The First 30 Years of the Universal Hepatitis-B Vaccination-Program in Italy: A Health Strategy with a Relevant and Favorable Economic-Profile
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical Cases Related to HBV and Related Costs, with and without the Universal HBV Vaccination-Program Implementation
3.2. Economic Impact of the Immunization Program
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization (WHO). Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 7 December 2021).
- Haber, P.; Schillie, S.; Hepatitis, B. Chapter 10—Epidemiology and Prevention of Vaccine-Preventable Diseases. Available online: https://www.cdc.gov/vaccines/pubs/pinkbook/hepb.html (accessed on 7 December 2021).
- Trépo, C.; Chan, H.L.; Lok, A. Hepatitis B virus infection. Lancet 2014, 384, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Hoofnagle, J.H. Chronic hepatitis B. N. Engl. J. Med. 1990, 323, 337–339. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). The Immunological Basis for Immunization Series: Module 22: Hepatitis B. Available online: https://www.who.int/publications/i/item/who-immunological-basis-for-immunization-series-module-22-hepatitis-b (accessed on 23 November 2022).
- World Health Organization (WHO). Global Hepatitis Report. 2017. Available online: https://www.who.int/publications/i/item/9789241565455 (accessed on 23 November 2022).
- European Centre for Disease Prevention and Control (ECDC). Hepatitis B-Annual Epidemiological Report for 2019. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER-Hepatitis-B-2019.pdf (accessed on 23 November 2022).
- Istituto Superiore di Sanità (ISS). Bollettino SEIEVA Epidemiologia Delle Epatiti Virali Acute in Italia, Numero 10-aggiornamento 2021. March 2022. Available online: https://www.epicentro.iss.it/epatite/bollettino/Bollettino-n-10-marzo-2022.pdf (accessed on 27 July 2022).
- Istituto Superiore di Sanità (ISS). Epidemiologia-dati SEIEVA. Available online: https://www.epicentro.iss.it/epatite/dati-seieva (accessed on 27 July 2022).
- Law 27 May 1991, n. 165. Obbligatorietà Della Vaccinazione Contro L’epatite Virale B. Gazz. Uff. 1° giugno 1991, n. 127. Available online: https://www.gazzettaufficiale.it/eli/id/1991/06/01/091G0201/sg (accessed on 27 July 2022).
- Da Villa, G.; Sepe, A. Immunization programme against hepatitis B virus infection in Italy: Cost-effectiveness. Vaccine 1999, 17, 1734–1738. [Google Scholar] [CrossRef]
- Boccalini, S.; Taddei, C.; Ceccherini, V.; Bechini, A.; Levi, M.; Bartolozzi, D.; Bonanni, P. Economic analysis of the first 20 years of universal hepatitis B vaccination program in Italy: An a posteriori evaluation and forecast of future benefits. Hum. Vaccin Immunother. 2013, 9, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Istituto Nazionale di Statistica (ISTAT); GeoDemo. Popolazione Residente. Available online: https://demo.istat.it/index.php (accessed on 27 July 2022).
- Istituto Nazionale di Statistica (ISTAT). GeoDemo. Speranza di Vita Alla Nascita. Available online: https://demo.istat.it/tvm2016/index.php?lingua=ita (accessed on 27 July 2022).
- Stroffolini, T.; Pasquini, P.; Mele, A. HBsAg carriers among pregnant women in Italy: Results from the screening during a vaccination campaign against hepatitis B. Public Health 1988, 102, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Stroffolini, T.; Pasquini, P.; Mele, A. A nationwide vaccination programme in Italy against hepatitis B virus infection in infants of hepatitis B surface antigen-carrier mothers. Vaccine 1989, 7, 152–154. [Google Scholar] [CrossRef]
- Stroffolini, T.; Pasquini, P. Five years of vaccination campaign against hepatitis B in Italy in infants of hepatitis B surface antigen carrier mothers. Ital. J. Gastroenterol. 1990, 22, 195–197. [Google Scholar] [PubMed]
- Ministry of Health. Vaccinazioni dell’età Pediatrica. Available online: https://www.salute.gov.it/portale/documentazione/p6_2_8_3_1.jsp?lingua=italiano&id=20 (accessed on 27 July 2022).
