Using Alternative Sources of Energy for Decarbonization: A Piece of Cake, but How to Cook This Cake?
Abstract
1. Introduction
1.1. The Hydrocarbon Civilization
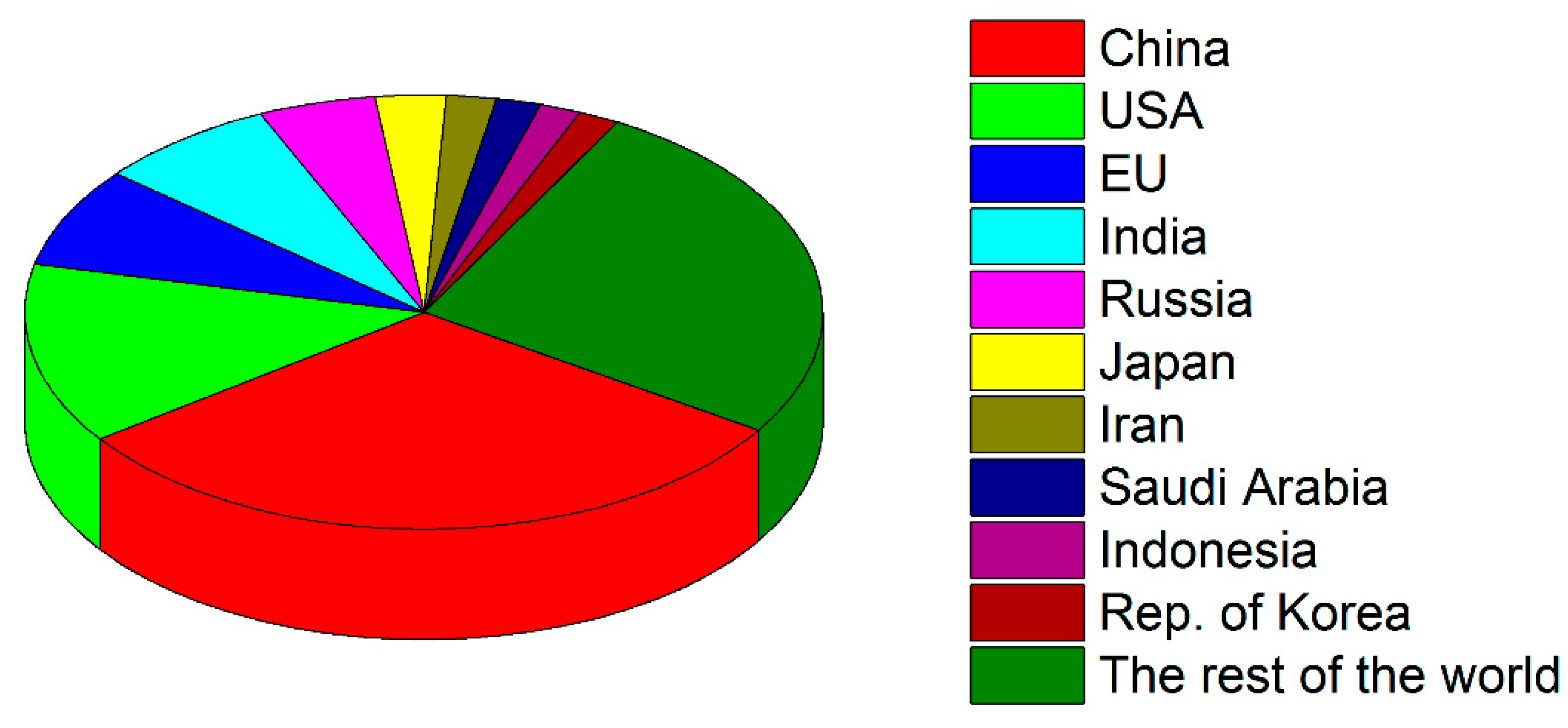
1.2. Carbonization of the Earth
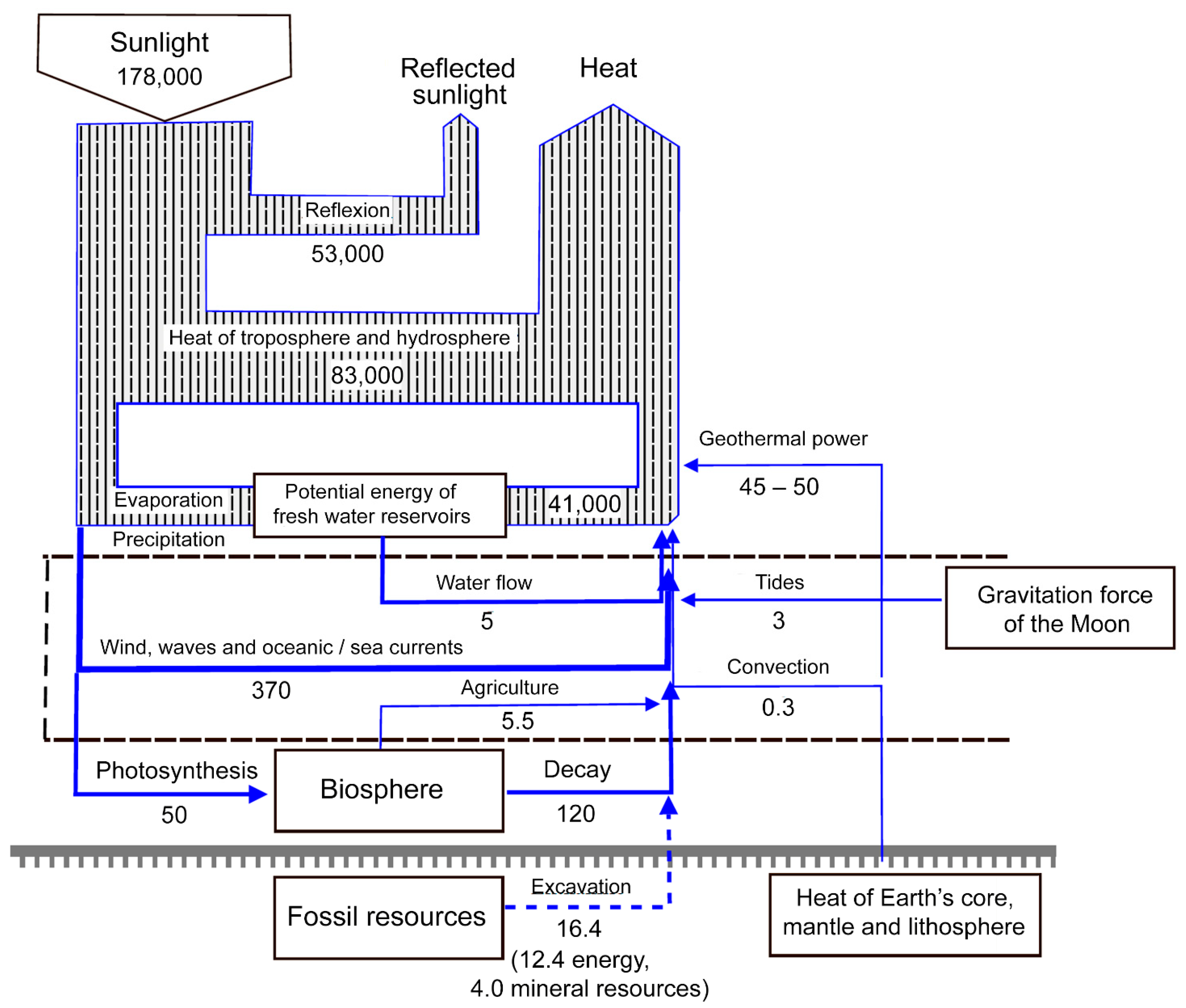
1.3. The Spaceship Earth Paradigm and the Alternative Energetics
2. Materials and Methods
3. Conditionally Renewable Alternative Sources of Energy
3.1. Biofuels
3.1.1. Solid Biofuels
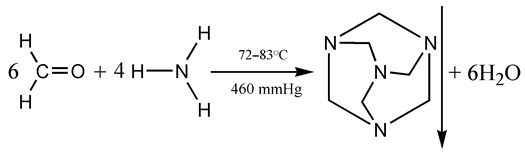
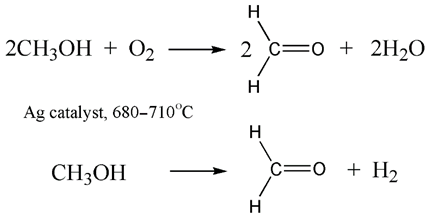
3.1.2. Liquid Biofuels
3.1.3. Gaseous Biofuels

3.1.4. Summary for Biofuels
3.2. Nuclear Energy
4. Renewable Alternative Sources of Energy
4.1. Hydrogen: Splendor and Misery of Colored Classification
- (a)
- Grey—H2 produced from or with the use of fossil fuels with carbon emissions to the troposphere.
