Associations between Muscle-Tendon Morphology and Functional Movements Capacity, Flexibility, and Balance in Older Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Measurements
2.3.1. Musculotendinous Ultrasound Imaging
2.3.2. Flexibility
2.3.3. Balance Performance
2.3.4. Functional Performance
2.4. Statistical Analysis
3. Results
3.1. Performance Variables
3.2. Associations between Musculotendinous Morphology and Functional Performance
3.3. Associations between Musculotendinous Morphology and Balance
3.4. Associations between Musculotendinous Morphology and Leg Flexibility
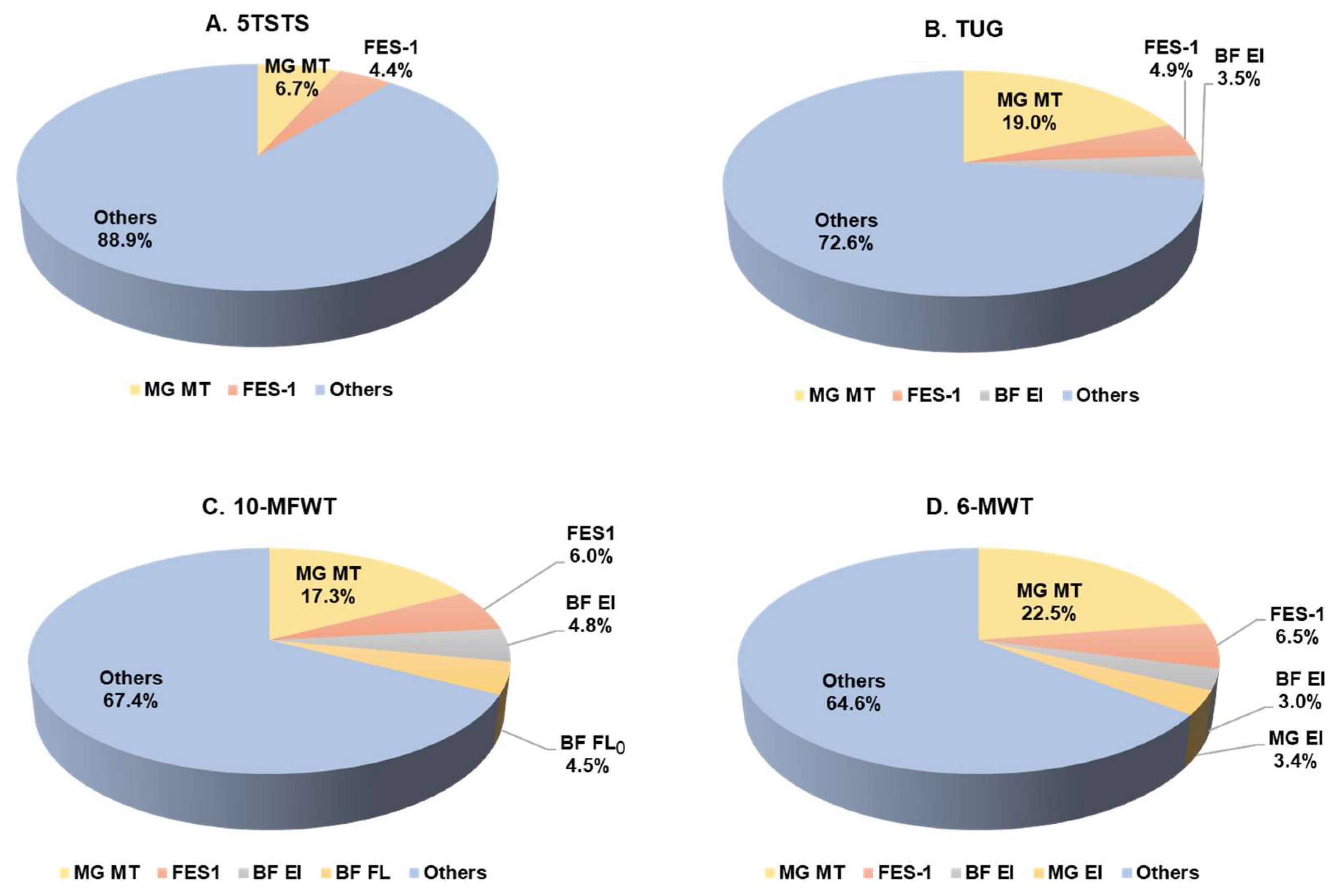
3.5. Functional Movement Performance Prediction
4. Discussion
Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogeri, P.S.; Zanella, R.; Martins, G.L.; Garcia, M.D.A.; Leite, G.; Lugaresi, R.; Gasparini, S.O.; Sperandio, G.A.; Ferreira, L.H.B.; Souza-Junior, T.P.; et al. Strategies to Prevent Sarcopenia in the Aging Process: Role of Protein Intake and Exercise. Nutrients 2021, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Avery, N.C.; Bailey, A.J. Enzymic and Non-Enzymic Cross-Linking Mechanisms in Relation to Turnover of Collagen: Relevance to Aging and Exercise. Scand. J. Med. Sci. Sports 2005, 15, 231–240. [Google Scholar] [CrossRef]
- Marcus, R.L.; Addison, O.; Kidde, J.P.; Dibble, L.E.; Lastayo, P.C. Skeletal Muscle Fat Infiltration: Impact of Age, Inactivity, and Exercise. J. Nutr. Health Aging 2010, 14, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, N.; Kimura, N.; Hirata, K. Non-Uniform Distribution of Passive Muscle Stiffness within Hamstring. Scand. J. Med. Sci. Sports 2020, 30, 1729–1738. [Google Scholar] [CrossRef]
- Mecagni, C.; Smith, J.P.; Roberts, K.E.; O’Sullivan, S.B. Balance and Ankle Range of Motion in Community-Dwelling Women Aged 64 to 87 Years: A Correlational Study. Phys. Ther. 2000, 80, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Rustani, K.; Kundisova, L.; Capecchi, P.L.; Nante, N.; Bicchi, M. Ultrasound Measurement of Rectus Femoris Muscle Thickness as a Quick Screening Test for Sarcopenia Assessment. Arch. Gerontol. Geriatr. 2019, 83, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Messina, C.; Vitale, J.; Sconfienza, L.M. Imaging of Sarcopenia: Old Evidence and New Insights. Eur. Radiol. 2020, 30, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Madden, K.M.; Feldman, B.; Arishenkoff, S.; Meneilly, G.S. A Rapid Point-of-Care Ultrasound Marker for Muscle Mass and Muscle Strength in Older Adults. Age Ageing 2021, 50, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Wilhelm, E.N.; Rech, A.; Minozzo, F.; Radaelli, R.; Pinto, R.S. Echo Intensity Independently Predicts Functionality in Sedentary Older Men. Muscle Nerve 2017, 55, 9–15. [Google Scholar] [CrossRef]
