Association between Exposure to Particulate Matter Air Pollution during Early Childhood and Risk of Attention-Deficit/Hyperactivity Disorder in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population, Outcome, and Comorbidities
2.3. Statistical Analysis
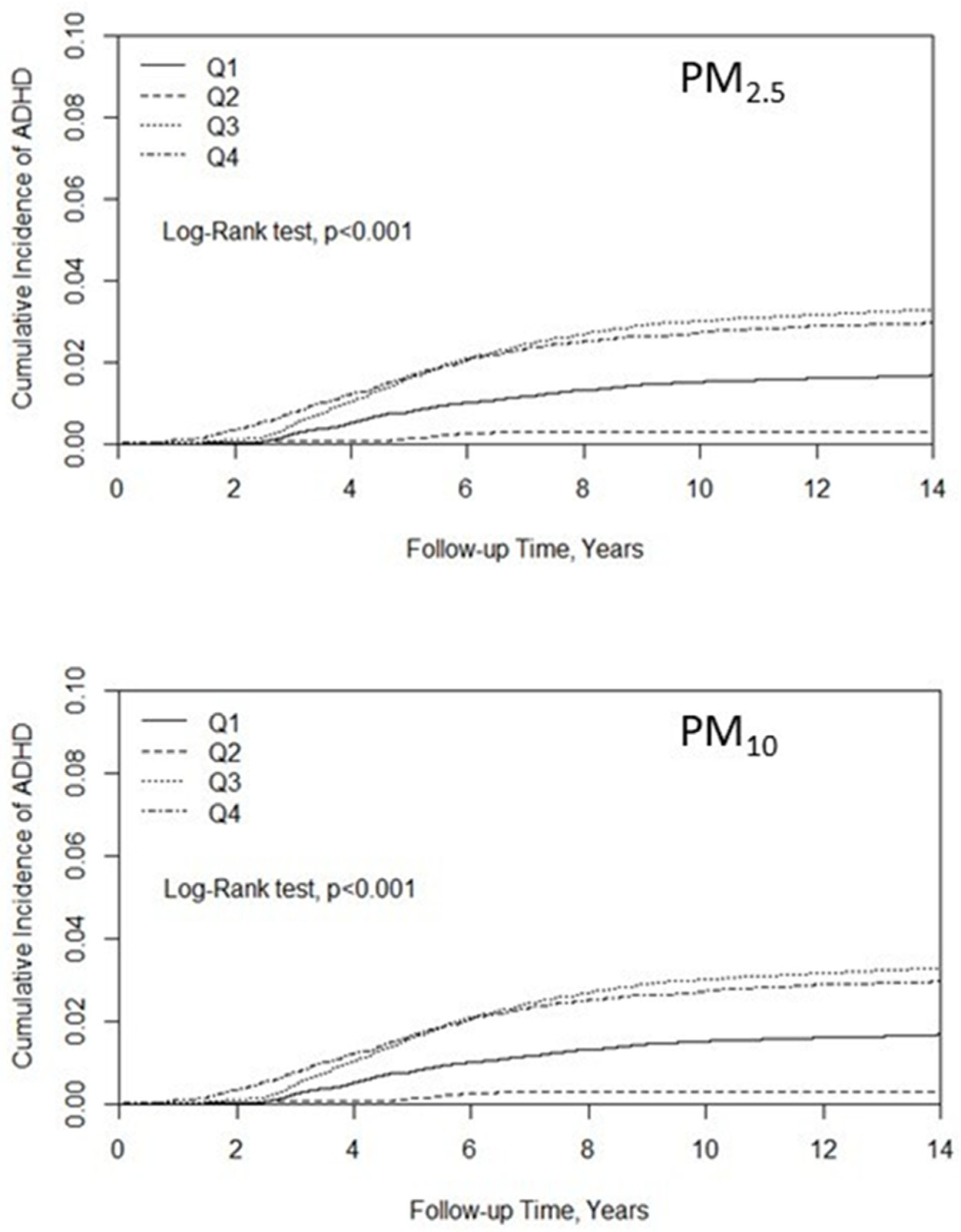
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayal, K.; Prasad, V.; Daley, D.; Ford, T.; Coghill, D. ADHD in children and young people: Prevalence, care pathways, and service provision. Lancet Psychiatry 2018, 5, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Sanders, S.; Doust, J.; Beller, E.; Glasziou, P. Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics 2015, 135, e994–e1001. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Xu, Y.; Yan, Q.; Tong, L. The Prevalence of Attention Deficit/Hyperactivity Disorder among Chinese Children and Adolescents. Sci. Rep. 2018, 8, 11169. [Google Scholar] [CrossRef] [PubMed]
- Magnus, W.; Nazir, S.; Anilkumar, A.C.; Shaban, K. Attention Deficit Hyperactivity Disorder. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Shaw, M.; Hodgkins, P.; Caci, H.; Young, S.; Kahle, J.; Woods, A.G.; Arnold, L.E. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: Effects of treatment and non-treatment. BMC Med. 2012, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.; Chadwick, O.; Heptinstall, E.; Danckaerts, M. Hyperactivity and conduct problems as risk factors for adolescent development. J. Am. Acad. Child Adolesc. Psychiatry 1996, 35, 1213–1226. [Google Scholar] [CrossRef]
- Willoughby, M.T. Developmental course of ADHD symptomatology during the transition from childhood to adolescence: A review with recommendations. J. Child Psychol. Psychiatry 2003, 44, 88–106. [Google Scholar] [CrossRef]
- Wilens, T.E.; Spencer, T.J. Understanding attention-deficit/hyperactivity disorder from childhood to adulthood. Postgrad. Med. 2010, 122, 97–109. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, H.; Sun, H.; Zou, L.; Zhu, L.Q. Role of dopamine receptors in ADHD: A systematic meta-analysis. Mol. Neurobiol. 