Clinical Outcomes of a Non-Compliant Balloon Dilatation Catheter: MOZEC™ NC Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Study Device
2.3. Interventional Procedure and Concomitant Medications
2.4. Study Endpoints and Study Definitions
2.5. Data Collection
2.6. Statistical Analysis
3. Results
3.1. Baseline and Demographic Characteristics
3.2. Lesion and Procedural Characteristics
3.3. Outcomes
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barton, M.; Grüntzig, J.; Husmann, M.; Rösch, J. Balloon angioplasty—The legacy of Andreas Grüntzig, M.D. (1939–1985). Front. Cardiovasc. Med. 2014, 1, 15. [Google Scholar] [CrossRef]
- Cuculi, F.; Bossard, M.; Zasada, W.; Moccetti, F.; Voskuil, M.; Wolfrum, M.; Malinowski, K.P.; Toggweiler, S.; Kobza, R. Performing percutaneous coronary interventions with predilatation using non-compliant balloons at high-pressure versus conventional semi-compliant balloons: Insights from two randomised studies using optical coherence tomography. Open Heart 2020, 7, e001204. [Google Scholar] [CrossRef] [PubMed]
- Safian, R.D.; Hoffmann, M.A.; Almany, S.; Kahn, J.; Reddy, V.; Marsalese, D.; Gangadharan, V.; Ajluni, S.; Friedman, H.Z.; Schreiber, T.L.; et al. Comparison of coronary angioplasty with compliant and non-compliant balloons (the angioplasty compliance trial). Am. J. Cardiol. 1995, 76, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Keeble, T.R.; Khokhar, A.; Akhtar, M.M.; Mathur, A.; Weerackody, R.; Kennon, S. Percutaneous balloon aortic valvuloplasty in the era of transcatheter aortic valve implantation: A narrative review. Open Heart 2016, 3, e000421. [Google Scholar] [CrossRef] [PubMed]
- Mach, M.; Szalkiewicz, P.; Poschner, T.; Hasan, W.; Andreas, M.; Winkler, B.; Hasimbegovic, E.; Steinkellner, T.; Strouhal, A.; Adlbrecht, C.; et al. The use of semi-compliant versus non-compliant balloon systems for predilatation during the implantation of self-expandable transcatheter aortic valves. Eur. J. Clin. Investig. 2021, 51, e13570. [Google Scholar] [CrossRef]
- Secco, G.G.; Ghione, M.; Mattesini, A.; Dall’Ara, G.; Ghilencea, L.; Kilickesmez, K.; De Luca, G.; Fattori, R.; Parisi, R.; Marino, P.N.; et al. Very high-pressure dilatation for undilatable coronary lesions: Indications and results with a new dedicated balloon. EuroIntervention 2016, 12, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Bil, J.; Pawłowski, T.; Gil, R.J. Short-term results of the First-in-Man new Non-Complaint Balloon Catheter clinical study. Kardiol. Inwazyjna 2018, 13, 4–9. [Google Scholar]
- Foin, N.; Secco, G.G.; Ghilencea, L.; Krams, R.; Di Mario, C. Final proximal post-dilatation is necessary after kissing balloon in bifurcation stenting. EuroIntervention 2011, 7, 597–604. [Google Scholar] [CrossRef]
- Tanaka, A.; Jabbour, R.J.; Kawamoto, H.; Latib, A.; Colombo, A. A super high-pressure balloon solution for a non-dilatable in-stent restenosis. Int. J. Cardiol. 2016, 203, 357–359. [Google Scholar] [CrossRef]
- Secco, G.G.; Buettner, A.; Parisi, R.; Pistis, G.; Vercellino, M.; Audo, A.; Kambis, M.; Garbo, R.; Porto, I.; Tarantini, G.; et al. Clinical Experience with Very High-Pressure Dilatation for Resistant Coronary Lesions. Cardiovasc. Revascularization Med. 2019, 20, 1083–1087. [Google Scholar] [CrossRef]