- (1)
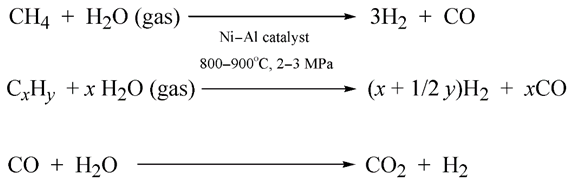
- The majority of industrial hydrogen (around ¾) is now obtained in the process of steam-reforming natural gas, pure methane or light alkanes such as propane–butane fractions [109]. Artificial syngas and carbon dioxide are obtained as the products of the following reactions:Water vapor further oxidizes the carbon monoxide in the syngas to carbon dioxide (the third reaction in Equation (4)).
- (2)
- Hydrogen is also currently derived in industrial amounts from water vapor over petroleum coke. This is the oldest means of industrial hydrogen production, which dates back to the early nineteenth century [109]:
- (3)
- A small part (less than 10%) of hydrogen is obtained by the catalytic or temperature-based partial oxidation of natural gas [109]:
- (b)
- Blue—Everything is the same as for the grey category, but carbon monoxide and carbon dioxide are stored in tanks.
- (c)
- Yellow—H2 is produced via the reaction of metals with acids.

- (d)
- Green—H2 is derived from the electrolysis of water:
- (e)
- Turquoise—H2 is obtained via the reaction of natural gas pyrolysis [111]:
- (f)
- Pink—H2 is derived from the high-temperature (800–1000 °C) electrolysis of water, using the electricity and heat generated in nuclear power plants.
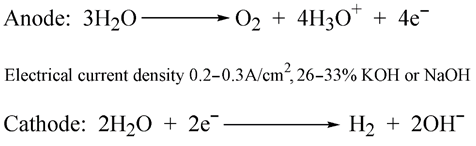
- (a)
- Nowadays, only “grey hydrogen” production is economically reasonable. “Green hydrogen” is still impossible to obtain in massive amounts due to its high production costs;
- (b)
- However, “grey hydrogen” is not at all environmentally friendly, as the very same hydrocarbons that lead to the greenhouse effect are used here as the reagents for hydrogen production;
- (c)
- Not all “green hydrogen” is truly “green” either. The “green hydrogen” obtained via water solution electrolysis entails the alkalization or acidification of the environment (with the dangerous contamination of soil and fresh water reservoirs, causing biota extinction). Using such “not-so-green” hydrogen in eco-friendly cars would also entail the operation of eco-hostile hydrogen-producing plants;
- (d)
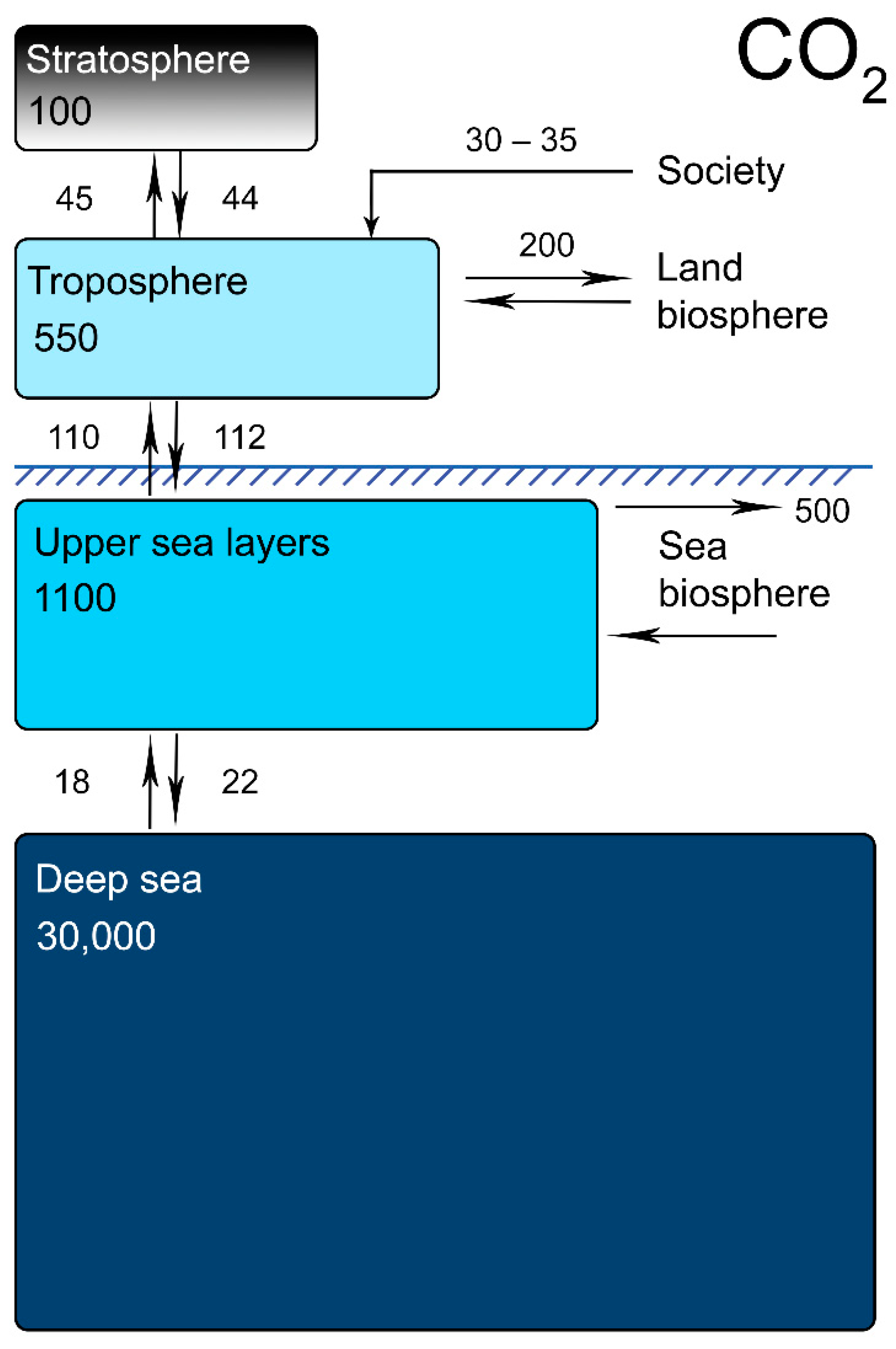
- “Blue hydrogen” is also not an eco-safe option, as the huge-scale pumping of CO2 into lithospheric splits would increase the total carbonization of the Earth. In time, the carbon dioxide pumped in such a way will be released into the troposphere due to natural gas exchange processes (Figure 1);
- (e)
- Let us assume a case in which humanity has successfully switched all its transport to operating on “green hydrogen”, having somehow dampened its cost inefficiency—this would still not solve the problem of the global energy demand for transport if we adopt the Spaceship Earth viewpoint. The global use of “green hydrogen” would, in fact, involve redistributing energy from one place to another.