- Istituto Nazionale di Statistica (ISTAT). Indice dei Prezzi al Consumo per le Rivalutazioni Monetarie. Available online: https://www.istat.it/it/archivio/30440 (accessed on 15 September 2022).
- Fattovich, G. Natural history of hepatitis B. J. Hepatol. 2003, 39 (Suppl. S1), S50–S58. [Google Scholar] [CrossRef]
- Gentilini, P.; Laffi, G.; La Villa, G.; Romanelli, R.G.; Buzzelli, G.; Casini-Raggi, V.; Melani, L.; Mazzanti, R.; Riccardi, D.; Pinzani, M.; et al. Long course and prognostic factors of virus-induced cirrhosis of the liver. Am. J. Gastroenterol. 1997, 92, 66–72. [Google Scholar]
- Lok, A.S.; McMahon, B.J. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Chronic hepatitis B. Hepatology 2001, 34, 1225–1241. [Google Scholar] [CrossRef]
- McMahon, B.J. The natural history of chronic hepatitis B virus infection. Hepatology 2009, 49 (Suppl. S5), S45–S55. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, F.E.; Janssen, H.L.; de Man, R.A.; Hop, W.C.; Schalm, S.W.; van Blankenstein, M. Survival and prognostic indicators in hepatitis B surface antigen-positive cirrhosis of the liver. Gastroenterology 1992, 103, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Santi, V.; Buccione, D.; Di Micoli, A.; Fatti, G.; Frigerio, M.; Farinati, F.; Del Poggio, P.; Rapaccini, G.; Di Nolfo, M.A.; Benvegnù, L.; et al. The changing scenario of hepatocellular carcinoma over the last two decades in Italy. J. Hepatol. 2012, 56, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Cucchetti, A.; Cescon, M.; Trevisani, F.; Morelli, M.C.; Ercolani, G.; Pellegrini, S.; Erroi, V.; Bigonzi, E.; Pinna, A.D. What is the probability of being too old for salvage transplantation after hepatocellular carcinoma resection? Dig. Liver Dis. 2012, 44, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Volk, M.L.; Pastorelli, D.; Lonardi, S.; Farinati, F.; Burra, P.; Angeli, P.; Cillo, U. Use of sorafenib in patients with hepatocellular carcinoma before liver transplantation: A cost-benefit analysis while awaiting data on sorafenib safety. Hepatology 2010, 51, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.T.; Zhou, F.; Hadler, S.C.; Bell, B.P.; Mast, E.E.; Margolis, H.S. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int. J. Epidemiol. 2005, 34, 1329–1339. [Google Scholar] [CrossRef]
- Boccalini, S.; Pellegrino, E.; Tiscione, E.; Pesavento, G.; Bechini, A.; Levi, M.; Rapi, S.; Mercurio, S.; Mannelli, F.; Peruzzi, M.; et al. Sero-epidemiology of hepatitis B markers in the population of Tuscany, Central Italy, 20 years after the implementation of universal vaccination. Hum. Vaccin Immunother. 2013, 9, 636–641. [Google Scholar] [CrossRef]
- Bonanni, P.; Pesavento, G.; Bechini, A.; Tiscione, E.; Mannelli, F.; Benucci, C.; Nostro, A.L. Impact of universal vaccination programmes on the epidemiology of hepatitis B: 10 years of experience in Italy. Vaccine 2003, 21, 685–691. [Google Scholar] [CrossRef]
- Zanella, B.; Bechini, A.; Boccalini, S.; Sartor, G.; Tiscione, E.; Working Group Dhs; Working Group AOUMeyer; Working Group Ausltc; Bonanni, P.; Hepatitis, B. Seroprevalence in the Pediatric and Adolescent Population of Florence (Italy): An Update 27 Years after the Implementation of Universal Vaccination. Vaccines 2020, 8, 156. [Google Scholar] [CrossRef]
- Mele, A.; Tosti, M.E.; Mariano, A.; Pizzuti, R.; Ferro, A.; Borrini, B.; Zotti, C.; Lopalco, P.; Curtale, F.; Balocchini, E.; et al. National Surveillance System for Acute Viral Hepatitis (SEIEVA) Collaborating Group. Acute hepatitis B 14 years after the implementation of universal vaccination in Italy: Areas of improvement and emerging challenges. Clin. Infect. Dis. 2008, 46, 868–875. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità (ISS). Bollettino SEIEVA Epidemiologia Delle Epatiti Virali Acute in Italia, Numero 8-aggiornamento 2020; March 2021. Available online: https://www.epicentro.iss.it/epatite/bollettino/Bollettino-n-8-marzo-2021.pdf (accessed on 27 July 2022).