- General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 2014, 81, 14–18. [Google Scholar]
- Dixon, J.R., Jr. The International Conference on Harmonization Good Clinical Practice guideline. Qual. Assur. 1998, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. ACC/AHA Guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2016, 68, 1082–1115. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; Dimaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef] [PubMed]
- Kandzari, D.E.; Teirstein, P.S.; Kereiakes, D.J.; Cannon, L.A.; Hearne, S.E.; Kuo, H.C.; Ying, S.W.; Cheong, W.F.; Popma, J.J. Procedural effectiveness of a novel 1.20 mm diameter angioplasty catheter: Clinical and angiographic outcomes. J. Interv. Cardiol. 2013, 26, 131–136. [Google Scholar] [CrossRef]
- Seth, A.; Gupta, S.; Singh, V.P.; Kumar, V. Expert Opinion: Optimising Stent Deployment in Contemporary Practice: The Role of Intracoronary Imaging and Non-compliant Balloons. Interv. Cardiol. 2017, 12, 81–84. [Google Scholar] [CrossRef]
- Özel, E.; Taştan, A.; Öztürk, A.; Özcan, E.E.; Uyar, S.; Şenarslan, Ö. What is better for predilatation in bioresorbable vascular scaffold implantation: A non-compliant or a compliant balloon? Anatol. J. Cardiol. 2016, 16, 244–249. [Google Scholar]
- Rana, O.; Shah, N.C.; Wilson, S.; Swallow, R.; O’Kane, P.; Levy, T. The impact of routine and intravascular ultrasound-guided high-pressure postdilatation after drug-eluting stent deployment: The STent OPtimization (STOP) study. J. Invasive Cardiol. 2014, 26, 640–646. [Google Scholar] [PubMed]
- Karamasis, G.V.; Kalogeropoulos, A.S.; Gamma, R.A.; Clesham, G.J.; Marco, V.; Tang, K.H.; Jagathesan, R.; Sayer, J.W.; Robinson, N.M.; Kabir, A.; et al. Effects of stent postdilatation during primary PCI for STEMI: Insights from coronary physiology and optical coherence tomography. Catheter. Cardiovasc. Interv. 2021, 97, 1309–1317. [Google Scholar] [CrossRef]
- Blachutzik, F.; Achenbach, S.; Tröbs, M.; Marwan, M.; Weissner, M.; Nef, H.; Schlundt, C. Effect of non-compliant balloon postdilatation on magnesium-based bioresorbable vascular scaffolds. Catheter. Cardiovasc. Interv. 2019, 93, 202–207. [Google Scholar] [CrossRef]
- Włodarczak, A.; Rola, P.; Barycki, M.; Kulczycki, J.J.; Szudrowicz, M.; Lesiak, M.; Doroszko, A. Rota-Lithotripsy’A Novel Bail-Out Strategy for Calcified Coronary Lesions in Acute Coronary Syndrome. The First-in-Man Experience. J. Clin. Med. 2021, 10, 1872. [Google Scholar] [CrossRef] [PubMed]
- Mendis, S.; Puska, P.; Norrving, B. Global Atlas on Cardiovascular Disease Prevention and Control; World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/handle/10665/44701 (accessed on 15 November 2022).
- Thomson, V.S.; Varghese, M.J.; Chacko, S.T.; Varghese, L.; Alex, A.G.; George, P.V.; George, O.K.; Joseph, G.; Yadav, B.K.; John, J. Coronary artery disease management and cost implications with fractional flow reserve guided coronary intervention in Indian patients with stable ischemic coronary artery disease. Catheter. Cardiovasc. Interv. 2021, 97, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Biotronik. Pantera LEO. Available online: https://biotronik.cdn.mediamid.com/cdn_bio_doc/bio23105/8615/bio23105.pdf (accessed on 24 April 2022).