2012, 45, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Demontis, D.; Walters, R.K.; Martin, J.; Mattheisen, M.; Als, T.D.; Agerbo, E.; Baldursson, G.; Belliveau, R.; Bybjerg-Grauholm, J.; Bækvad-Hansen, M.; et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 2019, 51, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Franke, B.; Neale, B.M.; Faraone, S.V. Genome-wide association studies in ADHD. Hum. Genet. 2009, 126, 13–50. [Google Scholar] [CrossRef] [PubMed]
- Gialluisi, A.; Andlauer, T.F.M.; Mirza-Schreiber, N.; Moll, K.; Becker, J.; Hoffmann, P.; Ludwig, K.U.; Czamara, D.; Pourcain, B.S.; Honbolygó, F.; et al. Genome-wide association study reveals new insights into the heritability and genetic correlates of developmental dyslexia. Mol. Psychiatry 2021, 26, 3004–3017. [Google Scholar] [CrossRef]
- Taylor, M.J.; Martin, J.; Lu, Y.; Brikell, I.; Lundstrom, S.; Larsson, H.; Lichtenstein, P. Association of Genetic Risk Factors for Psychiatric Disorders and Traits of These Disorders in a Swedish Population Twin Sample. JAMA Psychiatry 2019, 76, 280–289. [Google Scholar] [CrossRef]
- Soheilipour, F.; Shiri, S.; Ahmadkhaniha, H.R.; Abdollahi, E.; Hosseini-Baharanchi, F.S. Risk factors for attention-deficit/hyperactivity disorder: A case-control study in 5 to 12 years old children. Med. Pharm. Rep. 2020, 93, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, M.; Janjani, H.; Yousefian, F.; Jamal, A.; Yunesian, M. Association between ambient gaseous and particulate air pollutants and attention deficit hyperactivity disorder (ADHD) in children; a systematic review. Environ. Res. 2019, 173, 135–156. [Google Scholar] [CrossRef]
- Markevych, I.; Tesch, F.; Datzmann, T.; Romanos, M.; Schmitt, J.; Heinrich, J. Outdoor air pollution, greenspace, and incidence of ADHD: A semi-individual study. Sci. Total Environ. 2018, 642, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, M.; Holst, G.J.; Hansen, B.; Geels, C.; Kalkbrenner, A.; Schendel, D.; Brandt, J.; Pedersen, C.B.; Dalsgaard, S. Exposure to air pollution in early childhood and the association with Attention-Deficit Hyperactivity Disorder. Environ. Res. 2020, 183, 108930. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. EPA Particulate Matter (PM) Pollution US EPA. Available online: https://www.epa.gov/pm-pollution (accessed on 3 October 2022).