- Taniguchi, M.; Yamada, Y.; Fukumoto, Y.; Sawano, S.; Minami, S.; Ikezoe, T.; Watanabe, Y.; Kimura, M.; Ichihashi, N. Increase in Echo Intensity and Extracellular-to-Intracellular Water Ratio Is Independently Associated with Muscle Weakness in Elderly Women. Eur. J. Appl. Physiol. 2017, 117, 2001–2007. [Google Scholar] [CrossRef]
- Pillen, S.; Tak, R.O.; Zwarts, M.J.; Lammens, M.M.Y.; Verrijp, K.N.; Arts, I.M.P.; van der Laak, J.A.; Hoogerbrugge, P.M.; van Engelen, B.G.M.; Verrips, A. Skeletal Muscle Ultrasound: Correlation between Fibrous Tissue and Echo Intensity. Ultrasound Med. Biol. 2009, 35, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Baxter, J.R.; Hullfish, T.J.; Chao, W. Functional Deficits May Be Explained by Plantarflexor Remodeling Following Achilles Tendon Rupture Repair: Preliminary Findings. J. Biomech. 2018, 79, 238–242. [Google Scholar] [CrossRef]
- Van Melick, N.; Meddeler, B.M.; Hoogeboom, T.J.; Nijhuis-van der Sanden, M.W.G.; Van Cingel, R.E.H. How to Determine Leg Dominance: The Agreement between Self-Reported and Observed Performance in Healthy Adults. PLoS ONE 2017, 12, e0189876. [Google Scholar] [CrossRef] [PubMed]
- Young, H.-J.; Jenkins, N.T.; Zhao, Q.; Mccully, K.K. Measurement of Intramuscular Fat by Muscle Echo Intensity. Muscle Nerve 2015, 52, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Muanjai, P.; Mickevicius, M.; Sniečkus, A.; Sipavičienė, S.; Satkunskiene, D.; Kamandulis, S.; Jones, D.A. Low Frequency Fatigue and Changes in Muscle Fascicle Length Following Eccentric Exercise of the Knee Extensors. Exp. Physiol. 2020, 105, 502–510. [Google Scholar] [CrossRef]
- Komforti, D.; Joffe, C.; Magras, A.; Peller, A.; Garbe, E.; Garib, R.; Trapuzzano, A.; Dawson, N.; Stock, M.S. Does Skeletal Muscle Morphology or Functional Performance Better Explain Variance in Fast Gait Speed in Older Adults? Aging Clin. Exp. Res. 2021, 33, 921–931. [Google Scholar] [CrossRef]
- Schneebeli, A.; Visconti, L.; Cescon, C.; Clijsen, R.; Giardini, G.; Arizzio, M.E.; Barbero, M. Tendon Morphological Changes after a Prolonged Ski Race Can Be Detected by Ultrasound Echo Intensity. J. Foot Ankle Res. 2020, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Satkunskiene, D.; Mickevicius, M.; Snieckus, A.; Kamandulis, S. Leg Stiffness, Valgus Knee Motion, and Q-Angle Are Associated with Hypertrophic Soft Patella Tendon and Idiopathic Knee Pain in Adolescent Basketball Players. J. Sports Med. Phys. Fit. 2017, 57, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Ayala, F.; Sainz de Baranda, P.; De Ste Croix, M.; Santonja, F. Criterion-Related Validity of Four Clinical Tests Used to Measure Hamstring Flexibility in Professional Futsal Players. Phys. Ther. Sport 2011, 12, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Abdel-aziem, A.A.; Mohammad, W.S. Plantar-Flexor Static Stretch Training Effect on Eccentric and Concentric Peak Torque—A Comparative Study of Trained versus Untrained Subjects. J. Hum. Kinet. 2012, 34, 49–58. [Google Scholar] [CrossRef]