- Abbott. NC Trek™ Coronary Dilatation Catheter. 2022. Available online: https://www.cardiovascular.abbott/int/en/hcp/products/percutaneous-coronary-intervention/coronary-dilatation-catheters/nc-trek-coronary-dilatation-catheters.html (accessed on 26 April 2022).
- Boston Scientific NC EMERGE™ PTCA Dilatation Catheter. Available online: https://www.bostonscientific.com/en-IN/products/catheters-balloon/nc-emerge-balloon-catheter/performance-overview.html (accessed on 15 November 2022).
- Gil, R.J.; Pawlowski, T.; Bil, J. Feasibility and safety of the new coronary noncompliant balloon catheter River NC®. Futur. Cardiol. 2021, 17, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Abbott. Trek™ & Mini Trek™ Coronary Dilatation Catheters. 2022. Available online: https://www.cardiovascular.abbott/us/en/hcp/products/percutaneous-coronary-intervention/coronary-dilatation-catheters/trek-mini-coronary-dilatation-catheter.html (accessed on 26 April 2022).
- Medtronic. NC Sprinter Rx Noncompliant Balloon Dilatation Catheter. 2022. Available online: https://www.medtronic.com/ca-en/healthcare-professionals/products/cardiovascular/catheters/balloon-catheters/nc-sprinter-rx-noncompliant-balloon-dilatation-catheter.html (accessed on 26 April 2022).