- (1)
- Reducing the cost of solid polymer membrane electrolysis systems;
- (2)
- (3)
- Augmenting the membrane electrolysis efficiency, as well as the efficiency of hydrogen-powered engines.
4.2. Hydropower
- (a)
- The building of large hydroelectric dams on plain rivers has led to the submerging of vast areas, which are now occupied by shallow water storage reservoirs. This severely damages the land biota and ecosystems of these areas.
- (b)
- The overall rates of river flow decrease significantly. This leads to an increase in water temperature and the appearance of duckweed. Consequently, the distortion of river biota from the source to the estuary is a common effect and is not just seen in the reservoir.
- (c)
- Migratory fish populations usually go extinct in rivers with hydropower dams.
- (d)
- Mountainous hydroelectric stations often cause new lithospheric cracks, mud flows, the silting of the riverbed, shifting sands, earthquakes and avalanches.
- (e)
- The uncontrolled dumping of water from water reservoirs, which usually lasts for 10–15 days, results in stress perturbances of ecosystems. This may cause the irreversible contraction of trophic chains, the shrinking of freshwater fauna populations, the death of river invertebrates, the disappearance of nesting areas of migratory birds, the insufficient humidification of flood soil, the decrease in freshwater algae/river plant biodiversity, and the diminishing of the flow of biomass to the sea.
- (f)
- The most unexpected negative effect of hydropower stations is the fact that their water storage reservoirs may end up carbonizing the troposphere with natural gas that is synthesized during the anaerobic decay of flooded plants in shallow and warm waters. Methane causes the greenhouse effect to unfold even faster than carbon dioxide. Hydropower is an ASE that is expected to reduce global warming. Instead, it frequently contributes to it.
4.3. Wind Power
- (a)
- Perpetual high levels of noise. One middle-size wind generator with a 250 Kilowatt (kW) power output creates around 50–80 decibel (dB) of noise up to 0.5–1 km away and more than 100 dB in areas near it. Larger mills are much noisier [136].
- (b)
- It can generate infrasound that is especially hazardous to water and soil biota, as it swiftly spreads in dense media [137].
- (c)
- Vast zones of human and biotic displacement are being established near wind power plants.
- (d)
- (e)
- The rotation of the fans of turbines generates a substantial level of radio interference [139]. This necessitates building additional retranslating stations for TV, mobile networks and other communications around wind power plants, which may additionally perturb biota.
4.4. Solar Power
- (a)
- Multilevel rotating solar power modules are only effective in large solar power plants that track the daily movement of the sun [145]. This technique involves large rotational glass reflectors and metallic infrastructure. The areas around industrial solar power plants become enormously hot. The condensed sunlight causes much of the biota to migrate from the areas surrounding sun power plants [38]. Therefore, even greater zones of social and biotic displacement are being created;
- (b)
- In contrast to wind power, the operating of which causes the most harm, in solar power, preparing the solar elements is the most damaging to the environment.
- (c)
- Solar power plant equipment is complex. Automatic complex cleaning systems are necessary for removing atmospheric precipitation that may reduce the efficiency of solar energy conversion. The constant monitoring of solar panels is also a prerequisite for the normal maintenance of a solar power plant [145]. These factors increase the production costs of solar energy, which are substantially higher than the production costs of wind energy [140];
- (d)
- An unobvious yet serious limitation related to the largest solar power plants (dozens to hundreds of square kilometers) is their ability to cause reductions in air temperature in surrounding areas. The more solar energy that is being removed from tropospheric regions above a given area (to be converted to electricity), the cooler the air in this area [38].
4.5. Geothermal Power, Ocean Thermal Power and Sea Wave Power
4.6. Thermonuclear Power


5. Summary for ASE
6. Power Flows
- (1)
- its net use, i.e., its use on the scale of the Earth;
- (2)
- the density and distribution of its use across the Earth’s surface;
- (3)
- the harm that one ASE power plant causes or may cause.
7. Source of Energy—Carrier Problem
- (1)
- 100–130 TW power consumption in 2100;
- (2)
- total zero-carbon energy (the proportion of ASE use is 100%);
- (3)
- 50% presence of electric vehicles (with the remainder of vehicles powered by on-board hydrogen),
- (1)
- Most existing electrical networks are built as national projects. There are very few cross-border, large-scale electricity transmission projects, such as France–Italy or USA–Canada;
- (2)
- Most hydrocarbon-fueled heat power plants are spread evenly across the globe and, consequently, are easy to connect to any electrical network. There is no difference where to combust natural gas or fuel oil.
8. Limitations of the Review
- Not all ASEs received equal focus in our review. Some were discussed more carefully, and others more cursorily;
- We tried to ensure a more detailed consideration of ASEs that are usually deemed “more exotic”, and steered away from areas of mainstream research or media discourse. We confined ourselves to a briefer analysis of the more common ASEs, such as wind or solar energy, primarily focusing on their side effects and negative environmental impacts, as much has already been written about these ASEs;
- We did not provide exhaustive information about any of the ASEs. The size of this review would not have allowed it. In addition to this, there are plenty of books concerning all of the ASEs. Our intention was to consider using a combination of multiple ASEs together as the only possible means of decarbonization and to ensure the great energy transition of the twenty-first century;
- Our review included data and performed their analysis in a manner customary to environmentalist academia. The problem of the energy transition of the twenty-first century is much broader. It necessarily includes business entities, legal practitioners, politicians, policymakers, managers of different levels and government bodies. A truly comprehensive and systemic analysis of the ASEs must involve discussing the aspects of management, law, social attitudes and business planning. We have not considered these in our review. In our future work, we shall focus on some of these issues from the sociological viewpoint.
9. Conclusions
- (1)
- cost of construction of energy plants and infrastructure;
- (2)
- operational costs;
- (3)
- cost of the electricity generated;
- (4)
- local and global environmental impact of the ASE itself;
- (5)
- local and global environmental impact of the full cycle of the ASE;
- (6)
- ability to be switched to national electrical networks;
- (7)
- electrical battery/power stations requirements for energy transportation;
- (8)
- energy production efficiency coefficient;
- (9)
- energy output;
- (10)
- geographical distribution of such power plants and the ability of the ASE to solve the energy inequality problem.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lackner, M.; Sajjadi, B.; Chen, W.Y. Handbook of Climate Change Mitigation and Adaptation; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Glaubrecht, M. Das Ende der Evolution: Der Mensch und die Vernichtung der Arten; C. Bertelsmann Verlag: München, Germany, 2019. [Google Scholar]
- Smil, V. Growth: From Microorganisms to Megacities; MIT Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Sassin, W.; Donskikh, O.A.; Gnes, A.; Komissarov, S.A.; Liu, D. Evolutionary Environments: Homo sapiens–An Endangered Species? Studia: Innsbruck, Austria, 2018. [Google Scholar]
- Smil, V. Natural Gas in the New Energy World; Naturgy Foundation: Madrid, Spain, 2021. [Google Scholar]
- Smil, V. What we need to know about the pace of decarbonization. Substantia 2019, 3, 13–28. [Google Scholar]
- Smil, V. General Energetics: Energy in the Biosphere and Civilization; Wiley Interscience: New York, NY, USA, 1991. [Google Scholar]
- Smil, V. Energy Transitions: Global and National Perspectives; Praeger: Santa Barbara, CA, USA; Denver, CO, USA, 2016. [Google Scholar]
- Goeller, H.E.; Weinberg, A.M. Age of Substitutability: Or What Do We Do When the Mercury Runs out. Office of Scientific & Technical Information Report. 1975, p. 5045860. Available online: https://digital.library.unt.edu/ark:/67531/metadc1059064/ (accessed on 15 August 2022).
- Goeller, H.E.; Weinberg, A.M. The Age of Substitutability. Am. Econ. Rev. 1978, 68, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sassin, W. Er-Schöpfung der Schöpfung, oder Eine neue Kulturstufe in der Entwicklung des homo. Beacon J. Stud. Ideol. Ment. Dimens. 2019, 2, 020510203. [Google Scholar] [CrossRef]
- Sassin, W. Ursprung und Grenzen von Gemeinschaft: Eine eurasische Perspektive. Eur. Crossrd. 2021, 2, 010210217. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M.; Rosado, P. CO₂ and Greenhouse Gas Emissions. Our World in Data. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 30 August 2022).
