorogenic acid) *,+ | 2.15 | C16H18O9 | 354.0948 | 33.88 ± 0.36 | 27.73 ± 0.99 |
| Coumaroylquinic acid (isomer I) + | 2.50 | C16H18O8 | 338.1001 | 1.02 ± 0.09 | 1.32 ± 0.05 |
| Caffeoylquinic acid (isomer II) + | 2.69 | C16H18O9 | 354.0956 | 28.04 ± 0.15 | 20.98 ± 1.00 |
| Coumaric acid hexoside | 2.71 | C15H18O8 | 326.1004 | 0.02 ± 0.00 | 0.04 ± 0.00 |
| Caffeic acid *,+ | 2.76 | C9H8O4 | 180.0419 | 0.79 ± 0.24 | 1.27 ± 0.04 |
| Coumaroylquinic acid (isomer II) + | 3.09 | C16H18O8 | 338.1007 | 1.37 ± 0.01 | 2.23 ± 0.09 |
| Caffeoylquinic acid (isomer III) + | 3.16 | C16H18O9 | 354.0959 | 2.96 ± 0.82 | 3.88 ± 0.22 |
| Feruloylquinic acid (isomer I) + | 3.35 | C17H20O9 | 368.1111 | 1.75 ± 0.06 | 0.31 ± 0.02 |
| Coumaric acid *,+ | 3.47 | C9H8O3 | 164.0466 | ND | 0.02 ± 0.00 |
| Coumaroylquinic acid (isomer III) + | 3.66 | C16H18O8 | 338.0996 | 0.32 ± 0.00 | 0.26 ± 0.03 |
| Feruloylquinic acid (isomer II) + | 3.73 | C17H20O9 | 368.1121 | 0.42 ± 0.02 | ND |
| Ferulic acid *,+ | 3.90 | C10H10O4 | 194.0578 | 0.02 ± 0.00 | 0.03 ± 0.00 |
| Sinapic acid *,+ | 3.96 | C11H12O5 | 224.0687 | 0.03 ± 0.00 | 0.05 ± 0.00 |
| Dicaffeoylquinic acid (isomer I) + | 4.98 | C25H24O12 | 516.1281 | 0.07 ± 0.00 | ND |
| Organic acids 4 | |||||
| Quinic acid | 0.92 | C7H12O6 | 192.0264 | 0.042 ± 0.00 | ND |
| Hydroxycitric acid + | 2.14 | C6H8O8 | 208.0217 | 0.07 ± 0.00 | ND |
| Citric acid *,+ | 2.60 | C6H8O7 | 192.0628 | 19.42 ± 0.11 | 1.74 ± 0.10 |
| Hibiscus acid + | 2.70 | C6H8O8 | 190.0110 | 8.81 ± 1.12 | 6.37 ± 0.41 |
| Benzoic acid | 2.88 | C7H6O2 | 122.0364 | 0.06 ± 0.00 | ND |
| Aconitic acid | 3.09 | C6H6O6 | 174.0160 | 2.46 ± 0.27 | 1.99 ± 0.09 |
| Parameter | Standard Diet | HFFD | HFFD + White | HFFD + Purple |
|---|---|---|---|---|
| Serum FG 1 | 100.78 ± 18.2 a | 129.36 ± 17.0 a | 124.15 ± 15.2 a | 116.44 ± 17.6 a |
| Serum FINS 2 | 1.54 ± 0.06 bc | 1.88 ± 0.12 a | 1.25 ± 0.10 c | 1.83 ± 0.31 ab |
| HOMA-IR | 9.53 ± 1.39 b | 14.85 ± 1.08 a | 9.52 ± 1.39 b | 13.18 ± 3.25 ab |
| Serum FFA 1 | 18.74 ± 3.32 c | 27.69 ± 1.21 a | 21.59 ± 2.50 bc | 24.88 ± 1.20 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel-García, C.A.; Reynoso-Camacho, R.; Pérez-Ramírez, I.F.; Morales-Luna, E.; de los Ríos, E.A.; Salgado, L.M. Serum Phospholipids Are Potential Therapeutic Targets of Aqueous Extracts of Roselle (Hibiscus sabdariffa) against Obesity and Insulin Resistance. Int. J. Environ. Res. Public Health 2022, 19, 16538. https://doi.org/10.3390/ijerph192416538
Rangel-García CA, Reynoso-Camacho R, Pérez-Ramírez IF, Morales-Luna E, de los Ríos EA, Salgado LM. Serum Phospholipids Are Potential Therapeutic Targets of Aqueous Extracts of Roselle (Hibiscus sabdariffa) against Obesity and Insulin Resistance. International Journal of Environmental Research and Public Health. 2022; 19(24):16538. https://doi.org/10.3390/ijerph192416538
Chicago/Turabian StyleRangel-García, Carmen Alejandra, Rosalía Reynoso-Camacho, Iza F. Pérez-Ramírez, Elizabeth Morales-Luna, Ericka A. de los Ríos, and Luis M. Salgado. 2022. "Serum Phospholipids Are Potential Therapeutic Targets of Aqueous Extracts of Roselle (Hibiscus sabdariffa) against Obesity and Insulin Resistance" International Journal of Environmental Research and Public Health 19, no. 24: 16538. https://doi.org/10.3390/ijerph192416538
APA StyleRangel-García, C. A., Reynoso-Camacho, R., Pérez-Ramírez, I. F., Morales-Luna, E., de los Ríos, E. A., & Salgado, L. M. (2022). Serum Phospholipids Are Potential Therapeutic Targets of Aqueous Extracts of Roselle (Hibiscus sabdariffa) against Obesity and Insulin Resistance. International Journal of Environmental Research and Public Health, 19(24), 16538. https://doi.org/10.3390/ijerph192416538