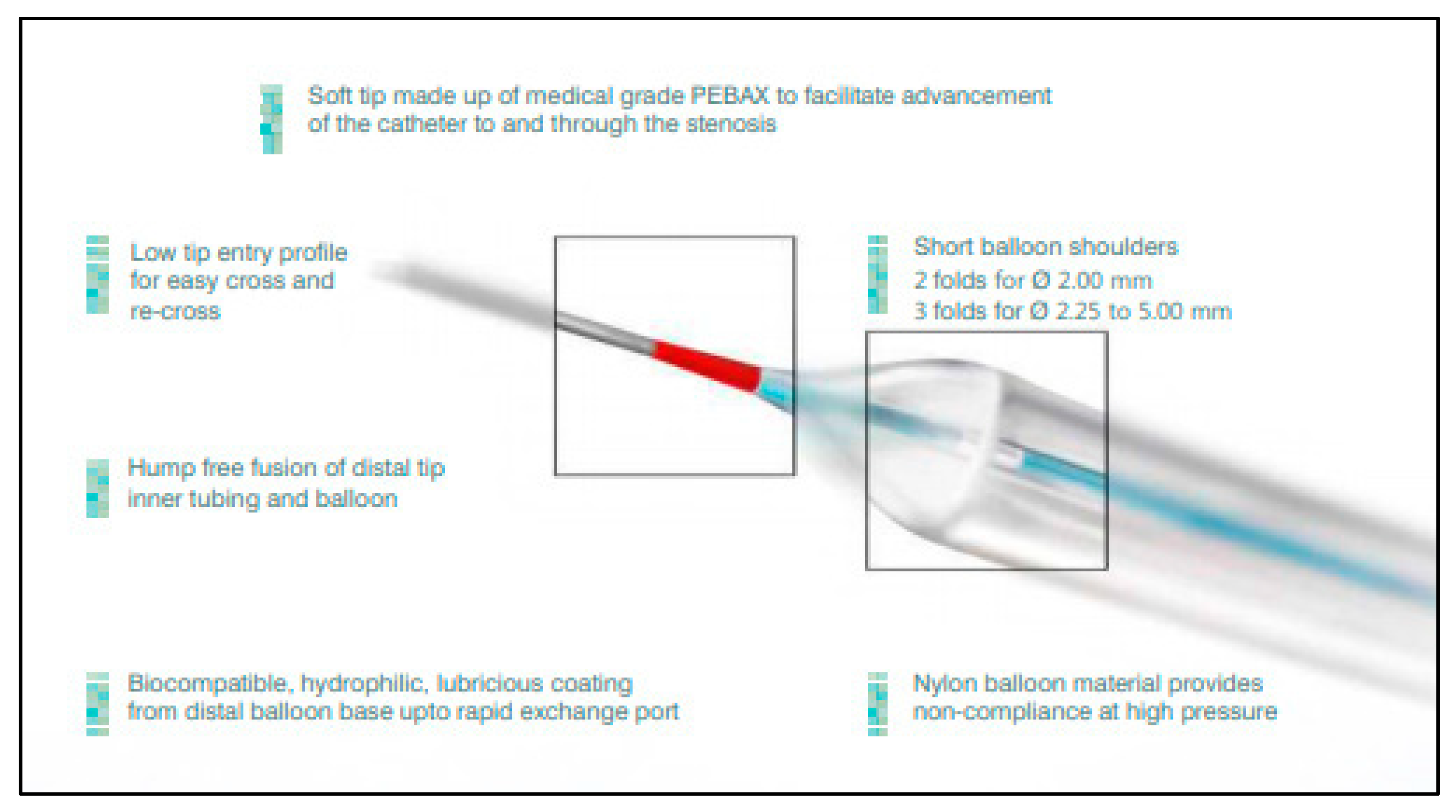
| Baseline Characteristics | Patients n = 57 |
|---|---|
| Age, years | 55.1 ± 11.5 |
| Males | 44 (77.2%) |
| Heart rate, beats per min | 77.9 ± 14.9 |
| Systolic blood pressure, mmHg | 121.1 ± 18.0 |
| Diastolic blood pressure, mmHg | 73.3 ± 17.0 |
| Medical History | |
| Hypertension | 18 (31.6%) |
| Diabetes mellitus | 13 (22.8%) |
| Smoking | 3 (5.26%) |
| Dyslipidaemia | 2 (3.5%) |
| Diagnosis at Presentation Stable angina | 20 (35.1%) |
| Acute coronary syndrome | 37 (64.9%) |
| ST-elevation MI | 17 (46) |
| Non-ST-elevation MI | 15 (40.5) |
| Unstable angina | 5 (13.5) |
| Single vessel disease | 11 (20) |
| Dual vessel disease | 23 (40) |
| Multivessel disease | 23 (40) |
| Previous coronary artery bypass graft | 0 (0%) |
| Previous myocardial infarction | 7 (12.3%) |
| Previous percutaneous coronary intervention | 1 (1.8%) |
| Clinical Characteristics | Patients n = 57 |
|---|---|
| Lesion details | |
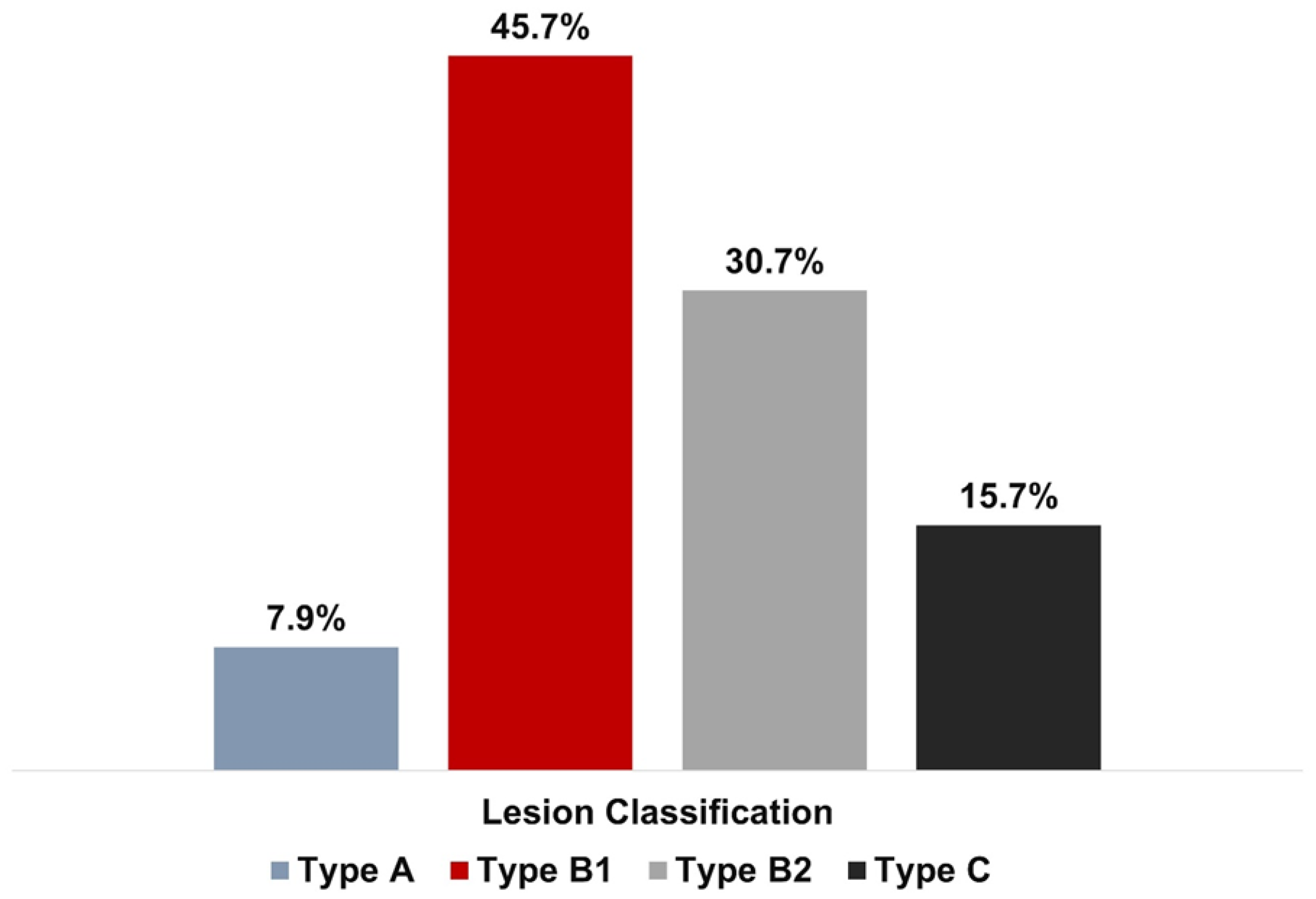
| Total number of lesions | 140 |
| Total number of lesions treated | 57 |
| Number of lesions per patient | 2.46 |