- Sonwani, S.; Saxena, P. Greenhouse Gases: Sources, Sinks and Mitigation; Springer: Singapore, 2022. [Google Scholar]
- Bandh, S. Climate Change: The Social and Scientific Construct; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Shea, J.J. Stone Tools in Human Evolution; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Volk, T. CO2 Rising; MIT Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Machta, L. Prediction of CO2 in the atmosphere. Brookhaven Symp. Biol. 1973, 30, 21–31. [Google Scholar]
- Sassin, W. Weltökologieprobleme. In Mut zur Zukunft-Über den Sinnvollen Umgang mit den Lebensmöglichkeiten auf der Erde; Schulte-Vieting, H.-J., Ed.; Einhard-Verlag: Aachen, Germany, 1983; pp. 101–116. [Google Scholar]
- Lemon, E.R. CO2 and Plants: The Response of Plants to Rising Levels of Atmospheric Carbon Dioxide; CRC Press: New York, NY, USA, 2020. [Google Scholar]
- McDonald, G.J. The Long-Term Impacts of Increasing Atmospheric Carbon Dioxide Levels; Ballinger: Cambridge, MA, USA, 1981. [Google Scholar]
- Demir, A.S. Modeling and forecasting of CO2 emissions resulting from air transport with genetic algorithms: The United Kingdom case. Theor. Appl. Climatol. 2022, 150, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.W. On the effect of international human migration on nations’ abilities to attain CO2 emission-reduction targets. PLoS ONE 2021, 16, e0258087. [Google Scholar] [CrossRef]
- Gurney, K.R.; Song, Y.; Liang, J.; Roest, G. Toward Accurate, Policy-Relevant Fossil Fuel CO2 Emission Landscapes. Environ. Sci. Technol. 2020, 54, 9896–9907. [Google Scholar] [CrossRef]
- Fossil Fuels Emissions. Available online: http://www.globalcarbonatlas.org/en/CO2-emissions (accessed on 23 November 2022).
- Miralles-Quirós, M.M.; Miralles-Quirós, J.L. Decarbonization and the Benefits of Tackling Climate Change. Int. J. Environ. Res. Public Health 2022, 19, 7776. [Google Scholar] [CrossRef]
- Carraro, C.; Canziani, P.O.; Nakicenovic, N.; McCarthy, J.J.; Goldemberg, J.; Hisas, L. The Truth About Climate Change. Available online: https://www.researchgate.net/publication/308750455_The_Truth_About_Climate_Change/link/57ee6b9508ae886b8973f34e/download (accessed on 20 August 2022).
- Rockström, J.; Gaffney, O.; Rogelj, J.; Meinshausen, M.; Nakicenovic, N.; Schellnhuber, H.J. A roadmap for rapid decarbonization. Science 2017, 355, 1269–1271. [Google Scholar] [CrossRef]
- Wheeler, N.; Watts, N. Climate Change: From Science to Practice. Curr. Environ. Health Rep. 2018, 5, 170–178. [Google Scholar] [CrossRef]
- Turner, M.G.; Calder, W.J.; Cumming, G.S.; Hughes, T.P.; Jentsch, A.; LaDeau, S.L.; Lenton, T.M.; Shuman, B.N.; Turetsky, M.R.; Ratajczak, Z.; et al. Climate change, ecosystems and abrupt change: Science priorities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190105. [Google Scholar] [CrossRef]
- Wang, X.; Meng, X.; Long, Y. Projecting 1 km-grid population distributions from 2020 to 2100 globally under shared socioeconomic pathways. Sci. Data 2022, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Sassin, W. Der Konflikt zwischen Ideologien und einer nüchternen und umfassenden Sichtweise: Nachhaltig gegen Resilient? Beacon J. Stud. Ideol. Ment. Dimens. 2020, 3, 020440211. [Google Scholar] [CrossRef]
- Davis, S.J.; Creutzig, F.; Fuss, S.; Minx, J.C.; Gabrielle, B.; Kato, E.; Jackson, R.; Cowie, A.L.; Kriegler, E.; Van Vuuren, D.P.; et al. Biophysical and economic limits to negative CO2 emissions. Nat. Clim. Change 2015, 6, 42–50. [Google Scholar] [CrossRef]
- Harrison, H. Make Room! Make Room! Doubleday: New York, NY, USA, 1966. [Google Scholar]
- Legach, E.I.; Sharov, K.S. (Eds.) SARS-CoV-2 and Coronacrisis: Epidemiological Challenges, Social Policies and Administrative Strategies; Springer: Singapore, 2021. [Google Scholar]
- Hou, Y.; Wang, Q. A bibliometric study about energy, environment, and climate change. Environ. Sci. Pollut. Res. Int. 2021, 28, 34187–34199. [Google Scholar] [CrossRef]
- Lisichkin, G.V. Ecological issues of alternative energetics. In The Basics of Ecological Safety; Bogdanovsky, G.A., Galaktionova, N.A., Eds.; MNEPU: Moscow, Russia, 2000; pp. 37–44. [Google Scholar]
- Ward, B. Spaceship Earth; Columbia University Press: New York, NY, USA, 1966. [Google Scholar]
- Fuller, R.B. Operating Manual for Spaceship Earth; Southern Illinois University Press: Carbondale, IL, USA, 1969. [Google Scholar]
- Boulding, K.E. The Economics of the Coming Spaceship Earth. In Environmental Quality in a Growing Economy; Jarrett, H., Ed.; Johns Hopkins University Press: Baltimore, MD, USA, 1966; pp. 3–14. [Google Scholar]
- Nazir, M.S.; Ali, Z.M.; Bilal, M.; Sohail, H.M.; Iqbal, H.M.N. Environmental impacts and risk factors of renewable energy paradigm-a review. Environ. Sci. Pollut. Res. Int. 2020, 27, 33516–33526. [Google Scholar] [CrossRef]
- Bildirici, M.; Kayıkçı, F. Renewable energy and current account balance nexus. Environ. Sci. Pollut. Res. Int. 2022, 29, 48759–48768. [Google Scholar] [CrossRef]
- Segreto, M.; Principe, L.; Desormeaux, A.; Torre, M.; Tomassetti, L.; Tratzi, P.; Paolini, V.; Petracchini, F. Trends in Social Acceptance of Renewable Energy Across Europe-A Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 9161. [Google Scholar] [CrossRef]
- Zimm, C.; Goldemberg, J.; Nakicenovic, N.; Busch, S. Is the renewables transformation a piece of cake or a pie in the sky? Energy Strat. Rev. 2019, 26, 100401. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Inegr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Dillman, J. Activated charcoal use. J. Emerg. Med. Serv. 2003, 28, 22. [Google Scholar]
- Wood, T.S.; Baldwin, S.F. Fuelwood and Charcoal Use in Developing Countries. Ann. Rev. Energy 1985, 10, 407–429. [Google Scholar] [CrossRef]
- Norgate, T.; Langberg, D. Environmental and Economic Aspects of Charcoal Use in Steelmaking. ISIJ Int. 2009, 49, 587–595. [Google Scholar] [CrossRef]
- Cordova-Aguilar, H. Firewood use and the effect on the ecosystem. GeoJournal 1992, 26, 297–309. [Google Scholar] [CrossRef]
- Lippincott, J.S. The History of Anthracite Coal in Nature and Art. Am. Nat. 1883, 17, 1–10. [Google Scholar] [CrossRef]