- Crinnion, W. Particulate Matter Is a Surprisingly Common Contributor to Disease. Integr. Med. 2017, 16, 8–12. [Google Scholar]
- Alexeeff, S.E.; Deosaransingh, K.; Liao, N.S.; van den Eeden, S.K.; Schwartz, J.; Sidney, S. Particulate Matter and Cardiovascular Risk in Adults with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2021, 204, 159–167. [Google Scholar] [CrossRef]
- Weuve, J.; Puett, R.C.; Schwartz, J.; Yanosky, J.D.; Laden, F.; Grodstein, F. Exposure to particulate air pollution and cognitive decline in older women. Arch. Intern. Med. 2012, 172, 219–227. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chen, W.T.; Su, S.H.; Jung, C.R.; Hwang, B.F. PM2.5 exposure and incident attention-deficit/hyperactivity disorder during the prenatal and postnatal periods: A birth cohort study. Environ. Res. 2022, 214 Pt 1, 113769. [Google Scholar] [CrossRef] [PubMed]
- Min, J.Y.; Min, K.B. Exposure to ambient PM10 and NO2 and the incidence of attention-deficit hyperactivity disorder in childhood. Environ. Int. 2017, 99, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Myhre, O.; Lag, M.; Villanger, G.D.; Oftedal, B.; Ovrevik, J.; Holme, J.A.; Aase, H.; Paulsen, R.E.; Bal-Price, A.; Dirven, H. Early life exposure to air pollution particulate matter (PM) as risk factor for attention deficit/hyperactivity disorder (ADHD): Need for novel strategies for mechanisms and causalities. Toxicol. Appl. Pharmacol. 2018, 354, 196–214. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- WHO Ambient (Outdoor) Air Pollution. Available online: Https://Www.Who.Int/En/News-Room/Fact-Sheets/Detail/Ambient-(Outdoor)-Air-Quality-and-Health (accessed on 3 October 2022).
- Curatolo, P.; D’Agati, E.; Moavero, R. The neurobiological basis of ADHD. Ital. J. Pediatr. 2010, 36, 79. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y. Association of Co-Exposure to Psychosocial Factors With Depression and Anxiety in Korean Workers. J. Occup. Environ. Med. 2020, 62, e498–e507. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Garciduenas, L.; Engle, R.; Mora-Tiscareno, A.; Styner, M.; Gomez-Garza, G.; Zhu, H.; Jewells, V.; Torres-Jardon, R.; Romero, L.; Monroy-Acosta, M.E.; et al. Exposure to severe urban air pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain Cogn. 2011, 77, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Banerjee, M.; Ray, M.R.; Lahiri, T. Attention-deficit hyperactivity disorder in children chronically exposed to high level of vehicular pollution. Eur. J. Pediatr. 2011, 170, 923–929. [Google Scholar] [CrossRef]
- Sunyer, J.; Esnaola, M.; Alvarez-Pedrerol, M.; Forns, J.; Rivas, I.; Lopez-Vicente, M.; Suades-Gonzalez, E.; Foraster, M.; Garcia-Esteban, R.; Basagana, X.; et al. Association between traffic-related air pollution in schools and cognitive development in primary school children: A prospective cohort study. PLoS Med. 2015, 12, e1001792. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Hu, C.; Chang, Q.; Deng, Q.; Yang, X.; Wu, Y. Study of the neurotoxicity of indoor airborne nanoparticles based on a 3D human blood-brain barrier chip. Environ. Int. 2020, 143, 105598. [Google Scholar] [CrossRef]
- Chen, C.H.; Wu, C.D.; Chiang, H.C.; Chu, D.; Lee, K.Y.; Lin, W.Y.; Yeh, J.I.; Tsai, K.W.; Guo, Y.L. The effects of fine and coarse particulate matter on lung function among the elderly. Sci. Rep. 2019, 9, 14790. [Google Scholar] [CrossRef]
- Kang, Y.J.; Tan, H.Y.; Lee, C.Y.; Cho, H. An Air Particulate Pollutant Induces Neuroinflammation and Neurodegeneration in Human Brain Models. Adv. Sci. 2021, 8, e2101251. [Google Scholar] [CrossRef] [PubMed]