- Istituto Superiore di Sanità (ISS). Bollettino SEIEVA Epidemiologia Delle Epatiti Virali Acute in Italia, Numero 6-aggiornamento 2019; March 2020. Available online: https://www.epicentro.iss.it/epatite/bollettino/Bollettino-n-6-marzo-2020.pdf (accessed on 27 July 2022).
- Istituto Superiore di Sanità (ISS). Bollettino SEIEVA Epidemiologia Delle Epatiti Virali Acute in Italia_Numero 4-aggiornamento 2018; march 2019. Available online: https://www.epicentro.iss.it/epatite/bollettino/Bollettino-4-marzo-2019.pdf (accessed on 27 July 2022).
- Pinon, M.; Giugliano, L.; Nicastro, E.; Kakaa, O.; Coscia, A.; Carbonara, C.; D’Antiga, L.; Calvo, P.L. Timely Birth Dose Vaccine to Prevent Vertical Transmission of Hepatitis B: A Single Center Experience on the Road to the WHO Elimination Goals in Italy. Vaccines 2021, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Conferenza Permanente Per i Rapporti Tra Lo Stato Le Regioni e Le Province Autonome di Trento e Bolzano. Intesa 19 gennaio 2017. Intesa, ai sensi dell’articolo 8, comma 6, della legge 5 giugno 2003, n. 131, tra il Governo, le regioni e le province autonome di Trento e Bolzano sul documento recante “Piano nazionale prevenzione vaccinale 2017–2019” (Rep. atti n. 10/CSR) (17A01195). (G.U. Serie Generale, n. 41 del 18 febbraio 2017). Available online: https://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=58185 (accessed on 28 July 2022).
- Istituto Nazionale di Statistica (ISTAT). Natalità e Fecondità Della Popolazione Residente. ANN0 2020. Statistiche report dicembre 2021. Available online: https://www.istat.it/it/files//2021/12/REPORT-NATALITA-2020.pdf (accessed on 21 October 2022).
- Ahmad, A.A.; Falla, A.M.; Duffell, E.; Noori, T.; Bechini, A.; Reintjes, R.; Veldhuijzen, I.K. Estimating the scale of chronic hepatitis B virus infection among migrants in EU/EEA countries. BMC Infect. Dis. 2018, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Istituto Superiore di Sanità (ISS). Epatite, B. Available online: https://www.epicentro.iss.it/epatite/epatite-b (accessed on 21 October 2022).
- Mariano, A.; Mele, A.; Tosti, M.E.; Parlato, A.; Gallo, G.; Ragni, P.; Zotti, C.; Lopalco, P.; Pompa, M.G.; Graziani, G.; et al. Role of beauty treatment in the spread of parenterally transmitted hepatitis viruses in Italy. J. Med. Virol. 2004, 74, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Bechini, A.; Bonanni, P.; Grazzini, M.; Paolini, D.; Arcangeli, G.; Mucci, N.; Bini, C.; Tiscione, E.; Zanella, B.; Boccalini, S. Need to take special care of non-responders to hepatitis B vaccination among health-care workers, students and chronic patients. Hum. Vaccin Immunother. 2021, 17, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Sixty-Third World Health Assembly–WHA63.18. Agenda Item 11.12. Viral Hepatitis. 21 May 2010. Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_R18-en.pdf (accessed on 30 December 2021).