- Eberling, E.J. The Issues of the Anthracite Problem. Curr. Hist. 1926, 24, 247–253. [Google Scholar] [CrossRef]
- Manandhar, A.; Mousavi-Avval, S.H.; Tatum, J.; Shrestha, E.; Nazemi, P.; Shah, A. Solid biofuels. In Biomass, Biofuels, Biochemicals: Green-Economy–Systems Analysis for Sustainability; Murthy, G.S., Gnansounou, E., Khanal, S.K., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 343–370. [Google Scholar]
- Grammelis, P. Solid Biofuels for Energy: A Lower Greenhouse Gas Alternative; Springer: Cham, Switzerland, 2011. [Google Scholar]
- Choudri, B.S.; Baawain, M. Bioenergy from Biofuel Residues and Wastes. Water Environ. Res. 2016, 88, 1446–14466. [Google Scholar] [CrossRef]
- Raju, S.S.; Shinoj, P.; Joshi, P.K. Sustainable Development of Biofuels: Prospects and Challenges. Econ. Pol. Wkly. 2010, 44, 65–72. [Google Scholar]
- Srikanta, P.; Sem, S.; Mahmoud, M.S. (Eds.) Smart Village Technology: Concepts and Developments; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Visvizi, A.; Lytras, M.D.; Mudri, G. (Eds.) Smart Villages in the EU and Beyond; Emerald: Bingley, UK, 2019. [Google Scholar]
- Heap, B. Smart Villages: New Thinking for Off-Grid Communities Worldwide; Banson: Cambridge, UK, 2015. [Google Scholar]
- Heap, B.; Hirmer, S. Smart villages. Horizons J. Int. Rel. Sustain. Dev. 2020, 15, 290–305. [Google Scholar]
- Tagami-Kanada, N.; Yoshikuni, K.; Mizuno, S.; Sawai, T. Combustion characteristics of densified solid biofuel with different aspect ratios. Renew. Energy 2022, 197, 1174–1182. [Google Scholar] [CrossRef]
- Sacramento Rivero, J.C.; Mwampamba, T.H.; Navarro-Pineda, F.S.; Musule, R.; García, C.A.; Martínez-Bravo, R.D.; Morales-García, A.L.; Equihua-Sánchez, M.; Fuentes-Gutiérrez, A.F.; Gallardo-Álvarez, R.M.; et al. A Methodological Framework for Assessing the Sustainability of Solid Biofuels Systems. BioEnergy Res. 2022, 15, 1797–1819. [Google Scholar] [CrossRef] [PubMed]
- Shadrin, D.V.; Daut, V.A.; Ozhegov, A.I.; Gusev, A.L.; Avramenko, E.V. Method of Synthesis of Hexamethylenetetramine (Urotropin). Available online: https://patents.google.com/patent/RU2198887C1/en (accessed on 7 July 2022).
- Tretyakov, V.F.; Talyshinnsky, R.M.; Ilolov, A.M. Method of Producing Formaldehyde. Available online: https://patents.google.com/patent/RU2404959C1/en (accessed on 5 July 2022).
- Method of Obtaining Formaldehyde. Available online: https://i.moscow/patents/SU255238A1_19691028 (accessed on 5 July 2022).
- Production of Ammonia. Available online: https://promplace.ru/himiya-i-proizvodstvo-plastmass-staty/proizvodstvo-ammiaka-1471.htm (accessed on 19 August 2022).
- Kovac Kralj, A. Energy-Efficient Hexamine Production Process. Adv. Chem. Eng. Res. 2013, 2, 51–54. [Google Scholar]
- Lago, C.; Caldés, M.; Lechón, Y. The Role of Bioenergy in the Emerging Bioeconomy: Resources, Technologies, Sustainability and Policy; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Nigam, P.S.; Singh, A. Production of liquid biofuels from renewable resources. Prog. Energy Combust. Sci. 2011, 37, 52–68. [Google Scholar] [CrossRef]
- Fivga, A.; Speranza, L.G.; Branco, C.M.; Ouadi, M.; Hornung, A. A review on the current state of the art for the production of advanced liquid biofuels. AIMS Energy 2019, 7, 46–76. [Google Scholar] [CrossRef]
- Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from algae: Challenges and potential. Biofuels 2010, 1, 763–784. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Qureshi, K.; Abro, R.; Harijan, K.; Zhao, Z.; Bazmi, A.A.; Abbase, T.; Yu, G. Progress in the production of biomass-to-liquid biofuels to decarbonize the transport sector–prospects and challenges. RSC Adv. 2016, 38, C5RA26459F. [Google Scholar] [CrossRef]
- Hunsberger, C.; German, L.; Götz, A. “Unbundling” the biofuel promise: Querying the ability of liquid biofuels to deliver on socio-economic policy expectations. En. Pol. 2017, 108, 791–805. [Google Scholar] [CrossRef]
- Sandesh, K.; Ujwal, P. Trends and perspectives of liquid biofuel–Process and industrial viability. En. Conv. Man. X 2021, 10, 100075. [Google Scholar] [CrossRef]
- Duque, A.; Álvarez, C.; Doménech, P.; Manzanares, P.; Moreno, A.D. Advanced Bioethanol Production: From Novel Raw Materials to Integrated Biorefineries. Processes 2021, 9, 206. [Google Scholar] [CrossRef]
- Transesterification. Available online: http://www.srsbiodiesel.com/technologies/transesterification (accessed on 27 August 2022).
- Hanafi, S.A.; Elmelawy, M.S.; Shalaby, N.H.; El-Syed, H.A.; Eshaq, G.; Mostafa, M.S. Hydrocracking of waste chicken fat as a cost effective feedstock for renewable fuel production: A kinetic study. Egypt. J. Petrol. 2016, 25, 531–537. [Google Scholar] [CrossRef]
- Bezergianni, S.; Kalogianni, A. Hydrocracking of used cooking oil for biofuels production. Bioresour. Technol. 2009, 100, 3927–3932. [Google Scholar] [CrossRef] [PubMed]
- Iyyappan, J.; Bharathiraja, B.; Vaishnavi, A.; Prathiba, S. Overview of Current Developments in Biobutanol Production Methods and Future Perspectives. Methods Mol. Biol. 2021, 2290, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Nimbalkar, P.R.; Khedkar, M.A.; Chavan, P.V.; Bankar, S.B. Enhanced Biobutanol Production in Folic Acid-Induced Medium by Using Clostridium acetobutylicum NRRL B-527. ACS Omega 2019, 4, 12978–12982. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Pereira, R.; Wahl, S.A.; Rocha, I. Metabolic engineering strategies for butanol production in Escherichia coli. Biotechnol. Bioeng. 2020, 117, 2571–2587. [Google Scholar] [CrossRef]
- Xin, F.; Dong, W.; Zhang, W.; Ma, J.; Jiang, M. Biobutanol Production from Crystalline Cellulose through Consolidated Bioprocessing. Trends Biotechnol. 2019, 37, 167–180. [Google Scholar] [CrossRef]
- Kolesárová, N.; Hutňan, M.; Bodík, I.; Špalková, V. Utilization of Biodiesel By-Products for Biogas Production. J. Biomed. Biotechnol. 2011, 2011, 126798. [Google Scholar] [CrossRef]
- Fuel Ethers’ Markets. Available online: https://www.sustainablefuels.eu/about-fuel-ethers/fuel-ether-markets (accessed on 25 August 2022).
- Bioethers. Available online: https://biofuel.org.uk/bioethers.html (accessed on 8 August 2022).