- Konrad, A.; Glashüttner, C.; Reiner, M.M.; Bernsteiner, D.; Tilp, M. The Acute Effects of a Percussive Massage Treatment with a Hypervolt Device on Plantar Flexor Muscles’ Range of Motion and Performance. J. Sports Sci. Med. 2020, 19, 690–694. [Google Scholar] [PubMed]
- Sipe, C.L.; Ramey, K.D.; Plisky, P.P.; Taylor, J.D. Y-Balance Test: A Valid and Reliable Assessment in Older Adults. J. Aging Phys. Act. 2019, 27, 663–669. [Google Scholar] [CrossRef]
- Thiamwong, L.; Suwanno, J. Fear of Falling and Related Factors in a Community-Based Study of People 60 Years and Older in Thailand. Int. J. Gerontol. 2017, 11, 80–84. [Google Scholar] [CrossRef]
- Namsawang, J.; Muanjai, P.; Luangpon, N.; Kiatkulanusorn, S. The Effects of Electrical Stimulation Program on Navicular Height, Balance, and Fear of Falling in Community-Dwelling Elderly. Int. J. Env. Res. Public Health 2021, 18, 9351. [Google Scholar] [CrossRef] [PubMed]
- Janyacharoen, T.; Yonglitthipagon, P.; Nakmareong, S.; Katiyajan, N.; Auvichayapat, P.; Sawanyawisuth, K. Effects of the Applied Ancient Boxing Exercise on Leg Strength and Quality of Life in Patients with Osteoarthritis. J. Exerc. Rehabil. 2018, 14, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-García, J.D.; Martínez-Amat, A.; De la Torre-Cruz, M.J.; Fábrega-Cuadros, R.; Cruz-Díaz, D.; Aibar-Almazán, A.; Achalandabaso-Ochoa, A.; Hita-Contreras, F. Suspension Training HIIT Improves Gait Speed, Strength and Quality of Life in Older Adults. Int. J. Sports Med. 2019, 40, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Thaweewannakij, T.; Wilaichit, S.; Chuchot, R.; Yuenyong, Y.; Saengsuwan, J.; Siritaratiwat, W.; Amatachaya, S. Reference Values of Physical Performance in Thai Elderly People Who Are Functioning Well and Dwelling in the Community. Phys. Ther. 2013, 93, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Boukabous, I.; Marcotte-Chénard, A.; Amamou, T.; Boulay, P.; Brochu, M.; Tessier, D.; Dionne, I.; Riesco, E. Low-Volume High-Intensity Interval Training Versus Moderate-Intensity Continuous Training on Body Composition, Cardiometabolic Profile, and Physical Capacity in Older Women. J. Aging Phys. Act. 2019, 27, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013; ISBN 978-1-4832-7648-9. [Google Scholar]
- Reeves, N.D.; Maganaris, C.N.; Narici, M.V. Ultrasonographic Assessment of Human Skeletal Muscle Size. Eur. J. Appl. Physiol. 2004, 91, 116–118. [Google Scholar] [CrossRef]