- Sixty-Seventh World Health Assembly-WHA67.6. Agenda item 12.3. Hepatitis. 24 May 2014. Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA67/A67_R6-en.pdf (accessed on 30 December 2021).
- World Health Organization (WHO). Global Health Sector Strategy on Viral Hepatitis 2016–2021 towards Ending Viral Hepatitis. Available online: https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf?sequence=1&isAllowed=y (accessed on 30 December 2021).
- Ministry of Health. Piano Nazionale Per La Prevenzione Delle Epatiti Virali da Virus B e C (PNEV). 27 October 2015. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2437_allegato.pdf (accessed on 30 December 2021).
- World Health Organization (WHO). Interim Guidance for Country Validation of Viral Hepatitis Elimination. World Health Organization: Geneva, Switzerland. Available online: https://www.who.int/publications/i/item/9789240028395 (accessed on 28 July 2022).
- Signorelli, C. Quarant’anni (1978–2018) di Politiche Vaccinali in Italia [Forty Years (1978–2018) of Vaccination Policies in Italy]. Acta Biomed. 2019, 90, 127–133. [Google Scholar] [CrossRef]
- Presidente della Repubblica Italiana. Law 31 July 2017, n. 119. Conversione in Legge, con Modificazioni, del Decreto-Legge 7 Giugno 2017, n. 73, Recante Disposizioni Urgenti in Materia di Prevenzione Vaccinale. In Gazzetta Ufficiale Serie Generale, n. 182 del 5 August 2017. Available online: https://www.gazzettaufficiale.it/eli/id/2017/08/5/17G00132/sg (accessed on 28 July 2022).
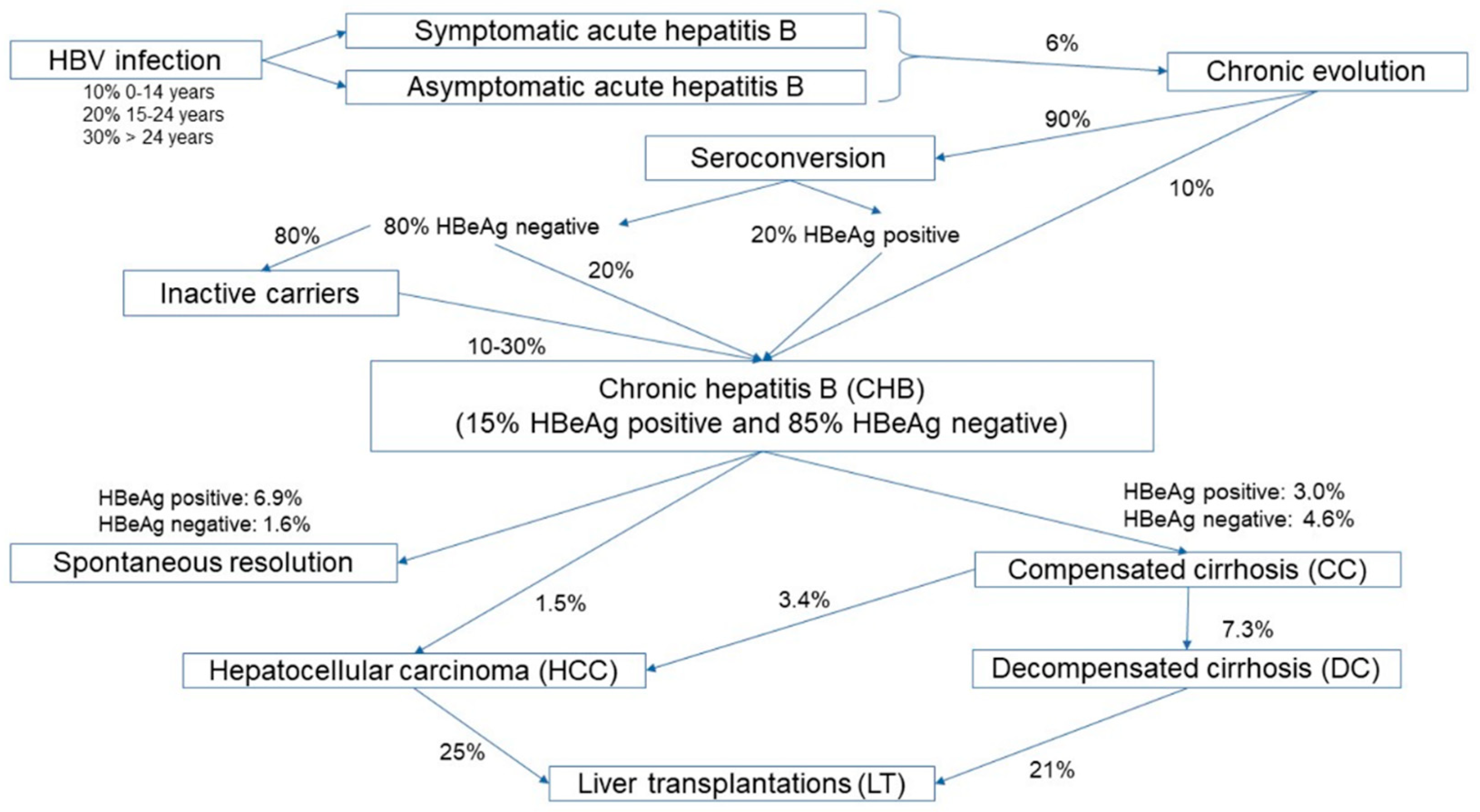
| Stage of the Disease | Time Interval Between Stages | Median Age of Patients | Survival Rate | Reference |
|---|---|---|---|---|
| CHB | - | 31 | Equal to life expectancy for the general population | [14,20] |
| CC | 15 years from CHB | 46 | 99.1% at 5 years | [20,21,22,23,24] |
| 76.8% at 10 years | ||||