- Arpia, A.A.; Nguyen, T.B.; Chen, W.H.; Dung, C.D. Microwave-assisted gasification of biomass for sustainable and energy-efficient biohydrogen and biosyngas production: A state-of-the-art review. Chemosphere 2022, 287, 132014. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, M.; Bolan, N.S.; Kapley, A.; Kumar, R.; Singh, L. Multidimensional approaches of biogas production and up-gradation: Opportunities and challenges. Bioresour. Technol. 2021, 338, 125514. [Google Scholar] [CrossRef]
- Kapoor, R.; Ghosh, P.; Kumar, M.; Vijay, V.K. Evaluation of biogas upgrading technologies and future perspectives: A review. Environ. Sci. Pollut. Res. Int. 2019, 26, 11631–11661. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wu, N.; Lan, C.Q.; Dubois-Calero, N. Biofuels from microalgae. Biotechnol. Prog. 2008, 24, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Pacioni, T.R.; Soares, D.; Domenico, M.D.; Rosa, M.F.; Moreira, R.F.P.M.; José, H.J. Bio-syngas production from agro-industrial biomass residues by steam gasification. Waste Manag. 2016, 58, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Wijeyekoon, S.L.J.; Vaidya, A.A. Woody biomass as a potential feedstock for fermentative gaseous biofuel production. World J. Microbiol. Biotechnol. 2021, 37, 134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cruz-Morales, P.; Zargar, A.; Belcher, M.S.; Pang, B.; Englund, E.; Dan, Q.; Yin, K.; Keasling, J.D. Biofuels for a sustainable future. Cell 2021, 184, 1636–1647. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- Kramm, L. The German Nuclear Phase-Out After Fukushima: A Peculiar Path or an Example for Others? Renew. Ener. Law Pol. Rev. 2012, 3, 251–262. [Google Scholar]
- When the Steam Clears. Available online: https://www.economist.com/briefing/2011/03/24/when-the-steam-clears (accessed on 14 July 2022).
- Joint Declaration for a Nuclear-Free EU Taxonomy. Available online: https://www.bmuv.de/meldung/joint-declaration-for-a-nuclear-free-eu-taxonomy-de (accessed on 21 September 2022).
- Nuclear Power in the World Today. Available online: https://world-nuclear.org/information-library/current-and-future-generation/nuclear-power-in-the-world-today.aspx (accessed on 16 September 2022).
- Ritchie, H.; Roser, M. Nuclear Energy. Available online: https://ourworldindata.org/nuclear-energy (accessed on 1 August 2022).
- Paillere, H.; Donovan, J. Nuclear Power 10 Years After Fukushima: The Long Road Back. Available online: https://www.iaea.org/newscenter/news/nuclear-power-10-years-after-fukushima-the-long-road-back (accessed on 17 September 2022).
- Joskow, P.; Parsons, J.E. The Future of Nuclear Power after Fukushima. Econ. Ener. Environ. Pol. 2012, 1, 99–114. [Google Scholar] [CrossRef]
- Frankel, D.R. Nuclear waste management. Ecol. Law Quart. 1980, 8, 790–800. [Google Scholar]
- Shorkin, R.A. Thermal-Neutron Reactors. Available online: http://nuclphys.sinp.msu.ru/students/nphm/04_tt.htm (accessed on 10 September 2022).
- Kleykamp, H. The chemical state of the fission products in oxide fuels. J. Nucl. Mater. 1985, 131, 221–246. [Google Scholar] [CrossRef]
- Teaguea, M.; Gorman, B.; King, J.; Portera, D.; Hayesa, S. Microstructural characterization of high burn-up mixed oxide fast reactor fuel. J. Nucl. Mater. 2013, 441, 267–273. [Google Scholar] [CrossRef]
- Cameron, I.R. Nuclear Fission Reactors; Springer: New York, NY, USA, 1982. [Google Scholar]
- Radioactive Waste Management. Available online: https://world-nuclear.org/information-library/nuclear-fuel-cycle/nuclear-wastes/radioactive-waste-management.aspx (accessed on 3 September 2022).
- Mahaffey, J. Atomic Accidents. A History of Nuclear Meltdowns and Disasters from the Ozark Mountains to Fukushima; Pegasus Books: New York, NY, USA, 2015. [Google Scholar]
- The Future of Hydrogen. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 22 August 2022).
- The ZEROe Demonstrator Has Arrived. Available online: https://www.airbus.com/en/newsroom/stories/2022-02-the-zeroe-demonstrator-has-arrived (accessed on 22 August 2022).
- Hydrogen Colours Codes. Available online: https://www.h2bulletin.com/knowledge/hydrogen-colours-codes (accessed on 25 August 2022).
- Production of Hydrogen. Available online: http://www.hemi.nsu.ru/ucheb172.htm (accessed on 24 August 2022).
- Carbon Capture, Utilisation and Storage. Available online: https://www.iea.org/fuels-and-technologies/carbon-capture-utilisation-and-storage (accessed on 25 August 2022).
- Porsin, A.; Kulikov, A.; Amosov, Y.; Rogozhnokov, V. Acetylene synthesis by methane pyrolysis on a tungsten wire. Theor. Found. Chem. Engineer. 2014, 48, 397–403. [Google Scholar] [CrossRef]
- Hydrogen Energetics. Available online: https://energy.hse.ru/hydrenergy (accessed on 25 August 2022).
- Kamada, R. How Green Is ‘Green’ Hydrogen, Really? Available online: https://www.researchgate.net/publication/363235330_How_Green_is_‘Green’_Hydrogen_Really (accessed on 24 September 2022).
- Zhu, B.; Chu, W. A Green Hydrogen Era: Hope or Hype? Environ. Sci. Technol. 2022, 56, 11107–11110. [Google Scholar] [CrossRef] [PubMed]
- Energy Spending during Water Electrolysis. Available online: https://chem21.info/info/441187 (accessed on 25 August 2022).
- Hydrogen Production Cost. Available online: https://www.sciencedirect.com/topics/engineering/hydrogen-production-cost (accessed on 26 August 2022).
- Singla, M.K.; Nijhawan, P.; Oberoi, A.S. Hydrogen fuel and fuel cell technology for cleaner future: A review. Environ. Sci. Pollut. Res. Int. 2021, 28, 15607–15626. [Google Scholar] [CrossRef] [PubMed]
- Ajanovic, A.; Sayer, M.; Haas, R. The economics and the environmental benignity of different colors of hydrogen. Int. J. Hydrog. Energy 2022, 47, 24136–24154. [Google Scholar] [CrossRef]
- Understanding the Resistivity of Water. Available online: https://sensorex.com/2020/12/29/resistivity-of-water (accessed on 27 August 2022).
- Rusanov, V.D. Hydrogen Energy. Available online: https://bigenc.ru/chemistry/text/1922269 (accessed on 27 August 2022).
- Solid Polymer Electrolyte Membrane. Available online: https://www.sciencedirect.com/topics/engineering/solid-polymer-electrolyte-membrane (accessed on 27 August 2022).