- Goulaouic, S.; Foucaud, L.; Bennasroune, A.; Laval-Gilly, P.; Falla, J. Effect of polycyclic aromatic hydrocarbons and carbon black particles on pro-inflammatory cytokine secretion: Impact of PAH coating onto particles. J. Immunotoxicol. 2008, 5, 337–345. [Google Scholar] [CrossRef]
- Cortese, A.; Lova, L.; Comoli, P.; Volpe, E.; Villa, S.; Mallucci, G.; la Salvia, S.; Romani, A.; Franciotta, D.; Bollati, V.; et al. Air pollution as a contributor to the inflammatory activity of multiple sclerosis. J. Neuroinflammation. 2020, 17, 334. [Google Scholar] [CrossRef]
- Ferraro, S.A.; Astort, F.; Yakisich, J.S.; Tasat, D.R. Particulate matter cytotoxicity in cultured SH-SY5Y cells is modulated by simvastatin: Toxicological assessment for oxidative damage. Neurotoxicology 2016, 53, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, P.; Tajuba, J.; Lippmann, M.; Chen, L.C.; Veronesi, B. Particulate matter neurotoxicity in culture is size-dependent. Neurotoxicology 2013, 36, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.C.; Coburn, J.; Garrick, J.M. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol. Ther. 2020, 210, 107523. [Google Scholar] [CrossRef]
- Lewis, J.; Bench, G.; Myers, O.; Tinner, B.; Staines, W.; Barr, E.; Divine, K.K.; Barrington, W.; Karlsson, J. Trigeminal uptake and clearance of inhaled manganese chloride in rats and mice. Neurotoxicology 2005, 26, 113–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, M.; Wang, C.; Zhang, X.; Song, H.; Li, Y. Association between exposure to air pollutants and attention-deficit hyperactivity disorder (ADHD) in children: A systematic review and meta-analysis. Int. J. Environ. Health Res. 2022, 32, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Forns, J.; Sunyer, J.; Garcia-Esteban, R.; Porta, D.; Ghassabian, A.; Giorgis-Allemand, L.; Gong, T.; Gehring, U.; Sorensen, M.; Standl, M.; et al. Air Pollution Exposure During Pregnancy and Symptoms of Attention Deficit and Hyperactivity Disorder in Children in Europe. Epidemiology 2018, 29, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Almqvist, C.; Bölte, S.; Lichtenstein, P.; Anckarsäter, H.; Lind, T.; Lundholm, C.; Pershagen, G. Exposure to air pollution from traffic and neurodevelopmental disorders in Swedish twins. Twin Res. Hum. Genet. 2014, 17, 553–562. [Google Scholar] [CrossRef]
- Setton, E.; Marshall, J.D.; Brauer, M.; Lundquist, K.R.; Hystad, P.; Keller, P.; Cloutier-Fisher, D. The impact of daily mobility on exposure to traffic-related air pollution and health effect estimates. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kwan, M.P. Assessment of sociodemographic disparities in environmental exposure might be erroneous due to neighborhood effect averaging: Implications for environmental inequality research. Environ. Res. 2021, 195, 110519. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Kwan, M.P.; Zhou, S. The Uncertain Geographic Context Problem in the Analysis of the Relationships between Obesity and the Built Environment in Guangzhou. Int. J. Environ. Res. Public Health 2018, 15, 308. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.W.; Carlsten, C.; Karlen, B.; Leckie, S.; van Eeden, S.; Vedal, S.; Wong, I.; Brauer, M. An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am. J. Respir. Crit. Care Med. 2011, 183, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
| N = 98,177 | Subgroups | n | % |
|---|---|---|---|
| Male | 49,740 | 50.7 | |
| Age, years | Mean ± SD | 9.66 ± 4.27 | |
| Urbanization level | 1 (highest) | 60,260 | 61.4 |