| Percentage diameter stenosis | 86.0 ± 15.0 |
| Pre-dilatation | 57 (100) |
| Post-dilatation | 57 (100) |
| Lesion features | |
| Bifurcation | 19 (33.33) |
| Calcified | 10 (17.5) |
| Tortuosity | 7 (12.3) |
| Chronic total occlusion | 4 (7) |
| Lesion locations | |
| Left main coronary artery | 5 (9) |
| Left anterior descending artery | 22 (38.6) |
| Left circumflex artery | 10 (17.5) |
| Right coronary artery | 18 (31.6) |
| Ramus intermedius | 06 (10.5) |
| Procedural details | |
| Procedural success | 57 (100) |
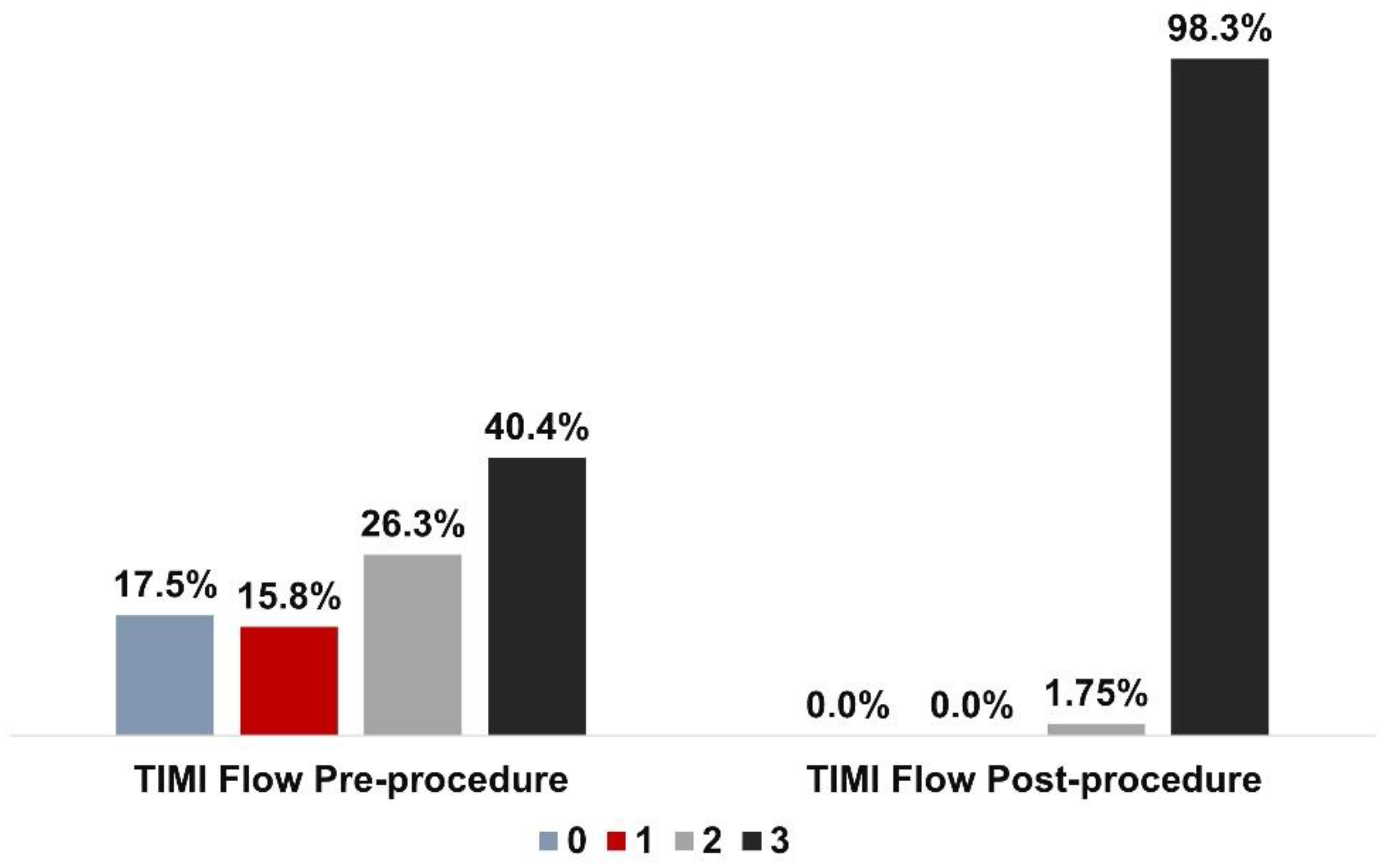
| Post Mozec™ NC Rx balloon TIMI III flow | 56 (98.3) |
| Major adverse cardiac event | 0 (0.0%) |
| Target lesion failure | 0 (0.0%) |
| Parameter | n (%) |
|---|---|
| Total stents | 86 (100) |
| Stent per patient | 1.5 |
| DES, n (%) | 85 (98.8) |
| Everolimus-eluting stent | 47 (56) |
| Sirolimus-eluting stent | 28 (33) |
| Zotarolimus-eluting stent | 9 (11) |
| BMS, n (%) | 1 (1.2) |
| Balloon details | |
| Balloon length, mm | 12.5 ± 2.4 |
| Balloon diameter, mm | 2.8 ± 0.5 |
| Post-dilatation balloon | |
| Length, mm, n (%) | |
| 8 | 5 (8.77) |
| 10 | 21 (36.84) |
| 13 | 16 (28.07) |
| 15 | 15 (26.32) |
| Diameter, mm, n (%) | |