- Kamaroddin, M.F.A.; Sabli, N.; Abdullah, T.A.T.; Siajam, S.I.; Abdullah, L.C.; Jalil, A.A.; Ahmad, A. Membrane-Based Electrolysis for Hydrogen Production: A Review. Membranes 2021, 11, 810. [Google Scholar] [CrossRef]
- Bao, L.Z.; Sun, B.G.; Luo, Q.H.; Li, J.C.; Qian, D.C.; Ma, H.Y.; Guo, Y.J. Development of a turbocharged direct-injection hydrogen engine to achieve clean, efficient, and high-power performance. Fuel 2022, 324, 124713. [Google Scholar] [CrossRef]
- Kufudakis, A. Equipment for continuous measurement of cathodic hydrogen diffusion through metal membranes. Czechoslov. J. Phys. 1970, 20, 1319–1324. [Google Scholar] [CrossRef]
- Zohuri, B. Hydrogen Energy Challenges and Solutions for a Cleaner Future; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Xiao, J.; Bénard, P.; Chahine, R. Adsorption-desorption cycle thermodynamics for adsorptive hydrogen storage system. Int. J. Hydrog. Energy 2014, 2, 1326–1351. [Google Scholar] [CrossRef]
- Glante, S.; Fischer, M.; Hartmann, M. Investigation of the optimum conditions for adsorptive hydrogen storage. Em. Mater. 2021, 4, 1295–1303. [Google Scholar] [CrossRef]
- Breeze, P. Hydropower; Academic Press: New York, NY, USA, 2018. [Google Scholar]
- Venus, T.E.; Hinzmann, M.; Gerdes, H. Public Acceptance of Hydropower. In Novel Developments for Sustainable Hydropower; Rutschmann, P., Kampa, E., Wolter, C., Albayrak, I., David, L., Stoltz, U., Schletterer, M., Eds.; Springer: Cham, Switzerland, 2022; pp. 29–40. [Google Scholar]
- Rutschmann, P.; Kampa, E.; Wolter, C.; Albayrak, I.; David, L.; Stoltz, U.; Schletterer, M. (Eds.) Novel Developments for Sustainable Hydropower; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Schroeder, U. Identification of the Hydropower Potential of Switzerland. Wasserwirtsch 2011, 101, 19–23. [Google Scholar]
- Manwell, J.F.; McGowan, J.G.; Rogers, A.L. Wind Energy Explained: Theory, Design and Application; Wiley: New York, NY, USA, 2010. [Google Scholar]
- Maegaard, P.; Krenz, A.; Palz, W. Wind Power for the World. The Rise of Modern Wind Energy; Jenny Stanford Publishing: Singapore, 2013. [Google Scholar]
- Gipe, P. Wind Energy for the Rest of Us: A Comprehensive Guide to Wind Power and How to Use It; Wind-Works Publ.: Denver, CO, USA, 2016. [Google Scholar]
- Anderson, C. Wind Turbines: Theory and Practice; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Letcher, T.M. (Ed.) Wind Energy Engineering: A Handbook for Onshore and Offshore Wind Turbines; Academic Press: New York, NY, USA, 2017. [Google Scholar]
- Boczar, T.; Zmarzły, D.; Kozioł, M.; Wotzka, D. The application of time-frequency ridge transformation for the analysis of infrasound signals generated by wind turbines. Appl. Acoust. 2021, 177, 107961. [Google Scholar] [CrossRef]
- Vishnoi, B.N.; Singh, V. Wind analysis for wind power at Jaisalmer. Mausam 2005, 56, 1087. [Google Scholar] [CrossRef]
- Angulo, I.; de la Vega, D.; Cascón, I.; Cañizo, J.; Wu, Y.; Guerra, D.; Angueira, P. Impact analysis of wind farms on telecommunication services. Renew. Sust. Ener. Rev. 2014, 32, 84–99. [Google Scholar] [CrossRef]
- Sivaram, V. Taming the Sun: Innovations to Harness Solar Energy and Power the Planet; MIT Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Nemet, G.F. How Solar Energy Became Cheap: A Model for Low-Carbon Innovation; Routledge: New York, NY, USA, 2019. [Google Scholar]
- Bed, K.C.; Shrestha, S.; Poudyal, K. Altitudes Variation of Solar Energy in Himalaya region, Nepal. Int. J. Adv. Soc. Sci. 2020, 3, 14–21. [Google Scholar]
- Verduci, R.; Romano, V.; Brunetti, G.; Nia, N.Y.; Di Carlo, A.; D’Angelo, G.; Ciminelli, C. Solar Energy in Space Applications: Review and Technology Perspectives. Adv. Ener. Mat. 2022, 12, 2200125. [Google Scholar] [CrossRef]
- Stagno, L.M. Marine solar energy. In Renewable Energy from the Oceans: From Wave, Tidal and Gradient Systems to Offshore Wind and Solar; Coiro, D., Sant, T., Eds.; IET Digital Library: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Gevorkian, P. Large-Scale Solar Power System Design: An Engineering Guide for Grid-Connected Solar Power Generation; McGraw Hill: New York, NY, USA, 2011. [Google Scholar]
- Letcher, T.M.; Fthenakis, V.M. (Eds.) A Comprehensive Guide to Solar Energy Systems; Academic Press: New York, NY, USA, 2018. [Google Scholar]
- Kontrosh, L.V.; Kalinovsky, V.S.; Khramov, A.V.; Kontrosh, E.V. Estimation of the chemical materials volumes required for the post-growth technology manufacturing InGaP/GaAs/Ge with a concentrator and planar α–Si:H/Si solar cells for 1 MW solar power plants. Clean. Eng. Technol. 2021, 4, 100186. [Google Scholar] [CrossRef]
- Zipori, A.; Rosenfeld, D.; Shpund, J.; Steinberg, D.M.; Erel, Y. Targeting and impacts of AgI cloud seeding based on rain chemical composition and cloud top phase characterization. Atm. Res. 2012, 114–115, 119–130. [Google Scholar] [CrossRef]
- Choudhury, G.; Tyagi, B.; Singh, J.; Sarangi, C.; Tripathi, S.N. Aerosol-orography-precipitation–A critical assessment. Atm. Environ. 2019, 214, 116831. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nadeem, F.; Tariq, R.; Rashid, U. Renewable and Alternative Energy Resources; Academic Press: New York, NY, USA, 2021. [Google Scholar]
- Stober, I.; Bucher, K. Geothermal Energy: From Theoretical Models to Exploration and Development; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Boden, D.R. Geologic Fundamentals of Geothermal Energy; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Manzella, A.; Allansdottir, A.; Pelllizzone, A. (Eds.) Geothermal Energy and Society; Springer: Berlin, Germany, 2019. [Google Scholar]
- Kim, A.S.; Kim, H.J. (Eds.) Ocean Thermal Energy Conversion (OTEC): Past, Present, and Progress; IntechOpen: London, UK, 2020. [Google Scholar]
- Avery, W.H.; Wu, C. Renewable Energy From the Ocean: A Guide to OTEC; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Lopez, G.; de los Angeles Ortega Del Rosario, M.; James, A.; Alvarez, H. Site Selection for Ocean Thermal Energy Conversion Plants (OTEC): A Case Study in Panama. Energies 2022, 15, 3077. [Google Scholar] [CrossRef]
- Shetty, C.; Priyam, A. A review on tidal energy technologies. Mat. Today Proc. 2022, 56, 2774–2779. [Google Scholar] [CrossRef]
- Curto, D.; Franzitta, V.; Guercio, A. Sea Wave Energy. A Review of the Current Technologies and Perspectives. Energies. 2021, 14, 6604. [Google Scholar] [CrossRef]
- Dixit, S.; Badgaiyan, P. Overview of Tidal Energy with its Benefits and Drawbacks. Smart Mov. J. IJOSci. 2021, 7, 75–80. [Google Scholar] [CrossRef]
- Do, H.T.; Nguyen, T.B.; Ly, T.M. Tidal energy potential in coastal Vietnam. Viet. J. Sci. Technol. Eng. 2022, 64, 85–89. [Google Scholar] [CrossRef]
- Nuclear Reactions in Stars. Available online: http://nuclphys.sinp.msu.ru/nuclsynt/n03_1.htm (accessed on 5 September 2022).
- Parisi, J.; Ball, J. The Future of Fusion Energy; WSPC: Singapore, 2018. [Google Scholar]
- McCracken, H.; Stott, P. Fusion: The Energy of the Universe; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Buinevich, V.S.; Nepapushev, A.A.; Moskovskikh, D.O.; Trusov, G.V.; Kuskova, K.V.; Vadchenkob, S.G.; Rogachev, A.S.; Mukasyan, A.S. Fabrication of ultra-high-temperature nonstoichiometric hafnium carbonitride via combustion synthesis and spark plasma sintering. Ceram. Int. 2020, 46, 16068–16073. [Google Scholar] [CrossRef]
- Claessens, M. ITER: The Giant Fusion Reactor. Bringing a Sun to Earth; Springer: Cham, Switzerland, 2020. [Google Scholar]
- ITER. Available online: https://www.iter.org/ (accessed on 6 September 2022).
- Burning of Hydrogen. Available online: http://nuclphys.sinp.msu.ru/nuclsynt/n04.htm (accessed on 6 September 2022).
- Freidberg, J.P. Plasma Physics and Fusion Energy; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Abu-Shawareb, H.M.; Acree, R.; Adams, P.; Adams, J.; Addis, B. Lawson Criterion for Ignition Exceeded in an Inertial Fusion Experiment. Phys. Rev. Lett. 2022, 129, 075001. [Google Scholar] [CrossRef] [PubMed]
- Doran, D.G.; Guinan, M.W. Fusion Materials High Energy-Neutron Studies–A Status Report. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/11/556/11556527.pdf (accessed on 7 September 2022).