| 49.4% at 15 years | ||||
| 25% at 20 years | ||||
| 0% at 25 years | ||||
| DC | 3 years from CC | 49 | 14% at 5 years | [20] |
| HCC | 44 months from CC | 50 | 78.7% at 1 years | [20,25] |
| 50.4% at 3 years | ||||
| 28.9% at 5 years | ||||
| LT | 3 months from HCC or DC | - | 5% of patients with LT encounter transplant-related death | [26,27] |
| 72% post-transplant survival rate at 5 years |
| Annual Cost of HBV Diseases (1990) | |
|---|---|
| Annual assistance costs/case | |
| AHB | 9296 EUR |
| CHB and Cirrhosis (hospital and home assistance) | 7230 EUR |
| HCC | 27,889 EUR |
| Annual social costs/case | |
| AHB | 1916 EUR |
| CHB and Cirrhosis | 1277 EUR |
| HCC | 11,654 EUR |
| HBV Vaccination Costs (1991) | |
| Direct costs | |
| Vaccine | 6.20 EUR/dose (pediatric dose) |
| 9.30 EUR/dose (adult dose) | |
| Vaccine preservation | 0.09 EUR/dose |
| Vaccine administration | 6.45 EUR/person |
| Treatment of side effects (1% of vaccine doses administered) | 4.93 EUR/case |
| Immuno-prophylaxis treatment of babies born to HBsAg-positive mothers | 14.98 EUR/newborn |
| Indirect costs | |
| Lost working-days for vaccination | 7.98 EUR/dose |
| Missed working-days for treatment of side effects (1% of vaccine-doses administered) | 21.17 EUR/dose |
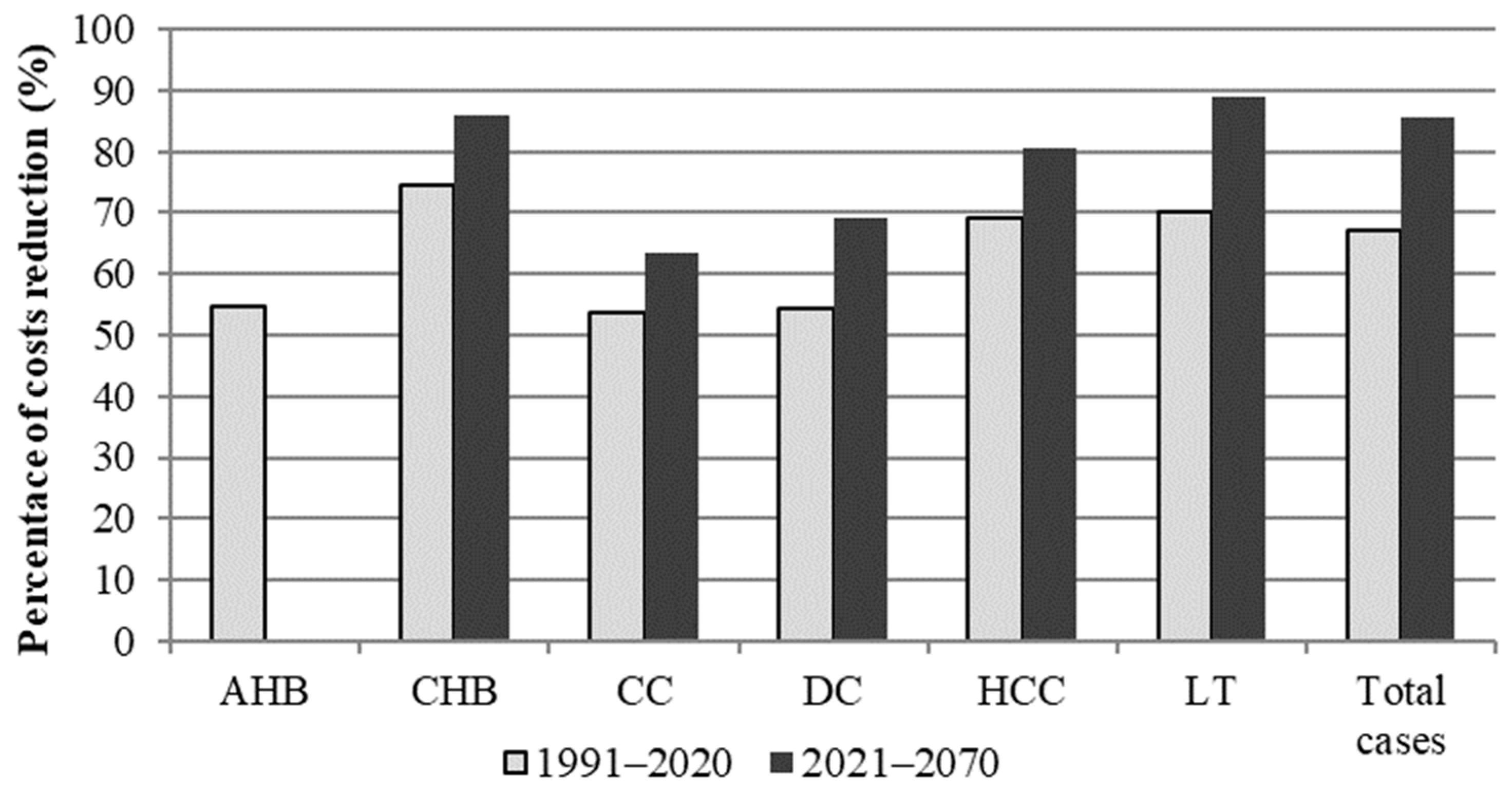
| 1991–2020 | No Vaccination | Vaccination | Prevented Cases | Reduction Rate (%) |
|---|---|---|---|---|
| HBV infection | 237,074 | 43,701 | 193,373 | 82 |
| AHB | 61,329 | 30,931 | 30,397 | 50 |
| CHB | 7670 | 1414 | 6256 | 82 |
| CC | 143 | 62 | 81 | 57 |
| DC | 10 | 4 | 6 | 57 |
| HCC | 120 | 23 | 97 | 81 |
| LT | 32 | 7 | 25 | 79 |
| 1991–2020 | Direct Costs (EUR) | Indirect Costs (EUR) | Total Costs (EUR) | Reduction of Direct Costs (%) | Reduction of Indirect Costs (%) | Total Reduction Rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Vaccination | Vaccination | Difference | No Vaccination | Vaccination | Difference | No Vaccination | Vaccination | Difference | ||||