| 2.50 | 1 (1.75) |
| 2.75 | 9 (15.79) |
| 3.00 | 24 (42.11) |
| 3.50 | 21(36.84) |
| 4.0 | 2 (3.51) |
| Features | Pantera LEO (Biotronik) [24] | NC Trek Rx (Abbott) [25] | NC Emerge™ (Boston Scientific Corporation) [26] | River (Balton) [27] | Mini Trek (Abbott) [28] | NC Sprinter Rx (Medtronic) [29] | Mozec NC (Meril Life Sciences) |
|---|---|---|---|---|---|---|---|
| Nominal pressure (atm) | 14 | 12 | 12 | 8 | 8 | 10 | 12 |
| Rated burst pressure (atm) | 18 & 20 | 18 | 18/20 | 18 | 14 | 18 | 20 |
| Guidewire compatibility (inch) | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.016 | 0.014 |
| Shaft length (cm) | 145 | 143 | 142 | 140 | 145 | 138 | 142 |
| Sheath compatibility (F) | 5 | - | 5,6 | 5 | 5,6 | - | 5 |
| Balloon length (mm) | 8–30 | 6–25 | 6–30 | 10–40 | 6–20 | 6–27 | 8–38 |
| Balloon diameter (mm) | 2.00–5.00 | 1.50–5.00 | 2.00–6.00 | 1.25–4.00 | 1.20–2.00 | 2.00–5.00 | 2–5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhan, A.; Vishwakarma, P.; Bhandari, M.; Sethi, R.; Chandra, S.; Chaudhary, G.; Sharma, A.; Perrone, M.A.; Dwivedi, S.; Narain, V. Clinical Outcomes of a Non-Compliant Balloon Dilatation Catheter: MOZEC™ NC Study. Int. J. Environ. Res. Public Health 2022, 19, 16231. https://doi.org/10.3390/ijerph192316231