| 2 | 31,108 | 31.7 | |
| 3 | 6046 | 6.16 | |
| 4 (lowest) | 763 | 0.78 | |
| Asthma | 21,024 | 21.4 | |
| AD | 9728 | 9.91 | |
| AR | 55,127 | 56.2 | |
| Temperature | Mean ± SD | 23.6 ± 1.35 | |
| PM2.5 (yearly average, μg/m3) | Mean ± SD | 29.3 ± 7.15 | |
| PM10 (yearly average, μg/m3) | Mean ± SD | 53.6 ± 12.1 | |
| ADHD | Yes | 2856 | 2.91 |
| Follow-up time, years | Mean ± SD | 14.7 ± 1.86 | |
| Pollutant Levels | N of ADHD | IR | cHR | (95% CI) |
|---|---|---|---|---|
| PM2.5 (μg/m3) | ||||
| Quartile 1, <25.5 | 426 | 1.15 | ||
| Quartile 2, 25.5–26.4 | 62 | 0.22 | 0.19 | (0.15, 0.25) |
| Quartile 3, 26.5–34.2 | 1045 | 2.25 | 1.96 | (1.75, 2.19) |
| Quartile 4, >34.2 | 656 | 2.03 | 1.77 | (1.56, 1.99) |
| PM10 (μg/m3) | ||||
| Quartile 1, <46.0 | 537 | 1.45 | ||
| Quartile 2, 46.0–50.7 | 513 | 1.39 | 0.96 | (0.85, 1.08) |
| Quartile 3, 50.8–60.4 | 962 | 2.87 | 1.96 | (1.77, 2.18) |
| Quartile 4, >60.4 | 844 | 2.30 | 1.58 | (1.42, 1.76) |
| Covariates | PM2.5 | PM10 | |||
|---|---|---|---|---|---|
| aHR | 95% CI | aHR | 95% CI | ||
| Age | 0.77 | (0.76, 0.78) | 0.79 | (0.78, 0.80) | |
| Gender | Female | 1.00 | 1.00 | ||
| Male | 2.98 | (2.70, 3.29) | 3.22 | (2.95, 3.51) | |
| Urbanization level | 1 (highest) | 1.00 | 1.00 | ||
| 2 | 0.90 | (0.82, 0.99) | 0.97 | (0.90, 1.06) | |
| 3 | 1.01 | (0.85, 1.20) | 0.94 | (0.80, 1.10) | |
| 4 (lowest) | 1.22 | (0.81, 1.85) | 1.23 | (0.86, 1.75) | |
| Asthma | No | 1.00 | 1.00 | ||
| Yes | 1.25 | (1.13, 1.37) | 1.21 | (1.11, 1.32) | |
| AD | No | 1.00 | 1.00 | ||
| Yes | 1.13 | (1.00, 1.28) | 1.09 | (0.98, 1.21) | |
| AR | No | 1.00 | 1.00 | ||
| Yes | 0.95 | (0.87, 1.04) | 0.84 | (0.78, 0.91) | |
| Pollutants | Quartile 1 | 1.00 | 1.00 | ||
| Quartile 2 | 0.20 | (0.15, 0.26) | 0.95 | (0.84, 1.07) | |
| Quartile 3 | 1.90 | (1.70, 2.13) | 2.02 | (1.82, 2.25) | |
| Quartile 4 | 1.79 | (1.58, 2.02) | 1.53 | (1.37, 1.70) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.-C.; Chen, C.-M.; Tsai, J.-D.; Chiang, K.-L.; Tsai, S.C.-S.; Huang, C.-Y.; Lin, C.-L.; Hsu, C.Y.; Chang, K.-H. Association between Exposure to Particulate Matter Air Pollution during Early Childhood and Risk of Attention-Deficit/Hyperactivity Disorder in Taiwan. Int. J. Environ. Res. Public Health 2022, 19, 16138. https://doi.org/10.3390/ijerph192316138
Fan H-C, Chen C-M, Tsai J-D, Chiang K-L, Tsai SC-S, Huang C-Y, Lin C-L, Hsu CY, Chang K-H. Association between Exposure to Particulate Matter Air Pollution during Early Childhood and Risk of Attention-Deficit/Hyperactivity Disorder in Taiwan. International Journal of Environmental Research and Public Health. 2022; 19(23):16138. https://doi.org/10.3390/ijerph192316138
Chicago/Turabian StyleFan, Hueng-Chuen, Chuan-Mu Chen, Jeng-Dau Tsai, Kuo-Liang Chiang, Stella Chin-Shaw Tsai, Ching-Ying Huang, Cheng-Li Lin, Chung Y. Hsu, and Kuang-Hsi Chang. 2022. "Association between Exposure to Particulate Matter Air Pollution during Early Childhood and Risk of Attention-Deficit/Hyperactivity Disorder in Taiwan" International Journal of Environmental Research and Public Health 19, no. 23: 16138. https://doi.org/10.3390/ijerph192316138
APA StyleFan, H.-C., Chen, C.-M., Tsai, J.-D., Chiang, K.-L., Tsai, S. C.-S., Huang, C.-Y., Lin, C.-L., Hsu, C. Y., & Chang, K.-H. (2022). Association between Exposure to Particulate Matter Air Pollution during Early Childhood and Risk of Attention-Deficit/Hyperactivity Disorder in Taiwan. International Journal of Environmental Research and Public Health, 19(23), 16138. https://doi.org/10.3390/ijerph192316138