- Franchi, M.V.; Reeves, N.D.; Narici, M.V. Skeletal Muscle Remodeling in Response to Eccentric vs. Concentric Loading: Morphological, Molecular, and Metabolic Adaptations. Front Physiol. 2017, 8, 447. [Google Scholar] [CrossRef]
- Kelp, N.Y.; Gore, A.; Clemente, C.J.; Tucker, K.; Hug, F.; Dick, T.J.M. Muscle Architecture and Shape Changes in the Gastrocnemii of Active Younger and Older Adults. J. Biomech. 2021, 129, 110823. [Google Scholar] [CrossRef] [PubMed]
- Strasser, E.M.; Draskovits, T.; Praschak, M.; Quittan, M.; Graf, A. Association between Ultrasound Measurements of Muscle Thickness, Pennation Angle, Echogenicity and Skeletal Muscle Strength in the Elderly. Age 2013, 35, 2377–2388. [Google Scholar] [CrossRef]
- Al-Ghamdi, N.S.; Shaheen, A.A.M. Reference Values and Regression Equations for Predicting the 6-Minute Walk Distance in Saudi Adults Aged 50-80 Years: A Cross- Sectional Study. J. Back Musculoskelet. Rehabil. 2021, 34, 783–793. [Google Scholar] [CrossRef]
- Wu, H.; Wei, Y.; Miao, X.; Li, X.; Feng, Y.; Yuan, Z.; Zhou, P.; Ye, X.; Zhu, J.; Jiang, Y.; et al. Characteristics of Balance Performance in the Chinese Elderly by Age and Gender. BMC Geriatr. 2021, 21, 596. [Google Scholar] [CrossRef] [PubMed]
- Aartolahti, E.; Lönnroos, E.; Hartikainen, S.; Häkkinen, A. Long-Term Strength and Balance Training in Prevention of Decline in Muscle Strength and Mobility in Older Adults. Aging Clin. Exp. Res. 2020, 32, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.J.; Conchola, E.C.; Palmer, T.B.; Stock, M.S. Effects of Aging on Maximal and Rapid Velocity Capacities of the Leg Extensors. Exp. Gerontol. 2014, 58, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A.; Patla, A.E.; Frank, J.S.; Walt, S.E. Biomechanical Walking Pattern Changes in the Fit and Healthy Elderly. Phys. Ther. 1990, 70, 340–347. [Google Scholar] [CrossRef]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef]
- Young, H.-J.; Southern, W.M.; Mccully, K.K. Comparisons of Ultrasound-Estimated Intramuscular Fat with Fitness and Health Indicators. Muscle Nerve 2016, 54, 743–749. [Google Scholar] [CrossRef]
- Manini, T.M.; Clark, B.C.; Nalls, M.A.; Goodpaster, B.H.; Ploutz-Snyder, L.L.; Harris, T.B. Reduced Physical Activity Increases Intermuscular Adipose Tissue in Healthy Young Adults. Am. J. Clin. Nutr. 2007, 85, 377–384. [Google Scholar] [CrossRef]