| AHB | 890,904,717 | 404,044,092 | 486,860,625 | 183,575,866 | 83,255,530 | 100,320,337 | 1,074,480,584 | 487,299,622 | 587,180,962 | 55 | 55 | 55 |
| CHB | 1,516,037,546 | 384,847,960 | 1,131,189,587 | 267,688,915 | 67,953,154 | 199,735,761 | 1,783,726,462 | 452,801,113 | 1,330,925,348 | 75 | 75 | 75 |
| CC | 34,501,507 | 15,965,699 | 18,535,808 | 6,091,980 | 2,819,086 | 3,272,894 | 40,593,487 | 18,784,785 | 21,808,702 | 54 | 54 | 54 |
| DC | 2,000,214 | 915,589 | 1,084,625 | 353,181 | 161,667 | 191,514 | 2,353,395 | 1,077,256 | 1,276,138 | 54 | 54 | 54 |
| HCC | 23,665,180 | 7,311,013 | 16,354,166 | 4,650,236 | 1,473,059 | 3,177,177 | 28,315,416 | 8,784,072 | 19,531,343 | 69 | 68 | 69 |
| LT | 8,145,359 | 2,427,413 | 5,717,945 | 1,294,608 | 364,472 | 930,137 | 9,439,967 | 2,791,885 | 6,648,082 | 70 | 72 | 70 |
| Total | 2,475,254,523 | 815,511,767 | 1,659,742,756 | 463,654,787 | 156,026,968 | 307,627,819 | 2,938,909,310 | 971,538,735 | 1,967,370,576 | 67 | 66 | 67 |
| 2021–2070 | No Vaccination | Vaccination | Difference | No Vaccination | Vaccination | Difference | No Vaccination | Vaccination | Difference | Reduction of Direct Costs (%) | Reduction of Indirect Costs (%) | Total Reduction Rate (%) |
| AHB | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CHB | 1,984,954,476 | 280,420,213 | 1,704,534,263 | 350,486,247 | 49,514,198 | 300,972,050 | 2,335,440,724 | 329,934,411 | 2,005,506,313 | 86 | 86 | 86 |
| CC | 13,827,330 | 5,061,534 | 8,765,796 | 2,441,511 | 893,722 | 1,547,789 | 16,268,842 | 5,955,256 | 10,313,585 | 63 | 63 | 63 |
| DC | 444,275 | 137,385 | 306,889 | 78,446 | 24,258 | 54,188 | 522,721 | 161,644 | 361,077 | 69 | 69 | 69 |