Pradhan A, Vishwakarma P, Bhandari M, Sethi R, Chandra S, Chaudhary G, Sharma A, Perrone MA, Dwivedi S, Narain V. Clinical Outcomes of a Non-Compliant Balloon Dilatation Catheter: MOZEC™ NC Study. International Journal of Environmental Research and Public Health. 2022; 19(23):16231. https://doi.org/10.3390/ijerph192316231
Chicago/Turabian StylePradhan, Akshyaya, Pravesh Vishwakarma, Monika Bhandari, Rishi Sethi, Sharad Chandra, Gaurav Chaudhary, Akhil Sharma, Marco Alfonso Perrone, Sudhanshu Dwivedi, and Varun Narain. 2022. "Clinical Outcomes of a Non-Compliant Balloon Dilatation Catheter: MOZEC™ NC Study" International Journal of Environmental Research and Public Health 19, no. 23: 16231. https://doi.org/10.3390/ijerph192316231
APA StylePradhan, A., Vishwakarma, P., Bhandari, M., Sethi, R., Chandra, S., Chaudhary, G., Sharma, A., Perrone, M. A., Dwivedi, S., & Narain, V. (2022). Clinical Outcomes of a Non-Compliant Balloon Dilatation Catheter: MOZEC™ NC Study. International Journal of Environmental Research and Public Health, 19(23), 16231. https://doi.org/10.3390/ijerph192316231