- Cruz-Montecinos, C.; Guajardo-Rojas, C.; Montt, E.; Contreras-Briceño, F.; Torres-Castro, R.; Díaz, O.; Cuesta-Vargas, A. Sonographic Measurement of the Quadriceps Muscle in Patients with Chronic Obstructive Pulmonary Disease: Functional and Clinical Implications. J. Ultrasound Med. 2016, 35, 2405–2412. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.W.; Roberts, M.; Price, M.J.; Kay, A.D. Association between Knee Extensor and Ankle Plantarflexor Muscle Thickness and Echo Intensity with Postural Sway, Mobility and Physical Function in Older Adults. Exp. Gerontol. 2021, 150, 111385. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-S.; Hsu, N.-W.; Lee, M.-J.; Lin, Y.-Y.; Tsai, C.-C.; Pan, P.-J. Correlation Analysis of Physical Fitness and Its Impact on Falls in 2130 Community- Dwelling Older Adults: A Retrospective Cross-Sectional Study. BMC Geriatr. 2022, 22, 447. [Google Scholar] [CrossRef]
- Kang, H.G.; Dingwell, J.B. Separating the Effects of Age and Walking Speed on Gait Variability. Gait Posture 2008, 27, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Itotani, K.; Kawahata, K.; Takashima, W.; Mita, W.; Minematsu, H.; Fujita, H. Myofascial Release of the Hamstrings Improves Physical Performance-A Study of Young Adults. Healthcare 2021, 9, 674. [Google Scholar] [CrossRef]
- Drew, R.C.; Sinoway, L.I.; White, M.J. The Two-Hour Marathon: Running Economy and Lower Body Flexibility. J. Appl. Physiol. 2011, 110, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Van Onsem, S.; Verstraete, M.; Dhont, S.; Zwaenepoel, B.; Van Der Straeten, C.; Victor, J. Improved Walking Distance and Range of Motion Predict Patient Satisfaction after TKA. Knee Surg Sports Traumatol. Arthrosc. 2018, 26, 3272–3279. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Martinez-Martin, P.; Habibi, S.-A.; Fereshtehnejad, S.-M.; Abasi, A.; Niazi Khatoon, J.; Saneii, S.H.; Taghizadeh, G. Reliability and Validity of Fall Efficacy Scale-International in People with Parkinson’s Disease during On- and Off-Drug Phases. Parkinsons Dis. 2019, 2019, 6505232. [Google Scholar] [CrossRef]
- Onat, Ş.Ş.; Polat, C.S.; Gürçay, E.; Özcan, D.S.; Orhan, A. Muscle Architecture and Clinical Parameters in Stroke Patients: An Ultrasonographic Study. J. Clin. Ultrasound 2022, 50, 713–718. [Google Scholar] [CrossRef]
- Yoshiko, A.; Ogawa, M.; Shimizu, K.; Radaelli, R.; Neske, R.; Maeda, H.; Maeda, K.; Teodoro, J.; Tanaka, N.; Pinto, R.S.; et al. Chair Sit-to-Stand Performance Is Associated with Diagnostic Features of Sarcopenia in Older Men and Women. Arch. Gerontol. Geriatr. 2021, 96, 104463. [Google Scholar] [CrossRef]
- Giuliani, H.K.; Shea, N.W.; Gerstner, G.R.; Mota, J.A.; Blackburn, J.T.; Ryan, E.D. The Influence of Age and Obesity-Altered Muscle Tissue Composition on Muscular Dimensional Changes: Impact on Strength and Function. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Muehlbauer, T.; Granacher, U.; Borde, R.; Hortobágyi, T. Non-Discriminant Relationships between Leg Muscle Strength, Mass and Gait Performance in Healthy Young and Old Adults. Gerontology 2018, 64, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Coletti, C.; Acosta, G.F.; Keslacy, S.; Coletti, D. Exercise-Mediated Reinnervation of Skeletal Muscle in Elderly People: An Update. Eur. J. Transl. Myol. 2022, 32, 10416. [Google Scholar] [CrossRef] [PubMed]