| HCC | 103,880,973 | 20,231,671 | 83,649,303 | 18,850,137 | 3,604,567 | 15,245,570 | 122,731,110 | 23,836,238 | 98,894,873 | 81 | 81 | 81 |
| LT | 6,206,322 | 695,148 | 5,511,174 | 446,907 | 27,434 | 419,473 | 6,653,229 | 722,582 | 5,930,647 | 89 | 94 | 89 |
| Total | 2,109,313,376 | 306,545,951 | 1,802,767,425 | 372,303,249 | 54,064,179 | 318,239,070 | 2,481,616,625 | 360,610,130 | 2,121,006,495 | 85 | 85 | 85 |
| 1991–2070 | No Vaccination | Vaccination | Difference | No Vaccination | Vaccination | Difference | No Vaccination | Vaccination | Difference | Reduction of Direct Costs (%) | Reduction of Indirect Costs (%) | Total Reduction Rate (%) |
| AHB | 890,904,717 | 404,044,092 | 486,860,625 | 183,575,866 | 83,255,530 | 100,320,337 | 1,074,480,584 | 487,299,622 | 587,180,962 | 55 | 55 | 55 |
| CHB | 3,500,992,022 | 665,268,173 | 2,835,723,850 | 618,175,163 | 117,467,352 | 500,707,811 | 4,119,167,185 | 782,735,524 | 3,336,431,661 | 81 | 81 | 81 |
| CC | 48,328,837 | 21,027,233 | 27,301,605 | 8,533,492 | 3,712,809 | 4,820,683 | 56,862,329 | 24,740,041 | 32,122,288 | 56 | 56 | 56 |
| DC | 2,444,488 | 1,052,975 | 1,391,514 | 431,627 | 185,925 | 245,702 | 2,876,115 | 1,238,900 | 1,637,215 | 57 | 57 | 57 |
| HCC | 127,546,153 | 27,542,684 | 100,003,469 | 23,500,373 | 5,077,626 | 18,422,747 | 151,046,526 | 32,620,310 | 118,426,216 | 78 | 78 | 78 |
| LT | 14,351,681 | 3,122,561 | 11,229,120 | 1,741,515 | 391,906 | 1,349,610 | 16,093,196 | 3,514,467 | 12,578,729 | 78 | 77 | 78 |
| Total | 4,584,567,899 | 1,122.057,718 | 3,462,510,182 | 835,958,036 | 210,091,147 | 625,866,889 | 5,420,525,935 | 1,332,148,865 | 4,088,377,071 | 76 | 75 | 75 |
| Period 1991–2020 | ||
|---|---|---|
| NHS Perspective | Societal Perspective | |
| Clinical savings (EUR) | 1,967,370,576 | 1,967,370,576 |
| Vaccination costs (EUR) | 1,263,247,830 | 1,484,792,906 |