- Ilgisonis, V.I. Managed Thermonuclear Synthesis. Available online: https://bigenc.ru/physics/text/4700247 (accessed on 9 September 2022).
- Dunlap, R. Energy from Nuclear Fusion; IOP Publishing: New York, NY, USA, 2021. [Google Scholar]
- Harms, A.A.; Kingdon, D.R.; Schoepf, K.F.; Miley, G.H. Principles of Fusion Energy; World Scientific: Singapore, 2000. [Google Scholar]
- Sassin, W. Zu den Grenzen menschlicher Erkenntnis. Beacon J. Stud. Ideol. Ment. Dimens. 2018, 1, 010310202. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Energy Production and Consumption. Our World in Data. Available online: https://ourworldindata.org/energy-production-consumption (accessed on 15 August 2022).
- Low, S. Climate Imagineering Practices and Politics of Sunlight Reflection and Carbon Removal Assessment. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2021. Available online: https://www.researchgate.net/publication/350966368_Climate_Imagineering_Practices_and_politics_of_sunlight_reflection_and_carbon_removal_assessment (accessed on 27 August 2022).
- Ignatieva, M.; Stewart, G.H.; Meurk, C. Planning and design of ecological networks in urban areas. Landscape Ecol. Eng. 2011, 7, 17–25. [Google Scholar] [CrossRef]
| Source of Energy | Positive Features | Negative Features |
|---|---|---|
| Biofuels | ||
| Solid | (1) simplicity of production, (2) ability to be a part of a closed resource cycle (within the borders of a self-sustained household), (3) broad availability of ingredients, (4) absence of special storage regime, (5) very long service lifetime, (6) inexpensiveness | (1) increase in carbonization, (2) deforestation, (3) practicability in limited regions of the Earth with forests |
| Liquid | (1) they are already widely used, (2) simplicity of equipment, (3) diversity of the fuels obtained | (1) relatively high cost of production, especially for biomethanol, (2) necessity in vast land or sea coast farming grounds, (3) large distortion of biosphere, (4) substantial increase in carbonization |
| Gaseous | (1) possibility to be received from waste (2) very low cost of production | (1) biomethane is a full analog of methane excavated from the Earth’s crust. Therefore, the carbonization is the same as for its industrial analog |
| Nuclear power | (1) large availability of the necessary elements (at least now), (2) simplicity of construction, (3) primitive operation cycle, (4) long service times, (5) it is already used for almost a century | (1) very high hazard of disaster events (meltdowns, leaks or explosions) with relatively small probability of these events, (2) necessity to dig exhausted radioactive fuel into the ground, (3) slow radioactive destruction of facilities, (4) vulnerability to human errors, (5) stimulation of energy inequality across the globe |
| Hydrogen | (1) eco-friendliness of the vehicles, (2) the safest fuel far to the environment known thus, (3) it may be stored in cylinders, absorption metal capacitors or generated on-board of vehicles | (1) perplexed colored classification, (2) complexity of production of “green” H2, (3) enormous production costs of “green” H2, (4) eco-hostility of production, hazardous technologies and reagents, involving alkalization of biosphere and pumping of gases to lithospheric splits, (5) “green” H2 is not a true source of energy; it is a means of re-pumping energy from one place to another on the planetary scale, (6) economical reasonability for production of mere “grey” H2, (7) large transportation costs, inability to build hydrogen pipelines due to physical properties of H2, (8) high explosiveness of its mixture with oxygen in 2:1 proportion |
| Hydropower | (1) very low production costs, (2) constant switching to national electrical networks, no battery stations are required, (3) little maintenance supervision is required, (4) long service times, (5) it is already sufficiently tested in our times | (1) severe destruction of biota that is observed in 100% cases, (2) change of river flow rate, increase in water temperature, (3) negative influence on lithosphere and soil, (4) indirect carbonization of atmosphere with natural gas synthesized during algae decay in shallow water reservoirs |
| Wind power | (1) it reduces carbonization significantly, (2) it is already sufficiently tested in our times | (1) perpetual high level of noise, (2) radio (UHF) waves interference, influence on mobile networks and TV, (3) ability to generate infrasound especially hazardous to water and soil biota, (4) creation of vast zones of human and biotic alienation, (5) changes of wind rose, (6) economic reasonability only on sea shores, (7) long periods of inactivity (no electricity generation). Therefore, it is difficult to switch wind power plants directly to national electrical networks. Battery stations are almost always required |
| Solar power | (1) easy installation and use on a little scale (households, small business, small agricultural farms), (2) practicability in almost any conditions whither sunlight reaches, independently of the temperature regime in an area (e.g., in mountains, under water, in cosmic space) | (1) complex maintenance systems are required in power plants of industrial size, (2) it can be used effectively only within 40° North to 40° South and a limited number of polar/circumpolar regions, (3) even greater zones of social and biotic alienation are being created than for wind power plants of comparable power output, (4) production cycle and installation of solar elements are dangerous to the environment, (5) ability to cause a drop in air temperatures in a local area and cloud formation that will decrease the efficiency of a solar power plant (self-suppression), (6) utilization of out-of-the-service power plants is environmentally dangerous, (7) long periods of inactivity (no electricity generation). Therefore, it is difficult to switch wind power plants directly to national electrical networks. Huge battery stations are always required |
| Geothermal power | (1) effectiveness in risky regions of elevated seismic and volcanic activity | (1) it is not widely used thus far, (2) the places where it can be used are spread very unevenly across the planetary surface, the regions of use are limited, (3) very high environmental impact, (4) high level of noise |
| Sea tidal power | (1) extensive use on the planetary scale will cause a decrease in the Earth’s rotation rate, (2) very high construction and maintenance costs | |
| Ocean thermal power | (1) it is insufficiently researched thus far, (2) it may be effective only in equatorial and tropical zones of the World ocean, i.e., in the places where the temperature difference between water surface and deeper layers is maximal (around 25 °C) | |
| Wind wave power | (1) it utilizes an unobvious but omnipresent source of energy | (1) it is insufficiently researched thus far, (2) cargo ships with batteries or constructing immense electrical networks on sea bottom are needed |
| Thermonuclear power | (1) it is almost a “perpetuum mobile” source, (2) it may cover energy demand of the whole humanity, (3) it exceeds any other ASE by efficiency coefficient and energy net value | (1) research and development (R&D) costs are tremendous; they exceed the total R&D costs for all other ASE, (2) it is not tested yet, either in laboratory or in industry, (3) risk of radioactive contamination is high, (4) hard-to-find or hard-to-synthesize substances are needed, (5) very short service life is foreseen due to high-energy neutron damage of facilities (less than a year), (6) electricity production costs are difficult to estimate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boguslavsky, D.V.; Sharov, K.S.; Sharova, N.P. Using Alternative Sources of Energy for Decarbonization: A Piece of Cake, but How to Cook This Cake? Int. J. Environ. Res. Public Health 2022, 19, 16286. https://doi.org/10.3390/ijerph192316286
Boguslavsky DV, Sharov KS, Sharova NP. Using Alternative Sources of Energy for Decarbonization: A Piece of Cake, but How to Cook This Cake? International Journal of Environmental Research and Public Health. 2022; 19(23):16286. https://doi.org/10.3390/ijerph192316286
Chicago/Turabian StyleBoguslavsky, Dmitry V., Konstantin S. Sharov, and Natalia P. Sharova. 2022. "Using Alternative Sources of Energy for Decarbonization: A Piece of Cake, but How to Cook This Cake?" International Journal of Environmental Research and Public Health 19, no. 23: 16286. https://doi.org/10.3390/ijerph192316286
APA StyleBoguslavsky, D. V., Sharov, K. S., & Sharova, N. P. (2022). Using Alternative Sources of Energy for Decarbonization: A Piece of Cake, but How to Cook This Cake? International Journal of Environmental Research and Public Health, 19(23), 16286. https://doi.org/10.3390/ijerph192316286