- Ando, R.; Taniguchi, K.; Saito, A.; Fujimiya, M.; Katayose, M.; Akima, H. Validity of Fascicle Length Estimation in the Vastus Lateralis and Vastus Intermedius Using Ultrasonography. J. Electromyogr. Kinesiol. 2014, 24, 214–220. [Google Scholar] [CrossRef] [PubMed]
| Participant Characteristics | |
|---|---|
| Age (years) | 66.6 ± 4.6 |
| 60–65 years (n) | 47 |
| 66–70 years (n) | 34 |
| 71–75 years (n) | 14 |
| 76–80 years (n) | 4 |
| Body mass (kg) | 57.3 ± 8.7 |
| Height (cm) | 156.1 ± 4.7 |
| BMI (kg∙m−2) | 23.5 ± 3.3 |
| Exercise volume (min∙week−1) | 200 ± 213 |
| Ultrasound Imaging | |||
|---|---|---|---|
| VL MT (cm) | 1.81 ± 0.30 | ||
| BF MT (cm) | 1.48 ± 0.32 | ||
| MG MT (cm) | 1.53 ± 0.24 | ||
| VL FL (cm) | 9.22 ± 1.85 | ||
| BF FL0 (cm) | 10.95 ± 2.60 | ||
| BF FLP (cm) | 18.98 ± 3.95 | ||
| MG FL0 (cm) | 5.07 ± 0.79 | ||
| MG FLP (cm) | 5.68 ± 0.75 | ||
| Passive BF FLs/FL0 | 0.78 ± 0.38 | ||
| Passive MG FLs/FL0 | 0.13 ± 0.09 | ||
| Corrected VL EI (A.U.) | 101.4 ± 21.8 | ||
| Corrected BF EI (A.U.) | 104.5 ± 17.2 | ||
| Corrected MG EI (A.U.) | 83.7 ± 14.9 | ||
| AT CSA (cm2) | 0.512 ± 0.114 | ||
| PT CSA (cm2) | 0.662 ± 0.127 | ||
| Flexibility | Balance | ||
| SLR (°) | 87.9 ± 11.0 | Single leg stance (s) | 30.0 ± 16.1 |
| Passive DF (°) | 12.9 ± 4.9 | FES-1 (points) | 23.6 ± 6.6 |
| Physical functions | |||
| 5TSTS (s) | 6.32 ± 1.46 | 10-MFWT (m∙s−1) | 1.92 ± 0.32 |
| TUG (s) | 6.44 ± 1.10 | 6-MWT (m) | 500.8 ± 67.7 |
| SLR | SLS | FES-1 | 5TSTS | TUG | 10-MFWT | 6-MWT | ||
|---|---|---|---|---|---|---|---|---|
| VL MTnor | 0.170, 0.093 | 0.227 *, 0.024 | 0.235 *, 0.019 | −0.228 *, 0.023 | −0.144, 0.154 | −0.185, 0.066 | 0.140, 0.168 | 0.208 *, 0.038 |
| BF MTnor | 0.331 **, 0.001 | 0.068, 0.508 | 0.093, 0.364 | −0.183, 0.074 | −0.061, 0.553 | −0.113, 0.269 | −0.019, 0.085 | 0.080, 0.436 |
| MG MTnor | 0.048, 0.639 | 0.128, 0.206 | 0.339 **, 0.001 | −0.207 *, 0.040 | −0.257 *, 0.010 | −0.444 **, 0.000 | 0.411 **, 0.000 | 0.480 **, 0.000 |
| VL SubcuTnor | 0.099, 0.327 | −0.352 **, 0.000 | −0.130, 0.200 | −0.014, 0.894 | −0.013, 0.901 | 0.031, 0.758 | −0.057, 0.557 | −0.088, 0.387 |
| BF SubcuTnor | 0.166, 0.030 | −0.084, 0.409 | 0.018, 0.858 | 0.002, 0.982 | 0.011, 0.915 | −0.117, 0.248 | 0.047, 0.642 | 0.080, 0.428 |
| MG SubcuTnor | 0.029, 0.775 | −0.231 *, 0.021 | −0.030, 0.765 | −0.037, 0.718 | −0.083, 0.414 | −0.095, 0.348 | 0.115, 0.257 | −0.111, 0.272 |