| Net costs (EUR) | −396,494,926 | −482,577,670 |
| ROI/BCR | 1.31 | 1.33 |
| Overall Period 1991–2070 | ||
| NHS Perspective | Societal Perspective | |
| Clinical savings (EUR) | 3,462,510,182 | 4,088,377,071 |
| Vaccination costs (EUR) | 1,263,247,830 | 1,484,792,906 |
| Net costs (EUR) | −2,199,262,351 | −2,603,584,165 |
| ROI/BCR | 2.74 | 2.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccalini, S.; Bonito, B.; Zanella, B.; Liedl, D.; Bonanni, P.; Bechini, A. The First 30 Years of the Universal Hepatitis-B Vaccination-Program in Italy: A Health Strategy with a Relevant and Favorable Economic-Profile. Int. J. Environ. Res. Public Health 2022, 19, 16365. https://doi.org/10.3390/ijerph192316365
Boccalini S, Bonito B, Zanella B, Liedl D, Bonanni P, Bechini A. The First 30 Years of the Universal Hepatitis-B Vaccination-Program in Italy: A Health Strategy with a Relevant and Favorable Economic-Profile. International Journal of Environmental Research and Public Health. 2022; 19(23):16365. https://doi.org/10.3390/ijerph192316365
Chicago/Turabian StyleBoccalini, Sara, Benedetta Bonito, Beatrice Zanella, Davide Liedl, Paolo Bonanni, and Angela Bechini. 2022. "The First 30 Years of the Universal Hepatitis-B Vaccination-Program in Italy: A Health Strategy with a Relevant and Favorable Economic-Profile" International Journal of Environmental Research and Public Health 19, no. 23: 16365. https://doi.org/10.3390/ijerph192316365
APA StyleBoccalini, S., Bonito, B., Zanella, B., Liedl, D., Bonanni, P., & Bechini, A. (2022). The First 30 Years of the Universal Hepatitis-B Vaccination-Program in Italy: A Health Strategy with a Relevant and Favorable Economic-Profile. International Journal of Environmental Research and Public Health, 19(23), 16365. https://doi.org/10.3390/ijerph192316365