| VL FL | 0.139, 0.171 | 0.023, 0.825 | −0.164, 0.107 | 0.016, 0.874 | −0.004, 0.968 | −0.122, 0.230 | 0.084, 0.410 | 0.010, 0.919 |
| BF FL0 | 0.189, 0.064 | 0.044, 0.667 | −0.045, 0.664 | −0.109, 0.228 | 0.159, 0.119 | 0.101, 0.324 | −0.217 *, 0.032 | −0.041, 0.689 |
| MG FL0 | −0.056, 0.582 | −0.423 **, 0.000 | 0.086, 0.397 | −0.163, 0.107 | 0.028, 0.781 | −0.186, 0.065 | 0.230 *, 0.022 | 0.139, 0.171 |
| BF FLs | 0.210 **, 0.039 | −0.093, 0.367 | 0.031, 0.762 | −0.129, 0.207 | −0.087, 0.398 | −0.158, 0.122 | 0.118, 0.249 | 0.033, 0.749 |
| MG FLs | −0.053, 0.601 | 0.499 **, 0.000 | 0.069, 0.499 | 0.079, 0.435 | −0.057, 0.575 | 0.040, 0.692 | −0.123, 0.224 | 0.000, 0.998 |
| VL EIcor | −0.055, 0.586 | −0.445 **, 0.000 | −0.342 **, 0.001 | 0.167, 0.099 | 0.045, 0.656 | 0.126, 0.215 | −0.060, 0.553 | −0.202 *, 0.045 |
| BF EIcor | −0.009, 0.926 | −0.091, 0.372 | −0.101, 0.319 | 0.015, 0.880 | −0.060, 0.555 | −0.116, 0.255 | 0.135, 0.184 | 0.108, 0.289 |
| MG EIcor | 0.022, 0.830 | −0.189, 0.065 | −0.107, 0.291 | 0.065, 0.520 | −0.009, 0.929 | 0.142, 0.161 | −0.076, 0.457 | −0.268 **, 0.007 |
| AT CSAnor | 0.024, 0.813 | 0.054, 0.596 | 0.338 **, 0.001 | −0.021, 0.837 | −0.010, 0.919 | −0.017, 0.865 | 0.043, 0.673 | 0.109, 0.281 |
| PT CSAnor | −0.036, 0.723 | 0.231 *, 0.022 | 0.221 *, 0.029 | 0.014, 0.895 | −0.026, 0.802 | −0.132, 0.196 | 0.046, 0.650 | 0.159, 0.117 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muanjai, P.; Namsawang, J.; Satkunskienė, D.; Kamandulis, S. Associations between Muscle-Tendon Morphology and Functional Movements Capacity, Flexibility, and Balance in Older Women. Int. J. Environ. Res. Public Health 2022, 19, 16099. https://doi.org/10.3390/ijerph192316099
Muanjai P, Namsawang J, Satkunskienė D, Kamandulis S. Associations between Muscle-Tendon Morphology and Functional Movements Capacity, Flexibility, and Balance in Older Women. International Journal of Environmental Research and Public Health. 2022; 19(23):16099. https://doi.org/10.3390/ijerph192316099
Chicago/Turabian StyleMuanjai, Pornpimol, Juntip Namsawang, Danguole Satkunskienė, and Sigitas Kamandulis. 2022. "Associations between Muscle-Tendon Morphology and Functional Movements Capacity, Flexibility, and Balance in Older Women" International Journal of Environmental Research and Public Health 19, no. 23: 16099. https://doi.org/10.3390/ijerph192316099
APA StyleMuanjai, P., Namsawang, J., Satkunskienė, D., & Kamandulis, S. (2022). Associations between Muscle-Tendon Morphology and Functional Movements Capacity, Flexibility, and Balance in Older Women. International Journal of Environmental Research and Public Health, 19(23), 16099. https://doi.org/10.3390/ijerph192316099





